- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Activity-based Anorexia for Modeling Vulnerability and Resilience in Mice
Published: Vol 11, Iss 9, May 5, 2021 DOI: 10.21769/BioProtoc.4009 Views: 3405
Reviewed by: Arnau Busquets-GarciaMario ValentinoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2670 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1644 Views
Abstract
Activity-based anorexia (ABA) is a widely used rodent model of anorexia nervosa. It involves combining limited access to food with unlimited access to a running wheel, leading to a paradoxical decrease in food intake, hyperactivity, and life-threatening weight loss. Although initially characterized in rats, ABA has been tested in mice with results that vary based on strain, sex, age, the amount of time food is available, and the number of days of food restriction. Here, we present our ABA protocol for modeling both vulnerability and resilience to diet and exercise in C57BL/6 female mice. While vulnerable mice exhibit the expected increase in running, reduction in food intake, and excessive weight loss, resilient mice exhibit an adaptive increase in food intake, decrease in total wheel running, and weight stabilization. In contrast to previous ABA studies in which resilience is defined by the relative rate of weight loss, our protocol leads to a resilient phenotype that more closely resembles the maintenance of a stable bodyweight exhibited by most humans who diet and exercise without developing anorexia nervosa. This protocol will be useful for future studies aimed at identifying the physiological and neural adaptations underlying both resilience and vulnerability to this eating disorder.
Keywords: Anorexia nervosaBackground
In the 1950s, Hall and Hanford first reported that rats placed on a restricted feeding schedule and given access to a running wheel exhibit a paradoxical increase in running and a decrease in food intake, leading to extreme weight loss (Hall et al., 1953; Hall and Hanford, 1954). The extent to which these responses are life-threatening was established in a later study, in which all the tested rats effectively ran themselves to death (Routtenberg and Kuznesof, 1967). Collectively, these findings formed the basis for the most commonly used animal model of anorexia nervosa, activity-based anorexia (ABA). ABA shares several characteristics with the human disorder, including extreme weight loss, increased voluntary physical activity, self-starvation, loss of estrus (Dixon et al., 2003), and greater vulnerability during adolescence (Woods and Routtenberg, 1971). As an animal model, it enables examination of how caloric restriction in combination with physical activity leads to neural and endocrine adaptations that may contribute to the development of the disorder. While most ABA studies have used rats, an increasing number are using mice, with results that vary based on strain, sex, age, the amount of time food is available, the number of days of food restriction, and how wheel-running is analyzed (Gelegen et al., 2007 and 2008; Klenotich et al., 2012). Given the number of variables affecting susceptibility to ABA, consistency across studies with respect to the parameters used and how the data are analyzed would enhance utility of the model.
A critical question in the field has been what factors promote vulnerability or resilience to anorexia nervosa. This has been difficult to model in ABA since most studies restrict food for 5 days or less, during which all animals show significant weight loss. Weight loss is particularly rapid in adolescent animals. Some investigators categorize animals as ‘vulnerable’ or ‘resilient’ based on how quickly they lose weight during this time period, either by evaluating individual differences within a group (Barbarich-Marsteller et al., 2013; Chowdhury et al., 2013) or by making strain comparisons (Gelegen et al., 2007). However, until recently no implementation of ABA has demonstrated a truly resilient phenotype, defined as adaptation and weight stabilization in the face of food restriction and exercise. We recently published our ABA study in which roughly half of the animals exhibit resilience by decreasing total running, increasing food intake, and reaching a stable bodyweight (Beeler et al., 2020). The other half of the animals are vulnerable to ABA, as demonstrated by increased wheel running, a failure to adapt food intake to increased energy expenditure, and excessive weight loss. Notably, we also detected vulnerability and resilience in food-restricted mice housed with a locked running wheel; although, wheel running does appear to accelerate weight loss. Here, we present the detailed protocol that we used to obtain distinct vulnerable and resilient phenotypes, which involves the use of young adult C57/BL6 female mice instead of adolescent mice, extending the number of days of food restriction from 5 to 10, and conducting a thorough analysis of individual differences.
This protocol will be useful for future studies aimed at characterizing vulnerability and resilience to anorexia nervosa. By generating both phenotypes, this protocol allows studies to examine the physiological, neural, and endocrine factors that differentiate the two, facilitating greater insight into the biological basis of this eating disorder. This protocol also provides an opportunity to evaluate the extent to which interventions decrease vulnerability and favor adaptation and resilience.
Materials and Reagents
Mouse cages: 19.56 cm (width) × 30.91 cm (length) × 14.94 cm (height) (Model #9, Thoren Caging Systems, Inc., Hazleton, PA)
Wire bar lids (WBL7115SMD-AMG, Allentown Inc., Allentown, NJ)
Beta Chip Hardwood bedding (Northeastern Products, WF Fisher and Sons, Somerville, NJ)
Prolab IsoPro rodent diet (WF Fisher & Son, Inc., catalog number: LD5P75) stored at 4°C
Mouse igloo (Bio-Serv, catalog number: K3327)
Clorox Fresh Scent Disinfecting Wipes
Paper towels
8-week-old female C57BL/6N mice (Taconic Biosciences, Germantown, NY)
Equipment
Low-profile wireless running wheels for mice (Med Associates, catalog number: ENV 047)
USB interface hub for wireless running wheels (Med Associates, catalog number: DIG-807)
PC computer
Green features turned ‘off’ so that the display does not go off, the PC does not hibernate or go to sleep, and the hard drive does not turn off
Analytical balance scale (1 mg)
Compact scale (0.1 g) (Fisher Scientific, catalog number: 0191933)
White noise machine (Marpac Dohm-Dual Speed)
Digital thermometer (VWR, catalog number: 35519-047)
External lights (GE Fluorescent Under Cabinet Light Fixtures)
Light timer (Indoor 24 Hour Mechanical Outlet Timer with 2-Outlets)
Red headlamp (Energizer headlamp with one red LED)
Software
Wheel Manager (SOF-860, Med Associates, Inc., St. Albans, VT)
Procedure
Order 8-week-old female C57BL/6N mice from Taconic Biosciences.
Upon arrival, group house mice (3-4/cage) with beta chip bedding, Isopro food, a water bottle, and an igloo. Place cages in a temperature-controlled room dedicated exclusively to ABA testing, with the white noise machine turned ‘on’ high to minimize exposure to animal facility noise. Set the light timers to the 12-h light/dark cycle that will be used throughout the duration of the experiment. If the overhead lights cannot be controlled by a timer, turn them ‘off’ and control the light cycle with external fluorescent light fixtures (18-22 inch) plugged into a mechanical timer. The number of lights required will be determined by the size of the room. Two fluorescent lights are adequate for a room with 2 racks containing a total of 30 cages.
Allow animals one week to acclimate with unlimited access to food and water.
Start the experiment (one week after arrival)
Weigh and allocate mice to one of four groups, counterbalancing weights across the groups. Information gained by testing each group is outlined in the Notes on Data Analysis section.
Activity-based anorexia (ABA, freely moving running wheel, food restriction)
Food-restricted control group (FR, locked wheel, food restriction)
Wheel control group (WH, freely moving running wheel, unlimited food)
Homecage control group (HC, locked wheel, unlimited food)
Individually/singly house mice with either a freely moving running wheel (ABA and WH groups) or a wheel locked with a pin (FR and HC groups). Provide food and water to all groups ad libitum.
Cage set-up: Remove all scents by wiping down each piece of the running wheel with a Clorox® wipe and drying with a paper towel. Place the base of the wheel flat against the bottom of the cage and cover with bedding. Orient the wire lid so that mice can access food or water without sitting on the wheel (Figure 1A). Verify that the wheel does not touch the wire lid and can freely turn (Figure 1B and Video 1). Limit the amount of bedding used so that a build-up of bedding underneath the wheel will not prevent it from turning.
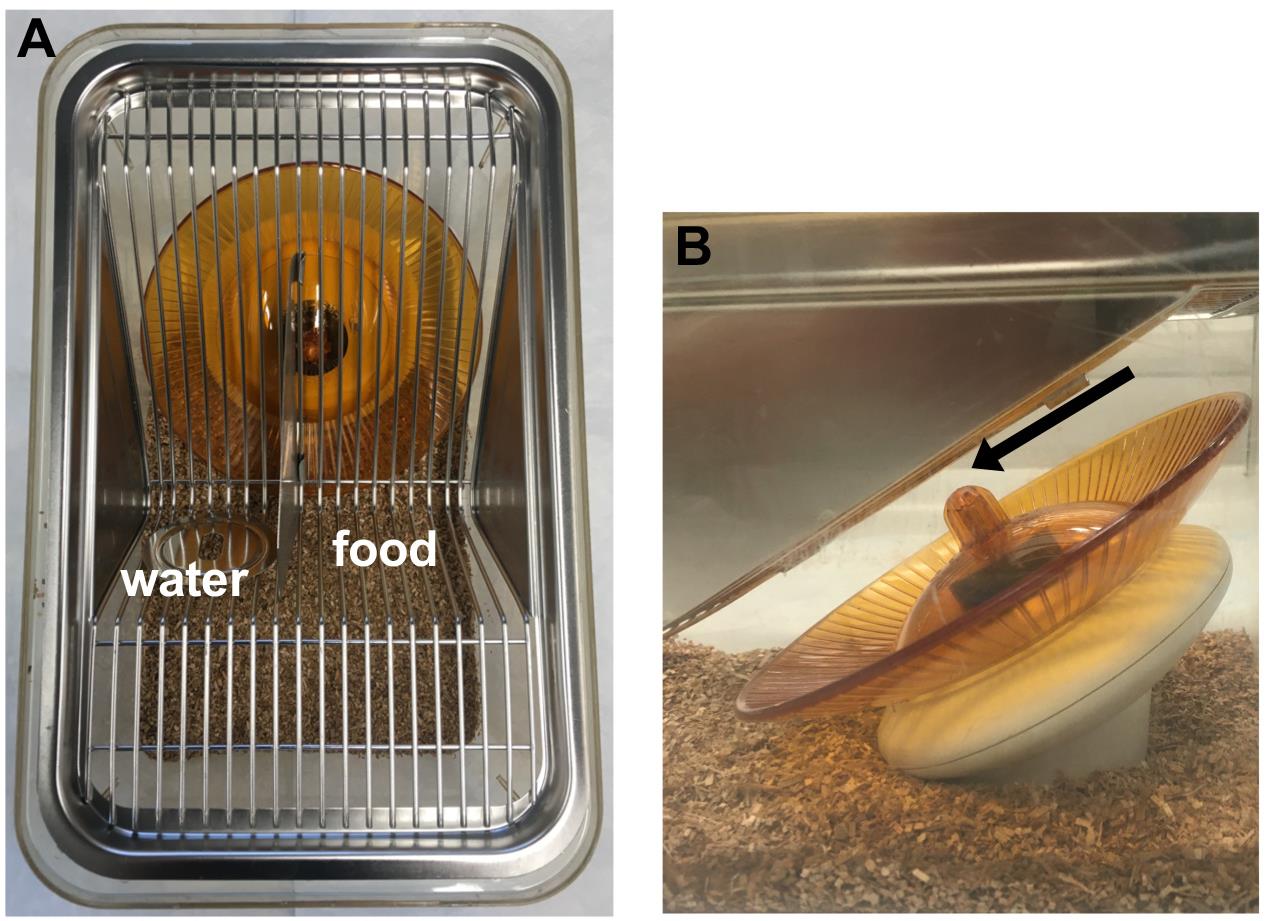
Figure 1. Overhead (A) and side (B) view of a running wheel inside a cage. Arrow indicates the space between the wire lid and the tip of the wheel.Video 1. Representative video of a mouse running on the wheel. Note that the water bottle and food were removed for illustrative purposes.Wheel set-up: Identify mice on the computer (Wheel Manager software) one at a time. Turn on one running wheel (top switch), enter the corresponding mouse ID into the computer, place the wheel in the cage, and then place the mouse in the cage with Isopro food and water before turning on the next wheel. After all IDs have been entered, click ‘Start Acquisition’ under the File menu.
Baseline Data Collection (3 days):
Baseline Day 1: Two days after individually housing the mice (Figure 2A), weigh the mice and food immediately prior to onset of the dark cycle. Different containers should be used (mouse only and food only) so that faeces and urine do not come into contact with the food. To minimize the number of times that each cage is moved, weigh the mouse and then the food from the same cage. When removing the mouse from the cage, place a finger on the running wheel to prevent it from rotating.
Baseline Day 2: Follow the same procedure as the previous day.
Baseline Day 3: Follow the same procedure as the previous day. Subsequently, remove the food from the ABA and FR groups 2 h after onset of the dark cycle. If the room does not contain an overhead red light, enter the room wearing a red headlamp so that the light/dark cycle is not disturbed.
ABA Data Collection (10 days):
Preparation: Each day, record the weight of large pellets of food (9-11 g) to the nearest 0.01 g for each ABA and FR mouse. Use fresh pellets in the event that a scent was left behind the previous day. Store food at room temperature in cups/containers labeled with the mouse ID and cover with foil.
Immediately prior to onset of the dark cycle, weigh all the mice and food in HC and WH groups.
Backup data without interrupting data collection by clicking ‘Export Data’ under the File menu.
Place the labeled food containers in front of the corresponding cages.
Once the lights have turned off, place the pre-weighed pellets in the overhead food bin of the ABA and FR mice and then leave the room for 2 h. Wear a red headlamp while administering the food, if necessary.
Calculate the % baseline (%B) bodyweight by dividing the current weight by the bodyweight recorded on baseline day 3 and multiplying by 100. Any mouse that is at 75% of the baseline weight or lower is at risk of death and must be removed from the experiment.
After 2 h of food availability (first 2-h of the dark cycle), remove the food pellets from the mice in the ABA and FR groups, place in the labeled containers, and cover with foil. Do not turn on the overhead lights when entering the ABA room; wear a red headlamp, if necessary. While in the room, fill the food bin (~40 g food) of mice that require removal from the experiment (≥ 25% bodyweight loss).
Record the post-intake weights of food pellets to 0.01 g for the ABA and FR groups and calculate the intake during the 2-h period of food availability for each mouse. Throw away partially eaten food and weigh fresh food the next day.
Repeat the steps above every day for 10 consecutive days.
On ABA day 10, remove all remaining mice from the experiment after 2 h of unlimited access to food.
Data analysis
Bodyweight data
Conduct a survival analysis (e.g., Kaplan-Meier) of the ABA and FR groups across all 10 days of food restriction (Figure 2B). This is a way of comparing groups based on when they lost 25% or more of their baseline bodyweight and were removed from the experiment. Percent survival indicates the percentage of animals within each group that remained in the experiment (i.e., not removed) on each day. Earlier removal from the experiment indicates a higher vulnerability to ABA.
For each day of food restriction, calculate the average percent baseline (%B) bodyweight for each group (Figure 2C). Given that mice are removed from the experiment on different days, use the linear mixed-effects model to analyze the data.
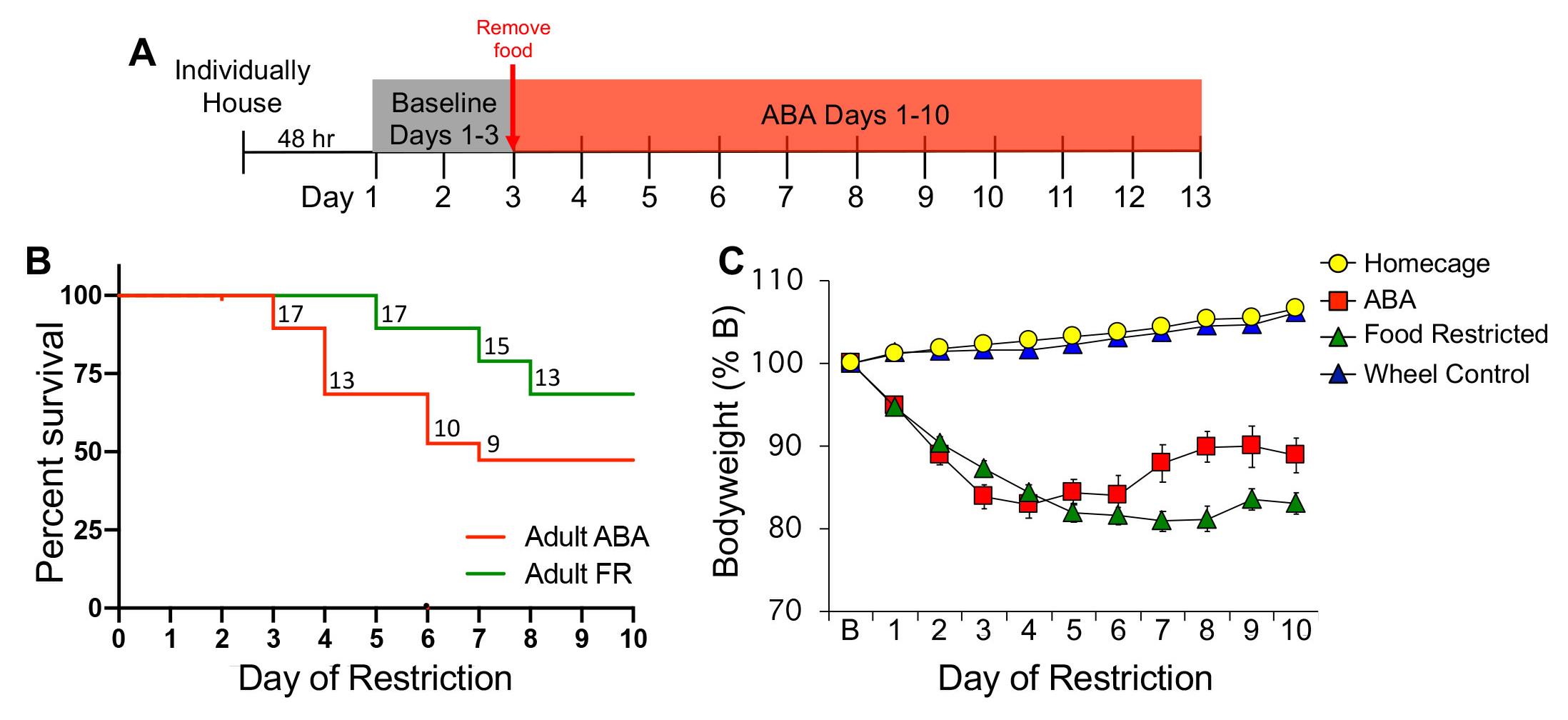
Figure 2. Experimental timeline (A), survival curve (B), and bodyweight data (C). Numbers inside the survival curve indicate the number of surviving mice on each day of food restriction. Error bars indicate the SEM; n = 19 (ABA), n = 19 (FR), n = 12 (homecage), n = 14 (wheel control).Identification of Vulnerable and Resilient Phenotypes: Within the ABA and FR groups, characterize mice that were removed from the experiment as ‘vulnerable’ and mice that remained throughout the duration of the experiment as ‘resilient.’ A comparison of bodyweight across the days of food restriction reveals rapid weight loss in vulnerable mice and maintenance of bodyweight in resilient mice (Figure 3).
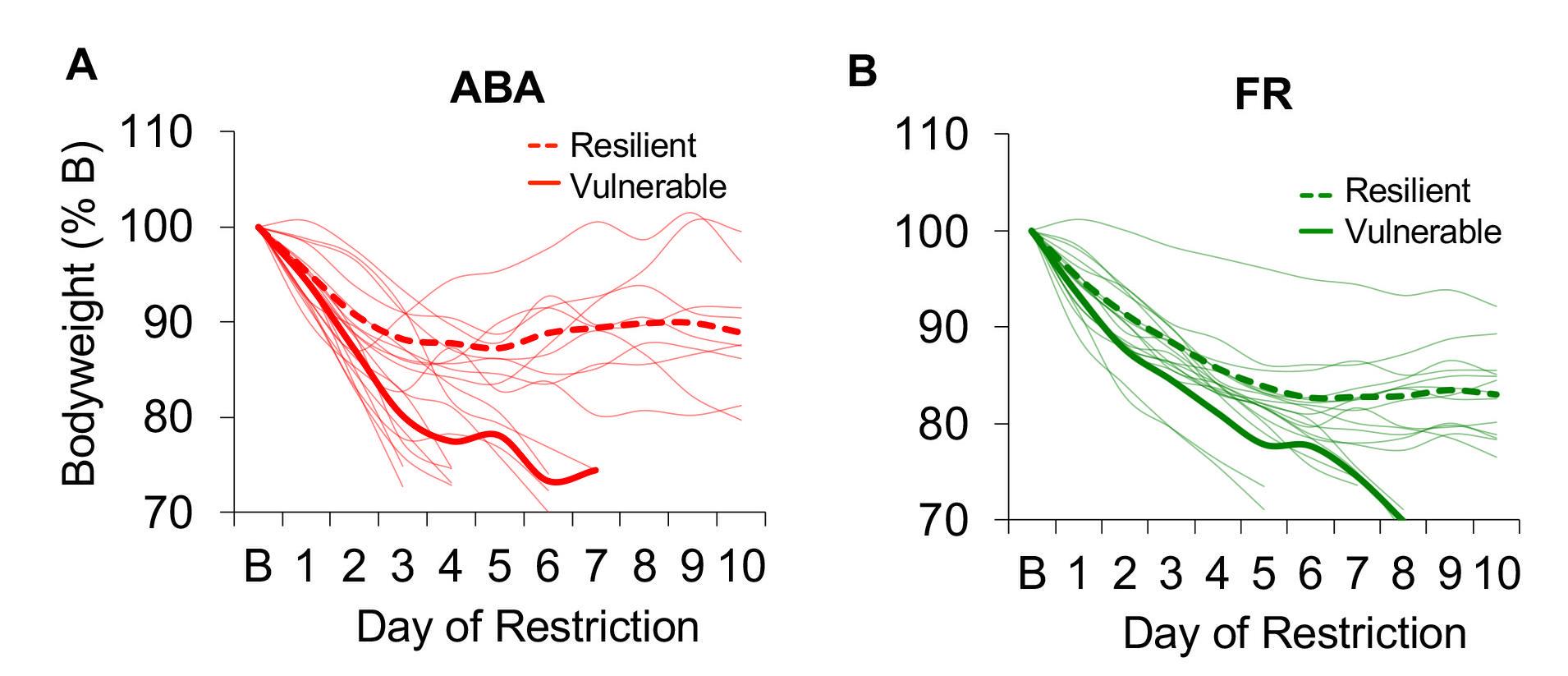
Figure 3. Bodyweight of individual mice (light traces) across the days of food restriction in (A) activity-based anorexia (ABA) and (B) food-restricted control (FR) groups. Group averages for resilient (dashed line) and vulnerable (solid line) mice are shown in bold. n = 10 vulnerable ABA, n = 9 resilient ABA, n = 6 vulnerable FR, n = 13 resilient FR.
Food intake data
Average the food intake across all 10 days of the experiment and use a one-way ANOVA for group comparisons (Figure 4A).
Compare the average intake of vulnerable and resilient ABA and FR mice across all 10 days of the experiment using a two-way ANOVA (Figure 4B).
Calculate the average intake before each animal’s last day in the experiment and the intake on the day of removal. Compare vulnerable and resilient ABA and FR mice using a two-way ANOVA (Figure 4C).
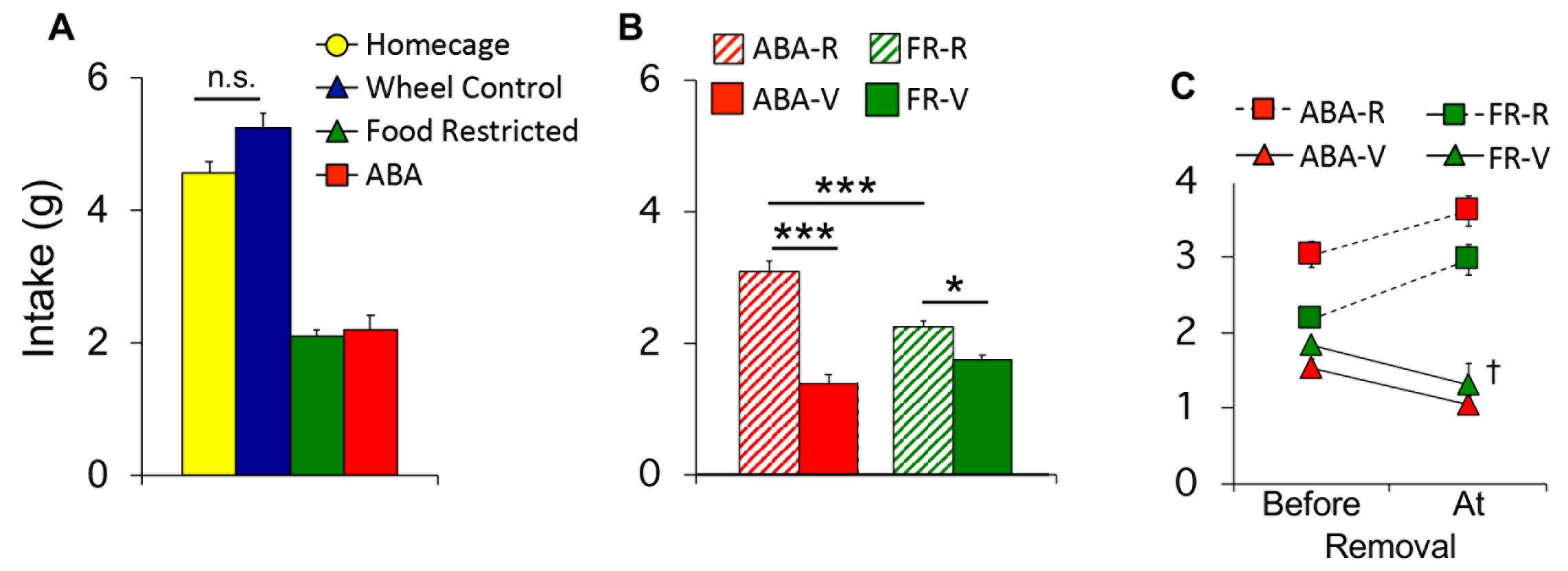
Figure 4. Food intake data. A. Food intake for all mice in each group averaged across 10 days of restriction. B. Average food intake of vulnerable (solid) and resilient (hatched) mice in the ABA and FR groups. C. Average food intake of vulnerable (triangle) and resilient (square) mice in the ABA and FR groups prior to removal vs. the day of removal. *P < 0.05; ***P < 0.001; †P < 0.01 vs. vulnerable groups before removal. Error bars indicate the SEM.Running wheel data
Use the Wheel Manager software (SOF-860, Med Associates, Inc., St. Albans, VT) to extract the running wheel data. Calculate the average total running across all 10 days of the experiment for wheel control, vulnerable ABA, and resilient ABA mice. Use a one-way ANOVA for group comparisons (Figure 5A).
Separately calculate the average light cycle (12-h) and dark cycle (12-h) running for each group and use a one-way ANOVA for group comparisons (Figure 5B, 5C).
Optional: Calculate the maximum increase in light cycle and dark cycle running across 2 consecutive days of the experiment. Our work indicates that vulnerability to ABA is associated with an abrupt increase in light cycle running, as indicated by a significantly larger increase in running over a 2-day period (Figure 5D). This abrupt increase in light cycle running can occur on any day of food restriction but precedes removal from the experiment by 1-3 days for most mice.
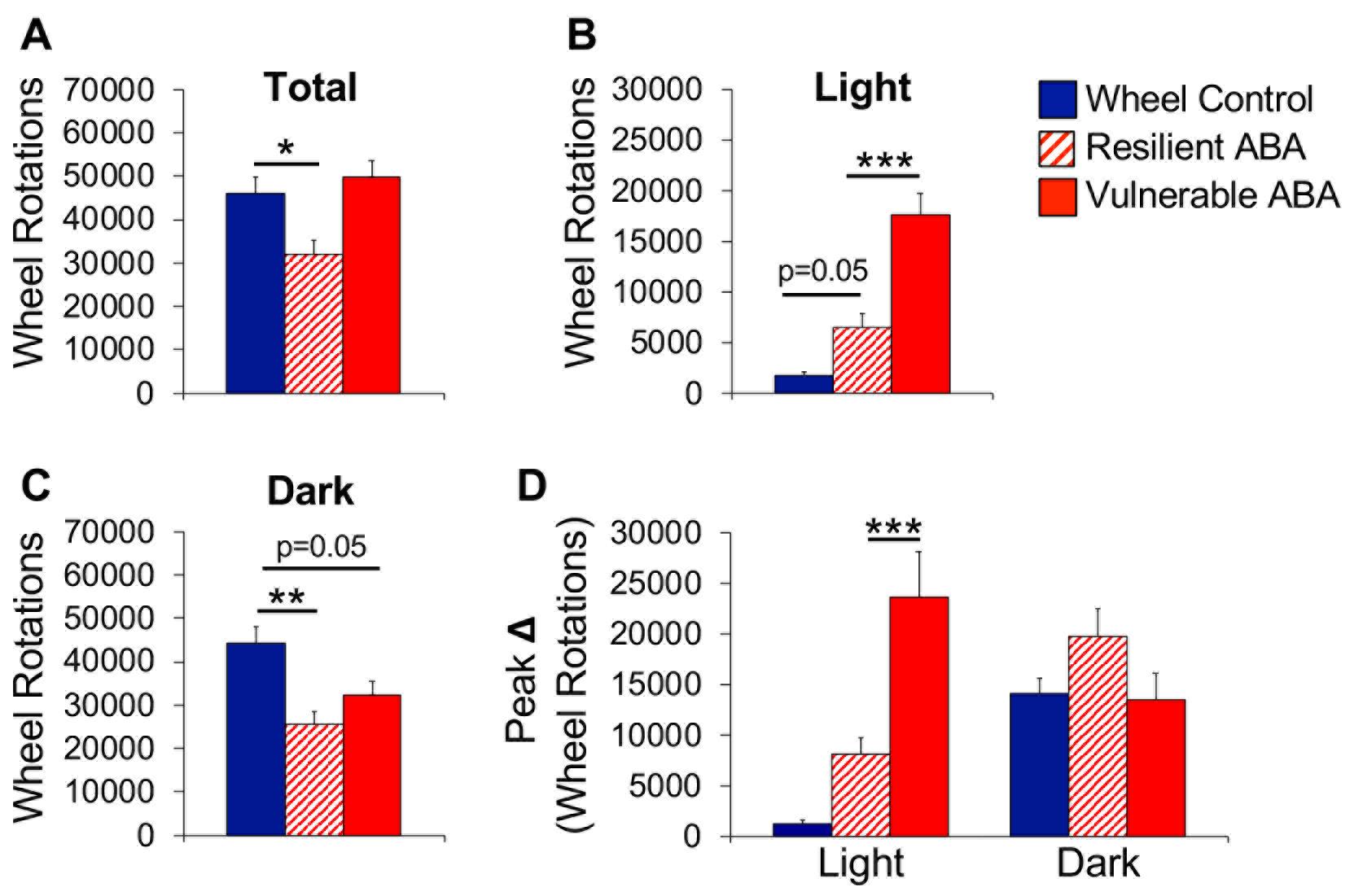
Figure 5. Wheel running data. (A) Total, (B) light cycle, and (C) dark cycle running averaged across the days of food restriction. (D) Maximum increase in wheel running across 2 consecutive days (averaged by group) during the light and dark cycles. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate the SEM.Optional: Collate the running wheel data into 5-min bins for each mouse on each day of the experiment (baseline day 1 to ABA day 10). Graph the data in a dot plot, with day-time on the x-axis and wheel revolutions on the y-axis; color code by group and indicate light and dark cycles (Figure 6A). Additionally, collate the running wheel data into 1-h blocks and then average across all baseline days (Figure 6B) and experimental/food restriction days (Figure 6C) for each group. The resulting graphs demonstrate group differences in running across the 24-h circadian cycle and can be used to characterize the extent of light cycle running. Running that precedes presentation of the food is referred to as food anticipatory activity.
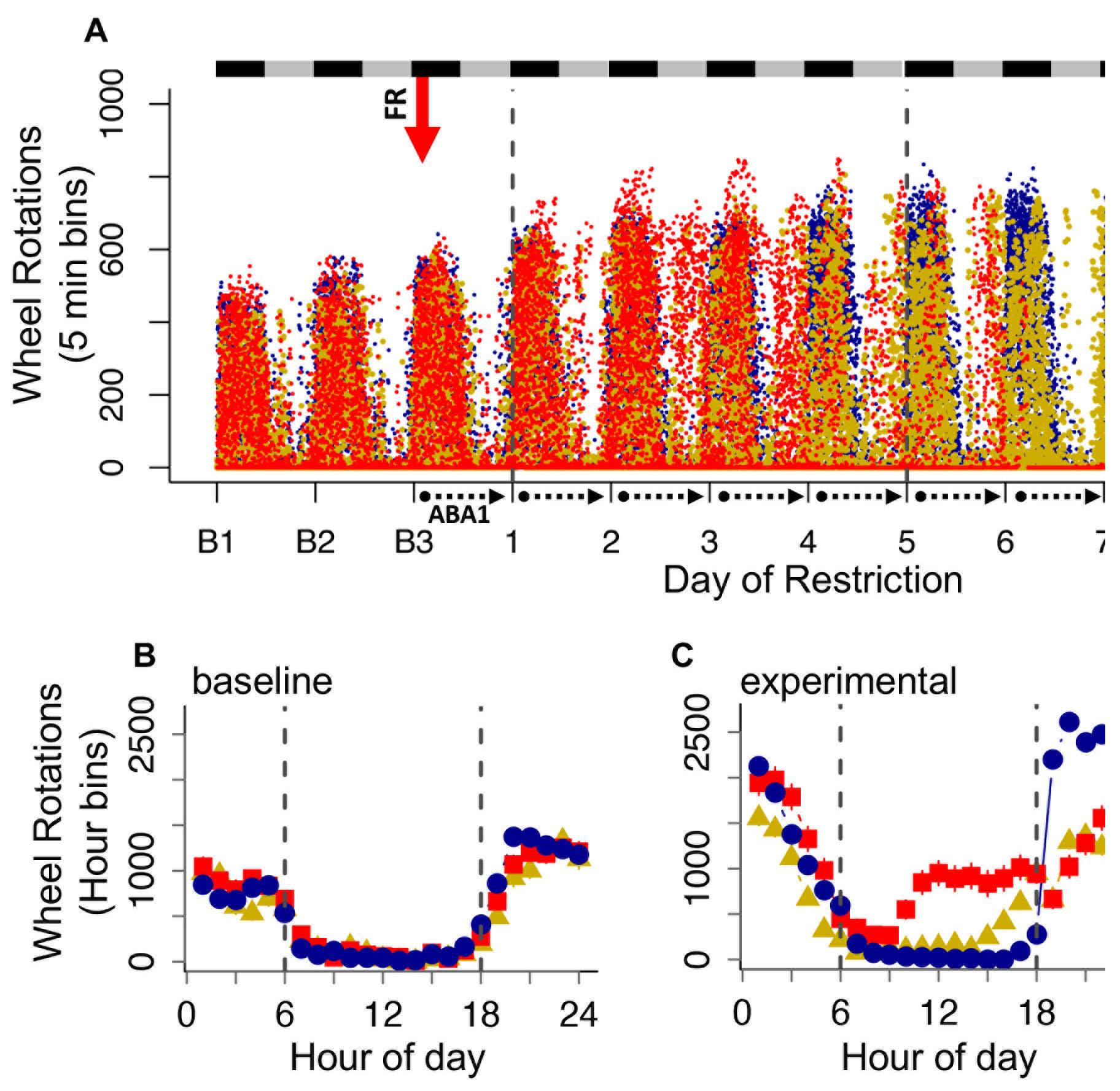
Figure 6. Analysis of the running wheel data across the 24-h circadian cycle. A. Dot plot of wheel running across the experimental days for all mice in 5-min bins. Black and gray bars indicate dark and light cycles, respectively. The red arrow indicates the start of food restriction (FR). B-C. Group averages across a 24-h period for (B) baseline days and (C) experimental days. The dotted lines mark the light cycle between 6 (lights on) and 18 (lights off) hours. Error bars indicate the SEM.
Notes
Notes on the Procedure:
Mice have been seen eating Bed-o’Cob bedding when hungry, which is why this protocol uses beta chip bedding.
Noise provokes activity in mice, including running behavior. To generate reproducible data, use a colony room that is dedicated solely to ABA testing so that the experimenter is the only person entering the room each day and noise is minimized.
When the lights are on, the experimenter should only enter the room shortly before the onset of the dark cycle to weigh the mice and food. Entering the room earlier may wake the mice and cause them to run, confounding the results. It can be challenging to weigh a large cohort of mice within this limited time period. We suggest entering the room no sooner than 45 min before the lights go off, which we find is enough time to weigh 20-30 mice, depending on whether they have unlimited access to food.
Room temperature has been shown to affect vulnerability to activity-based anorexia (Gutierrez et al., 2002). ABA should be tested in a room where the temperature is tightly controlled. In our experiments, the room temperature is monitored daily and ranges from 70-75°F.
Vulnerability to ABA varies based on the age and strain of the mouse (Gelegen et al., 2007; Klenotich and Dulawa, 2012; Klenotich et al., 2012). Using a strain and/or age group that differs from that specified in this protocol will likely affect the distribution of vulnerable and resilient mice, such that all mice may only exhibit one phenotype.
This protocol uses female mice since anorexia nervosa is more prevalent in women than men. It is possible that using male mice will lead to different results.
The vast majority of mice that have lost 25% of their baseline weight and are removed from the experiment will recover overnight; however, this is not true for all mice. If a mouse appears particularly weak, we recommend placing a moistened pellet of food on the floor of the cage (in addition to filling the food bin) during the recovery period.
Give food-restricted mice the largest pellets of food. This will reduce the possibility that a small piece of food will fall into the cage during feeding.
Notes on Data analysis:
A comparison of food intake in the ABA and FR groups reveals how much wheel running affects consumption when food is only available for 2 h. A comparison of wheel running in the ABA and WH groups reveals how much food restriction affects the level of activity. The HC control group provides a measure of bodyweight and food intake in the absence of food restriction and/or wheel running.
All data shown in Figures 1-6 have previously been published (Beeler et al., 2020).
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse, DA046058 (JAB), the National Institute of Mental Health, R21MH114182 (NSB), the National Institute on Minority Health and Health Disparities of the NIH, G12MD007599 (NSB), the PSC-CUNY Awards program jointly funded by the Professional Staff Congress and the City University of New York (JAB & NSB), and the Klarman Family Foundation Eating Disorders Research Grants Program (JAB & NSB). The described protocol was adapted from previous studies in which activity-based anorexia was tested in other mouse strains (Klenotich and Dulawa, 2012; Klenotich et al., 2012).
Competing interests
The authors have no financial or non-financial competing interests to report.
Ethics
This protocol was approved by the Institutional Animal Care and Use Committees of Hunter College (NB-Anorexia, 9/1/20-8/31/23) and Queens College (ACUP# 164, 2/26/20-2/25/23), USA.
References
- Barbarich-Marsteller, N. C., Underwood, M. D., Foltin, R. W., Myers, M. M., Walsh, B. T., Barrett, J. S. and Marsteller, D. A. (2013). Identifying novel phenotypes of vulnerability and resistance to activity-based anorexia in adolescent female rats. Int J Eat Disord 46(7): 737-746.
- Beeler, J. A., Mourra, D., Zanca, R. M., Kalmbach, A., Gellman, C., Klein, B. Y., Ravenelle, R., Serrano, P., Moore, H., Rayport, S., Mingote, S. and Burghardt, N. S. (2020). Vulnerable and Resilient Phenotypes in a Mouse Model of Anorexia Nervosa. Biol Psychiatry doi: 10.1016/j.biopsych.2020.06.030.
- Chowdhury, T. G., Wable, G. S., Sabaliauskas, N. A. and Aoki, C. (2013). Adolescent female C57BL/6 mice with vulnerability to activity-based anorexia exhibit weak inhibitory input onto hippocampal CA1 pyramidal cells. Neuroscience 241: 250-267.
- Dixon, D. P., Ackert, A. M. and Eckel, L. A. (2003). Development of, and recovery from, activity-based anorexia in female rats. Physiol Behav 80(2-3): 273-279.
- Gelegen, C., Collier, D. A., Campbell, I. C., Oppelaar, H., van den Heuvel, J., Adan, R. A. and Kas, M. J. (2007). Difference in susceptibility to activity-based anorexia in two inbred strains of mice. Eur Neuropsychopharmacol 17(3): 199-205.
- Gelegen, C., van den Heuvel, J., Collier, D. A., Campbell, I. C., Oppelaar, H., Hessel, E. and Kas, M. J. (2008). Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav 7(5): 552-559.
- Gutierrez, E., Vazquez, R. and Boakes, R. A. (2002). Activity-based anorexia: ambient temperature has been a neglected factor. Psychon Bull Rev 9(2): 239-249.
- Hall, J. F. and Hanford, P. V. (1954). Activity as a function of a restricted feeding schedule. J Comp Physiol Psychol 47(5): 362-363.
- Hall, J. F., Smith, K., Schnitzer, S. B. and Hanford, P. V. (1953). Elevation of activity level in the rat following transition from ad libitum to restricted feeding. J Comp Physiol Psychol 46(6): 429-433.
- Klenotich, S. J. and Dulawa, S. C. (2012). The activity-based anorexia mouse model. Methods Mol Biol 829: 377-393.
- Klenotich, S. J., Seiglie, M. P., McMurray, M. S., Roitman, J. D., Le Grange, D., Dugad, P. and Dulawa, S. C. (2012). Olanzapine, but not fluoxetine, treatment increases survival in activity-based anorexia in mice. Neuropsychopharmacology 37(7): 1620-1631.
- Routtenberg, A. and Kuznesof, A. W. (1967). Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol 64(3): 414-421.
- Woods, D. J. and Routtenberg, A. (1971). "Self-starvation" in activity wheels: developmental and chlorpromazine interactions. J Comp Physiol Psychol 76(1): 84-93.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Beeler, J. A. and Burghardt, N. S. (2021). Activity-based Anorexia for Modeling Vulnerability and Resilience in Mice. Bio-protocol 11(9): e4009. DOI: 10.21769/BioProtoc.4009.
Category
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









