- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Production of Phenotypically Uniform Human Cerebral Organoids from Pluripotent Stem Cells
(*contributed equally to this work) Published: Vol 11, Iss 8, Apr 20, 2021 DOI: 10.21769/BioProtoc.3985 Views: 5556
Reviewed by: Prashanth N SuravajhalaObul BandapalliAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assay for Site-Specific Homologous Recombination Activity in Adherent Cells, Suspension Cells, and Tumor Tissues
Yuki Yoshino [...] Natsuko Chiba
Apr 5, 2025 2432 Views

Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
Quang Bach Le [...] Deepak Choudhury
Nov 20, 2025 1536 Views

Vascularization of Human Pancreatic Islets With Adaptive Endothelial Cells for In Vitro Analysis and In Vivo Transplantation
Ge Li [...] Shahin Rafii
Dec 20, 2025 751 Views
Abstract
Recent advances in stem cell technology have allowed researchers to generate 3D cerebral organoids (COs) from human pluripotent stem cells (hPSCs). Indeed, COs have provided an unprecedented opportunity to model the developing human brain in a 3D context, and in turn, are suitable for addressing complex neurological questions by leveraging advancements in genetic engineering, high resolution microscopy, and tissue transcriptomics. However, the use of this model is limited by substantial variations in the overall morphology and cellular composition of organoids derived from the same pluripotent cell line. To address these limitations, we established a robust, high-efficiency protocol for the production of consistent COs by optimizing the initial phase of embryoid body (EB) formation and neural induction. Using this protocol, COs can be reproducibly generated with a uniform size, shape, and cellular composition across multiple batches. Furthermore, organoids that developed over extended periods of time (3–6 months) showed the establishment of relatively mature features, including electrophysiologically active neurons, and the emergence of oligodendrocyte progenitors. Thus, this platform provides a robust experimental model that can be used to study human brain development and associated disorders.
Graphic abstract:
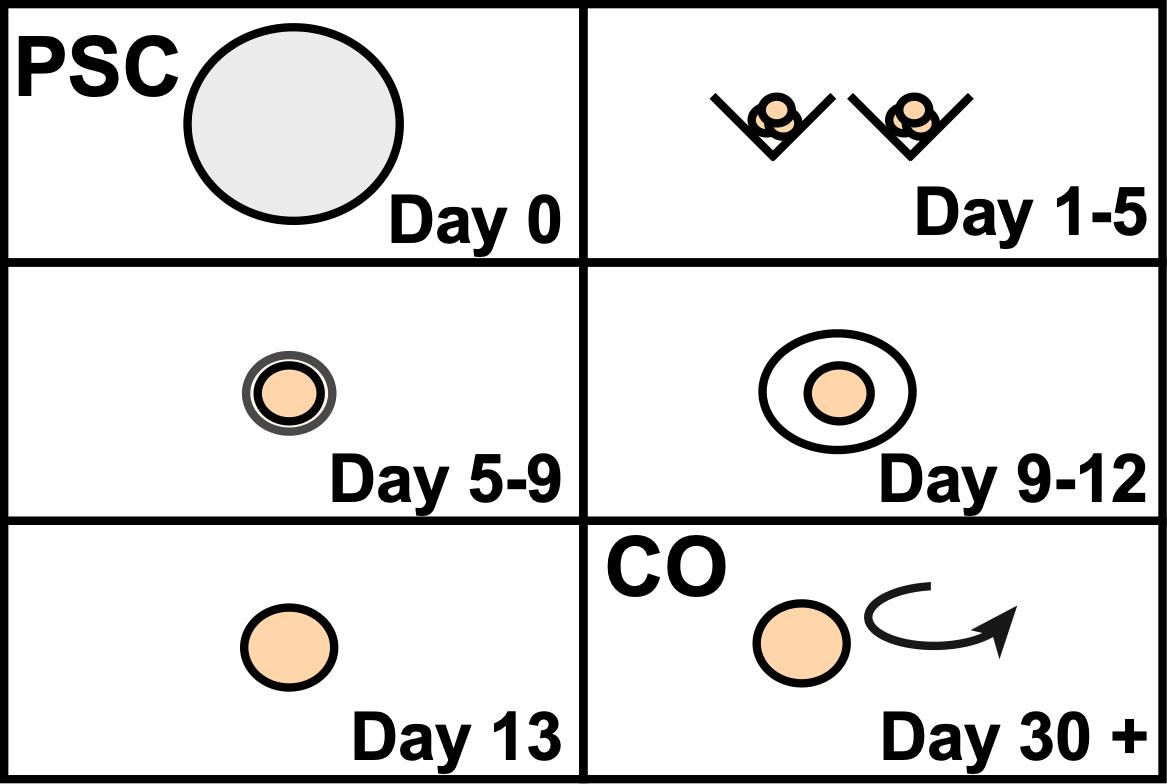
Overview of cerebral organoid development from pluripotent stem cells
Background
Recent advancements in the in vitro development of 3D cerebral organoids (COs) derived from human pluripotent stem cells (hPSCs) have provided an unprecedented opportunity to model the developing human brain and relevant complex diseases in an experimentally tractable system. Indeed, this approach has allowed researchers to study early brain development and the consequences of alterations associated with various human neurological disorders, such as Alzheimer’s, blindness, Autism Spectrum Disorder (ASD), and Zika virus infection (Lancaster and Knoblich, 2014b; Quadrato et al., 2016; Di and Kriegstein, 2017; Huch et al., 2017; Amin and Paşca, 2018; Rossi et al., 2018; Chen et al., 2019). In addition, several groups have applied COs to study and establish preclinical models of human brain cancers such as glioblastoma multiforme (Drost and Clevers, 2018; Linkous et al., 2018). In recent years, numerous protocols have emerged to facilitate the development of region specific-COs by controlling the underlying cell signaling pathways with exogenous growth factors and small molecule inhibitors to guide cell fate changes as the organoid matures (Lancaster et al., 2013; Mariani et al., 2015; Jo et al., 2016; Qian et al., 2016; Birey et al., 2017; Quadrato et al., 2017; Watanabe et al., 2017; Pollen et al., 2019; Velasco et al., 2019; Yoon et al., 2019). However, due to the fact that human whole-brain organoids are largely produced by intrinsic self-patterning and do not rely on controllable exogenous factors, stochastic differentiation often leads to cellular diversity, which is amplified with extended culture. Unfortunately, the considerable variability between individual organoids obtained using whole-brain differentiation platforms can therefore limit the utility of these COs for studying disease mechanisms or the development of potential therapeutics. Here, we describe our robust protocol for efficiently and reproducibly generating mature, uniform human COs (Figure 1). By optimizing an established protocol for the creation of self-patterned whole-brain organoids (Lancaster and Knoblich, 2014a; Lancaster et al., 2013), we successfully generated phenotypically uniform forebrain organoids with reproducible cell-type compositions.
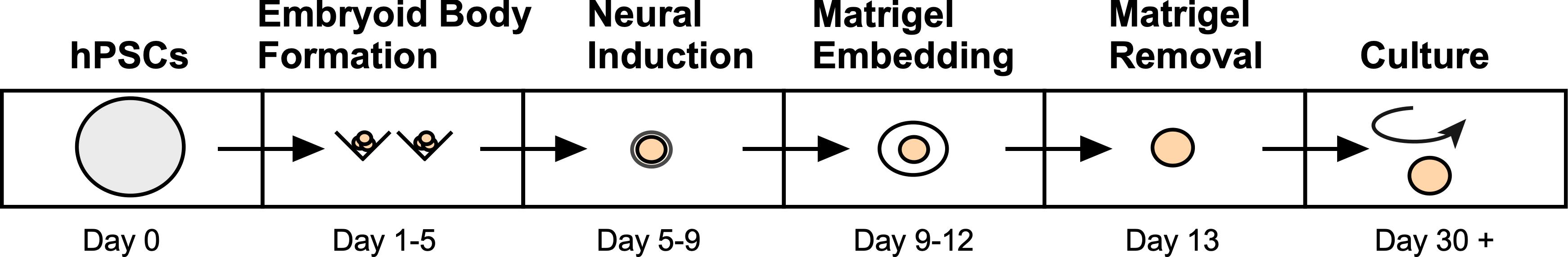
Figure 1. Overview of the developed method to generate human COs from pluripotent stem cells
Materials and Reagents
96-well V-bottomed non-binding plates (Greiner Bio-One, catalog number: 651970 )
24-well clear flat-bottomed ultra-low attachment plates (Corning, catalog number: 3473 )
4-well cell culture plates (ASI, catalog number: TP9004 )
50 ml and 15 ml conical centrifuge tubes (Corning, Falcon, catalog numbers: 352096, 352070 )
Low-retention microcentrifuge tubes (1.5 ml and 0.6 ml, Thermo Fisher Scientific, catalog numbers: 3451 and 3446 )
50 ml sterile disposable reagent reservoirs (Corning, catalog number: 4870 )
DMEM/F-12, HEPES (Thermo Fisher Scientific, Gibco, catalog number: 11330032 , store at 4 °C)
KnockOutTM Serum Replacement – Multi-Species (Thermo Fisher Scientific, Gibco, catalog number: A3181502 , store at -20°C)
MEM Non-Essential Amino Acid Solution (100×) (Thermo Fisher Scientific, Gibco, catalog number: 11140050 , store at 4°C)
2-Mercaptoethanol (1,000×) (Thermo Fisher Scientific, Gibco, catalog number: 21985023 , store at 4 °C)
Animal-Free Recombinant Human FGF-basic (Peprotech, catalog number: AF-100-18B , store at -80°C)
TrypLETM Express Enzyme (1×) (Thermo Fisher Scientific, Gibco, catalog number: 12604013 , stored at room temperature [22°C] in the dark)
Neural Basal Medium (Thermo Fisher Scientific, Gibco, catalog number: 21103049 , store at 4°C)
Y27632 ROCK Inhibitor (Cedarlane, catalog number: S1049-10MG , store at -80°C)
GlutaMAXTM Supplement (Thermo Fisher Scientific, Gibco, catalog number: 35050-061 , store at 4°C)
Heparin sodium salt (Sigma-Aldrich, catalog number: H4784 , store at -20°C)
Insulin solution human (Sigma-Aldrich, catalog number: I9278 , store at 4°C)
N-2 Supplement (100×) (Thermo Fisher Scientific, Gibco, catalog number: 17502001 , aliquots of 500 µl, store at -20°C)
B-27TM Supplement (50×), minus vitamin A (Thermo Fisher Scientific, Gibco, catalog number: 12587010 , aliquots of 500 µl, store at -20°C)
B-27TM Supplement (50×), serum-free (Thermo Fisher Scientific, Gibco, catalog number: 17504044 , aliquots of 500 µl, store at -20°C)
Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix (Corning, catalog number: 356231 , aliquots of 500 µl, store at -20°C)
D-PBS-/-, 1×, without calcium and magnesium (Wisent Bioproducts, catalog number: 311-425-CL , store at 4°C)
Embryoid Body Media (EB Media) (see Recipes)
Neural Induction Media (see Recipes)
Cerebral Organoid Differentiation Media without Vitamin A (CDM-A) (see Recipes)
Cerebral Organoid Differentiation Media with Vitamin A (CDM+A) (see Recipes)
Equipment
NalgeneTM Square PETG media bottles (250 ml) (Gibco, Thermo Fisher Scientific, catalog number: 2019-0250 )
Pipettes (5 ml, 10 ml, 25 ml, 50 ml), micro-pipettes (10 µl, 20 µl, 200 µl, 1,000 µl)
Multi-channel pipette (Eppendorf, model: Research plus 12 channel pipette, catalog number: ES-12-300 )
Water bath
Centrifuge
Phase contrast microscope
Hemocytometer
37°C, 5% CO2 cell culture incubator
Orbital shaker that can be installed inside the incubator (such as Thermo Fisher, catalog number: 88881101 )
Blade and scalpel
Procedure
Embryoid Body Production: Singularizing and Plating Human Embryonic Stem Cells (hESCs)
Prepare EB Media without growth factors and prewarm to 37°C using a water bath. EB Media (without growth factors) can be stored for up to 1 week at 4°C, and once warmed, the stock should be used or discarded.
Wash hESCs, which have reached 70–80% confluence, twice with 3 volumes of D-PBS-/-.
Note: This protocol was established using hESCs cultured in mouse embryoinic fibroblast (MEF) conditioned media supplemented with bFGF to a final concentration of 4 ng/ml (Sivitilli et al., 2020).
Add 1 ml TrypLE and incubate for 5 min at room temperature (22°C).
Gently pipette up and down to dissociate colonies to a single-cell suspension.
Neutralize TrypLE with 4 volumes (i.e., 4 ml) EB media and add the resulting suspension to a 15-ml Falcon tube.
Use a hemocytometer to count the cells. Avoid using an automated cell counter, which can result in inaccurate cell numbers because hESCs are prone to clumping.
Cenrifuge cells at 150 × g for 5 min at room temperature (22°C).
During centrifugation, prepare EB Media by supplementing with 4 ng/ml bFGF and 50 µM Y-27632 (ROCK inhibitor). For this, use 15 ml EB media with 1.5 µl bFGF (from a 40 ng/µl stock) and 7.5 µl Y-27635 (from a 100 mM stock).
Resuspend single hESCs to a final concentration of 80,000 cells/ml in the freshly prepared EB Media supplemented with bFGF and Y-27632.
Transfer the resuspended cells to a sterile disposable reagent reservoir and add 150 µl cell suspension (12,000 cells) to each well of a 96-well V-bottomed non-binding plate using a multi-channel pipette.
Place the plate at 37°C in a CO2 incubator for 2 days.
Embryoid Body Production: Feeding
Prepare EB Media fresh daily and prewarm to 37°C using a water bath.
On day 2, feed the EBs with EB media supplemented with 2 ng/ml bFGF. To reach the required concentration, mix 15 ml EB media with 0.75 µl bFGF (40 ng/ul).
Remove 135 µl media from each well of the incubated 96-well plate using the multi-channel pipette.
Transfer the EB media (supplemented with 2 ng/ml bFGF) into a sterile disposable reagent reservoir and add 150 µl EB media using the multi-channel pipette.
Return the plate to the CO2 incubator for a further 2 days.
Note: Due to evaporation, wells toward the peripheral edge of the 96-well plate may have a slightly lower volume. This is normal and EBs should still form correctly.
Embryoid Body Production: Assessment Checkpoint
On day 5, measure the EB size using a brightfield microscope to determine whether the EBs are ready for neural induction (Figure 2). If the size of the EBs is 350–600 µm, proceed to Procedure D (Neural Induction); if the size is below 350 µm, then repeat Procedure B; if the size is above 600 µm, we do not recommend continuing with the protocol. Restart the protocol and double check cell counting in Procedure A to ensure accuracy when seeding EBs and start another batch.
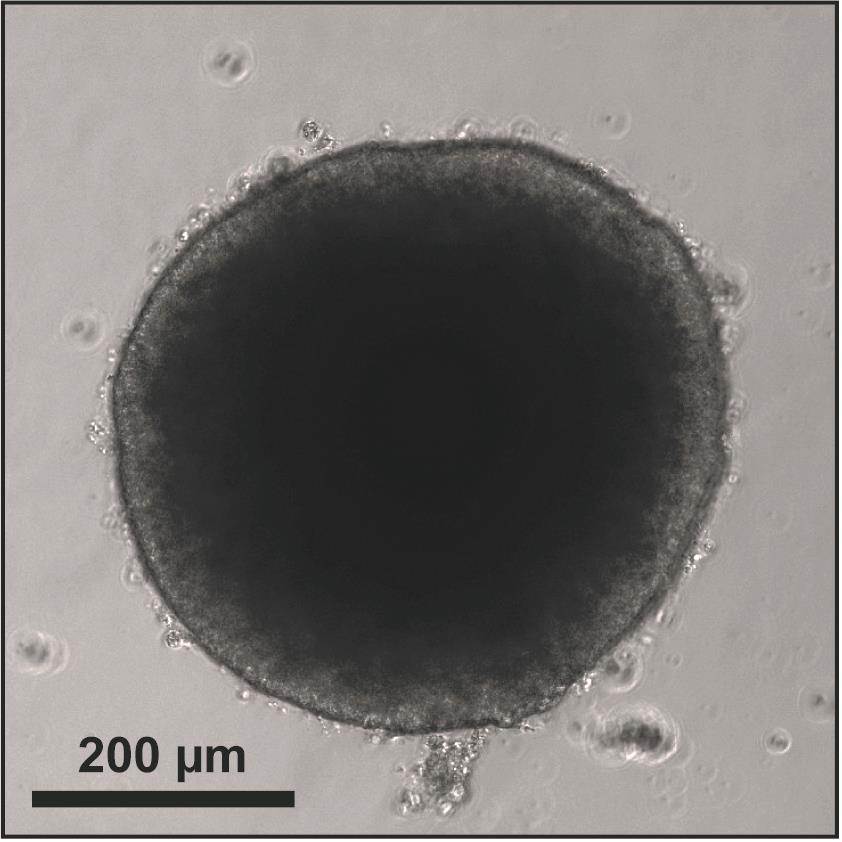
Figure 2. Representative EB derived from H9 hESCs on Day 5Neural Induction: Transfer to Neural Induction
Prepare Neural Induction Media fresh for each differentiation and prewarm an aliquot to 37°C using a water bath. Neural Induction Media can be stored for 1 week at 4°C. Once warmed, Neural Induction Media should not be reused.
Choose EBs that meet the size and visual criteria (Figure 2).
Cut 2–3 mm off the extremity of a 200-µl filtered pipette tip using a razor blade to make a wider opening.
Prepare a 24-well ultra-low attachment plate by adding 500 µl prewarmed Neural Induction Media to each well.
Using a dissecting scope and a cut 200 µl pipette tip, pick up the EB and transfer to a 24-well ultra-low attachment plate. Set the pipette to 20 µl, place the end at the bottom of the well and pipette up the EB. A maximum of 2 EBs per well can be added; additional EBs increase the likelihood of further aggregation.
Place the plate in a CO2 incubator for 2 days.
Neural Induction: Feeding
Prewarm Neural Induction Media to 37°C using a water bath.
Feed the EBs by adding an additional 500 µl Neural Induction Media to each well.
Return the plate to a CO2 incubator for a further 2 days.
Neural Induction: Assessment Checkpoint
Use a brightfield microscope to examine the neuralized EBs for optical clearing in the outer 100 µm of the EB (Figure 3). The outer ring structure should be prominent and marked by a stark change in opacity as compared with the central region of the EB. This is a critical checkpoint; EBs that do not have the correct organization of the neuroepithelial ring will not form organoids.
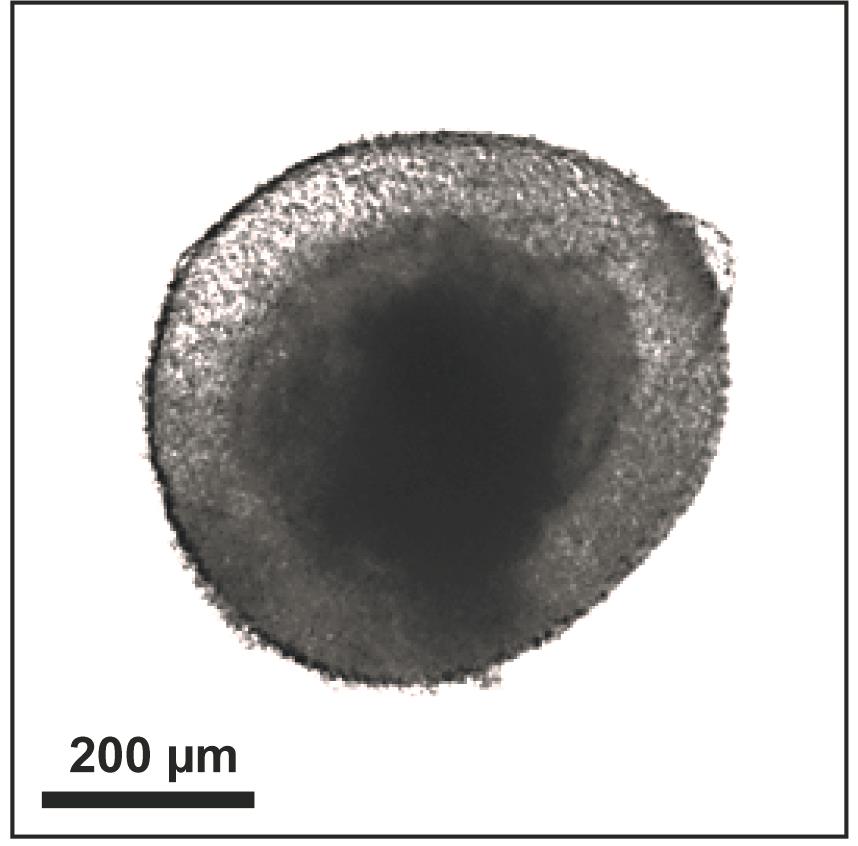
Figure 3. Neuroectoderm formation in an EB derived from H9 hESCs at Day 9
Neuroepithelial Expansion: Transfer to Solid Phase Matrigel Scaffold
Thaw Growth Factor Reduced Matrigel on ice for 2 h. Once thawed, Matrigel can be used for up to 6 h but must be kept on ice. Matrigel cannot be refrozen once thawed.
Prepare CO Differentiation Media without Vitamin A (CDM-A) fresh for each differentiation and prewarm an aliquot to 37°C using a water bath. CDM-A can be stored for 2 weeks at 4°C. Once warmed, CDM-A should not be reused.
Prepare a box of wide-bore tips by cutting 3–4 mm off the extremity of a 200 µl filtered pipette tip using a razor blade to make a wider opening. Pick up the EBs one by one using a 200 µl cut tip and transfer to the center of each well of the 4-well plate (1 EB per well).
Using an uncut 200 µl tip, draw 25 µl to aspirate the remaining Neural Induction Media from each well. It is critical to remove all media from the EB in the 4-well plate; failure to do so will prevent the Matrigel from attaching to the plate surface and prevent removal of the Matrigel in Procedure J.
Cover the neuralized EB with a drop of cold Matrigel (30 µl) using an uncut 200 µl tip.
Position the EB in the center of the Matrigel droplet using a 10 µl tip. Ensure that the EB is not resting on the bottom of the plate.
Once a plate has been filled (4 EBs), transfer the full plate to a CO2 incubator for 10 min at 37°C to polymerize the Matrigel.
After 10 min, carefully add 500 µl prewarmed CDM-A and return to a CO2 incubator for a futher 2 days.
Note: Be consistent with the amount of media collected when transferring the EB. We recommend collecting EBs with 25 µl media to create a droplet in the 4-well plates.
Neuroepithelial Expansion: Feeding
Prewarm an aliquot of CDM-A to 37°C using a water bath.
Carefully aspirate all media and add 500 µl CDM-A to each well of the 4-well plate.
Return the plate to the incubator for a further 2 days.
Neuroepithelial Expansion: Assessment of Checkpoint
Use a brightfield microscope to examine the COs for ring structures in the peripheral regions of the organoid (Figure 4). COs should be approximately 500–700 µm in diameter and have multiple ring structures throughout; this is a critical checkpoint, those without ring structures will not form organoids.
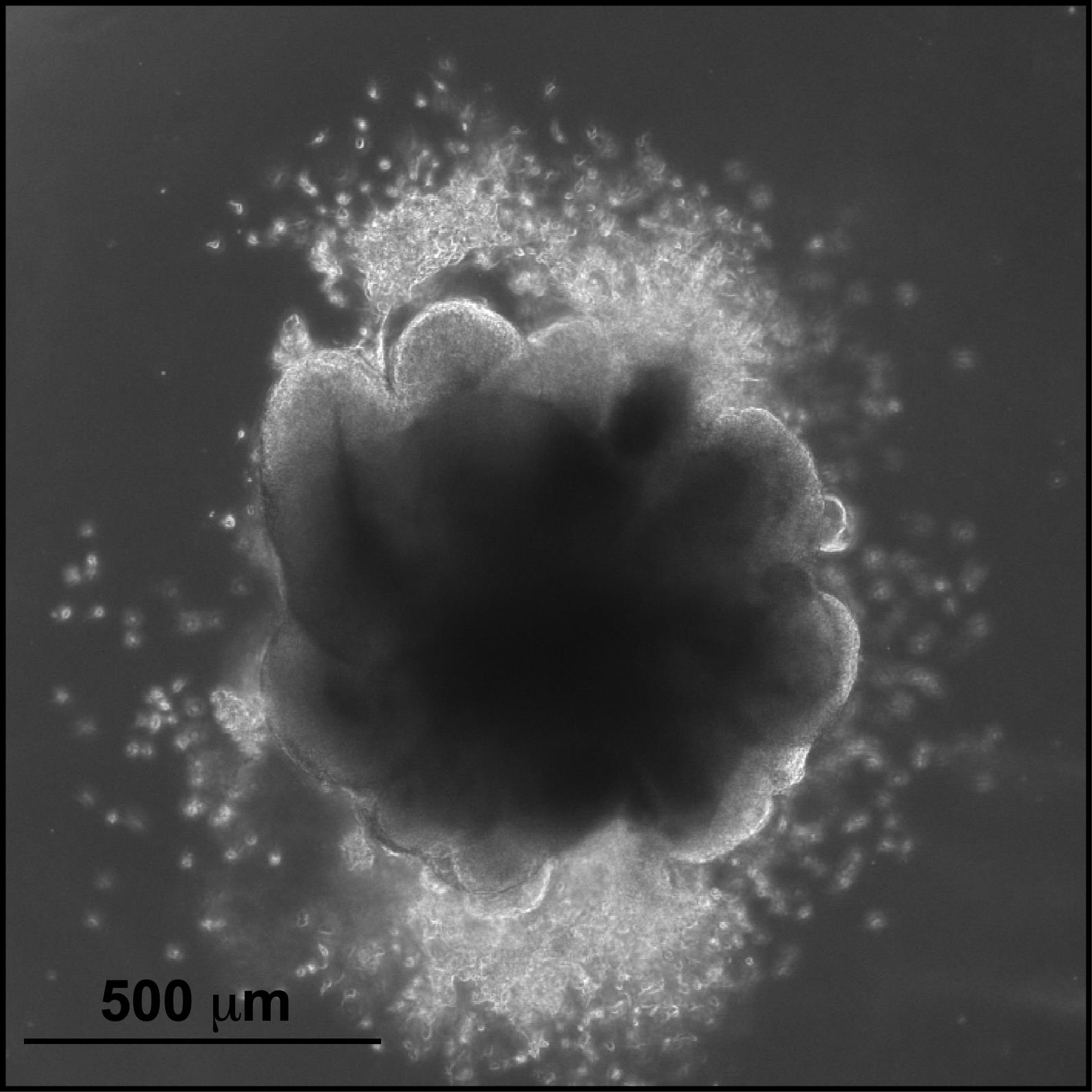
Figure 4. Representative hCOs derived from H9 hESCs on day 13 prior to Matrigel extraction
Transfer to Spinning Culture: Extraction from Solid Phase Matrigel
Prepare CO Differentiation Media (CDM) fresh and prewarm an aliquot to 37°C using a water bath. CDM can be stored for 2 weeks at 4°C. Once warmed, CDM should not be reused.
Prepare wide-bore pipette tips by cutting 4–5 mm off the extremity of 200 µl pipette tips to make a wider opening.
Prepare a 6-well plate with 3 ml prewarmed CDM per well.
Using a dissection microscope, carefully cut around the COs implanted in the Matrigel droplet; the goal here is to remove as much Matrigel as possible without damaging the CO. Excess Matrigel remaining on the CO at this stage will result in formation of cyst-like structures in the spinning culture.
Use a cut 200 µl tip to transfer the COs to the 6-well plate containing CDM; a maximum of 4 hCOs per well is acceptable.
Once all the COs have been transferred, place the 6-well plate on an orbital shaker (ThermoFisher Cat: 88881101 ) in a 37°C incubator at a speed of 80–90 rpm (rpm may vary if a different orbital shaker model is used).
Transfer to Spinning Culture: Feeding in Spinning Culture
Prewarm CDM using a 37°C water bath.
Pause the orbital shaker and collect the plate containing the COs.
Carefully aspirate 2/3 of the total media volume from each well; be mindful not to aspirate the COs with the media since they are in suspension.
Add 3 ml prewarmed CDM to each well.
Return the plate to the orbital shaker in the incubator and initialize (Figure 5).
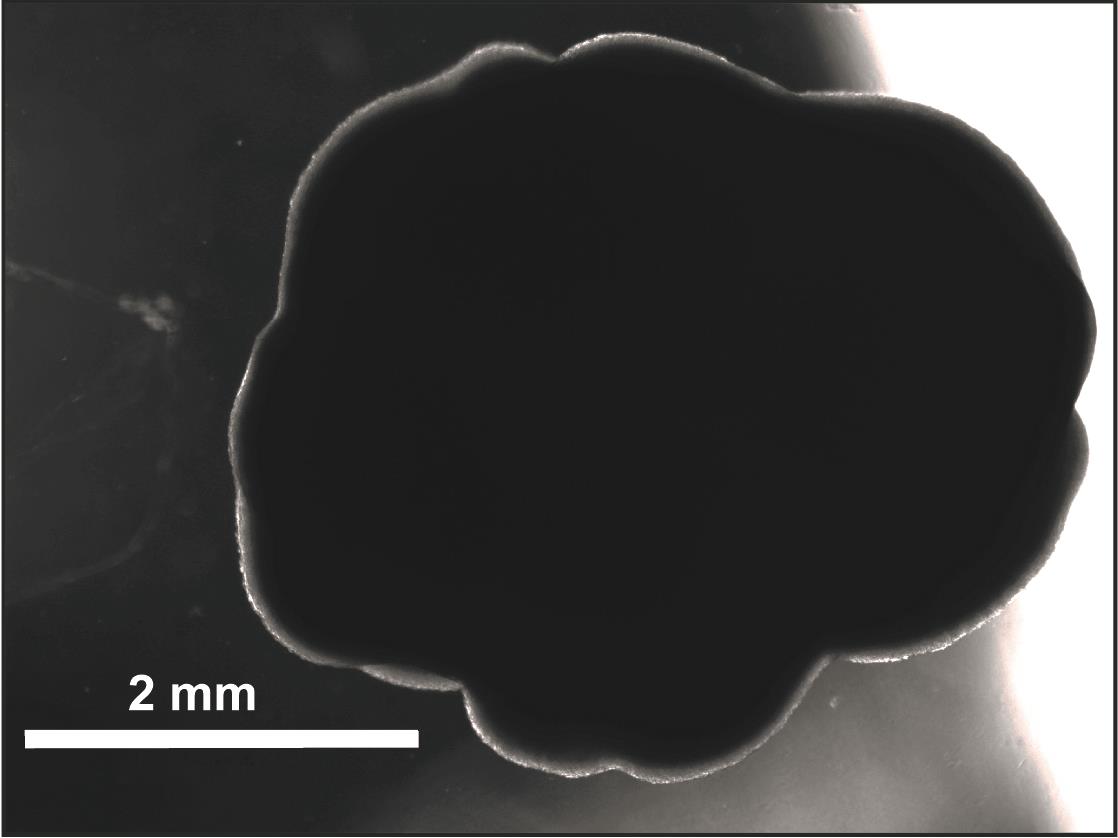
Figure 5. Representative hCO in a spinning culture derived from H9 hESCs at 12 weeks
Recipes
Embryoid Body Media (EB Media) (Table 1)
Table 1. EB Media Composition. A list of components, stock and final concentrations (Con.), and volumes for working stocks are indicated.
Components Stock Con. Final Con. /50 ml medium DMEM/F12 39.5 ml KOSR 10 ml MEM:NEAA 100× 1× 500 µl 2-mercaptoethanol 100× 1× 50 µl Neural Induction Media (prepared fresh every week) (Table 2)
Table 2. Composition of Neural Induction Media (prepared fresh every week). Once warmed, Neural Induction Media should not be reused. A list of components, stock and final concentrations (Con.), and volumes for working stocks are indicated.
Components Stock Con. Final Con. /50 ml medium DMEM/F12 48.5 ml GlutaMax 100× 1× 500 µl MEM:NEAA 100× 1× 500 µl N2 100× 1× 500 µl Heparin 10 mg/ml 0.001 mg/ml 5 µl Cerebral Organoid Differentiation Media without Vitamin A (CDM-A) (Table 3)
Table 3. Composition of Cerebral Organoid Differentiation Media without Vitamin A. A list of components, stock and final concentrations (Con.), and volumes for working stocks are indicated.
Components Stock Con. Final Con. /50 ml medium DMEM/F12 24 ml Neural Basal Media 24 ml MEM:NEAA 100× 0.5× 250 µl GlutaMax 100× 1× 500 µl B27-Vit A 50× 0.5× 500 µl N2 100× 0.5× 250 µl 2-mercaptoethanol 1,000× 1× 50 µl Insulin 9.5–11.5 mg/ml 2–3 µg/ml 12.5 µl Cerebral Organoid Differentiation Media with Vitamin A (CDM+A) (Table 4)
Table 4. Composition of Cerebral Organoid Differentiation Media with Vitamin A. A list of components, stock and final concentrations (Con.), and volumes for working stocks are indicated.
Components Stock Con. Final Con. /100 ml medium DMEM/F12 48 ml Neural Basal Media 48 ml MEM:NEAA 100× 0.5× 500 µl GlutaMax 100× 1× 1 ml B27 (with Vit A) 50× 0.5× 1 ml N2 100× 0.5× 500 µl 2-mercaptoethanol 1,000× 1× 100 µl Insulin 9.5–11.5 mg/ml 2–3 µg/ml 25 µl
Acknowledgments
This work was supported by funds from the Canada First Research Excellence Fund/Medicine by Design, the Krembil Foundation, and the Canadian Institute for Health Research (CIHR) Foundation grant FDN148455 to L.A. This protocol is adapted from the method published in Sivitilli et al., (2020). We thank Javier J. Hernandez and the staff of the Applied Organoid Core (ApOC) at the University of Toronto for advice.
Competing interests
The authors declare that they have no conflicts of interest.
References
- Amin, N. D. and Paşca, S. P. (2018). Building models of brain disorders with three-dimensional organoids. Neuron 100(2): 389-405.
- Drost, J. and Clevers, H. (2018). Organoids in Cancer Research. Nat Rev Cancer 18(7): 407-418.
- Linkous, A., Balamatsias, D., Snuderl, M., Edwards, L., Miyaguchi, K., Milner, T., Reich, B., Cohen-Gould, L., Storaska, A., Nakayama, Y., Schenkein, E., Singhania, R., Cirigliano, S., Magdeldin, T., Lin, Y., Nanjangud, G., Chadalavada, K., Pisapia, D., Liston, C., Fine, H. A. (2019). Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep. 26(12): 3203-3211.
- Birey, F., Andersen, J., Makinson, C. D., Islam, S., Wei, W., Huber, N., Fan, H. C., Metzler, K. R. C., Panagiotakos, G. and Thom, N. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545(7652): 54-59.
- Chen, H. I., Song, H. and Ming, G. l. (2019). Applications of human brain organoids to clinical problems. Dev Dyn 248(1): 53-64.
- Di, L. E. and Kriegstein, A. R. (2017). The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18(10): 573.
- Huch, M., Knoblich, J. A., Lutolf, M. P. and Martinez-Arias, A. (2017). The hope and the hype of organoid research. Development 144(6): 938-941.
- Jo, J., Xiao, Y., Sun, A. X., Cukuroglu, E., Tran, H.-D., Göke, J., Tan, Z. Y., Saw, T. Y., Tan, C. and Lokman, H. (2016). Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19(2): 248-257.
- Lancaster, M. A. and Knoblich, J. A. (2014a). Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9(10): 2329-2340.
- Lancaster, M. A. and Knoblich, J. A. (2014b). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345(6194).
- Lancaster, M. A., Renner, M., Martin, C.-A., Wenzel, D., Bicknell, L. S., Hurles, M. E., Homfray, T., Penninger, J. M., Jackson, A. P. and Knoblich, J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501(7467): 373-379.
- Mariani, J., Coppola, G., Zhang, P., Abyzov, A., Provini, L., Tomasini, L., Amenduni, M., Szekely, A., Palejev, D. and Wilson, M. (2015). FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162(2): 375-390.
- Pollen, A. A., Bhaduri, A., Andrews, M. G., Nowakowski, T. J., Meyerson, O. S., Mostajo-Radji, M. A., Di, L. E., Alvarado, B., Bedolli, M. and Dougherty, M. L. (2019). Establishing cerebral organoids as models of human-specific brain evolution. Cell 176(4): 743-756. e717.
- Qian, X., Nguyen, H. N., Song, M. M., Hadiono, C., Ogden, S. C., Hammack, C., Yao, B., Hamersky, G. R., Jacob, F. and Zhong, C. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165(5): 1238-1254.
- Quadrato, G., Brown, J. and Arlotta, P. (2016). The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat Med 22(11): 1220-1228.
- Quadrato, G., Nguyen, T., Macosko, E. Z., Sherwood, J. L., Yang, S. M., Berger, D. R., Maria, N., Scholvin, J., Goldman, M. and Kinney, J. P. (2017). Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545(7652): 48-53.
- Rossi, G., Manfrin, A. and Lutolf, M. P. (2018). Progress and potential in organoid research. Nat Rev Genet 19(11): 671-687.
- Sivitilli, A. A., Gosio, J. T., Ghoshal, B., Evstratova, A., Trcka, D., Ghiasi, P., Hernandez, J. J., Beaulieu, J. M., Wrana, J. L. and Attisano, L. (2020). Robust production of uniform human cerebral organoids from pluripotent stem cells. Life Sci Alliance 3(5).
- Velasco, S., Kedaigle, A. J., Simmons, S. K., Nash, A., Rocha, M., Quadrato, G., Paulsen, B., Nguyen, L., Adiconis, X. and Regev, A. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570(7762): 523-527.
- Watanabe, M., Buth, J. E., Vishlaghi, N., de la Torre-Ubieta, L., Taxidis, J., Khakh, B. S., Coppola, G., Pearson, C. A., Yamauchi, K. and Gong, D. (2017). Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep 21(2): 517-532.
- Yoon, S. J., Elahi, L. S., Pașca, A. M., Marton, R. M., Gordon, A., Revah, O., Miura, Y., Walczak, E. M., Holdgate, G. M. and Fan, H. C. (2019). Reliability of human cortical organoid generation. Nat Methods 16(1): 75-78.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sivitilli, A., Ghiasi, P. and Attisano, L. (2021). Production of Phenotypically Uniform Human Cerebral Organoids from Pluripotent Stem Cells. Bio-protocol 11(8): e3985. DOI: 10.21769/BioProtoc.3985.
Category
Developmental Biology > Morphogenesis
Neuroscience > Basic technology
Cell Biology > Cell engineering > Tissue engineering
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link