- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Multilayered Fabrication Assembly Technique to Engineer a Corneal Stromal Equivalent
Published: Vol 11, Iss 6, Mar 20, 2021 DOI: 10.21769/BioProtoc.3963 Views: 3453
Reviewed by: Alessandro DidonnaWilliam C. W. ChenKarem A Court
Abstract
Tissue engineering has emerged as a strategy to combat the donor shortage of human corneas for transplantation. Synthetic corneal substitutes are currently unable to support the normal phenotype of human cells and so decellularized animal corneas have been deployed to more closely provide the topographical and biochemical cues to promote cell attachment and function. Although full thickness decellularized corneas can support corneal cells, the cells are slow to populate the scaffold and density declines from the surface. To avoid these problems, this protocol describes the stacking of alternate layers of decellularized porcine corneal sheets and cell-laden collagen hydrogel to produce a corneal construct. The sheets are obtained by cryosectioning porcine corneas, decellularizing them with detergents and nucleases and finally air drying for storage and ease of manufacture. Corneal stromal cells are then encapsulated in a collagen type I solution and cast between these sheets. This protocol presents a rapid method to ensure high cellularity throughout the construct using tissue-derived materials alone.
Graphic abstract:
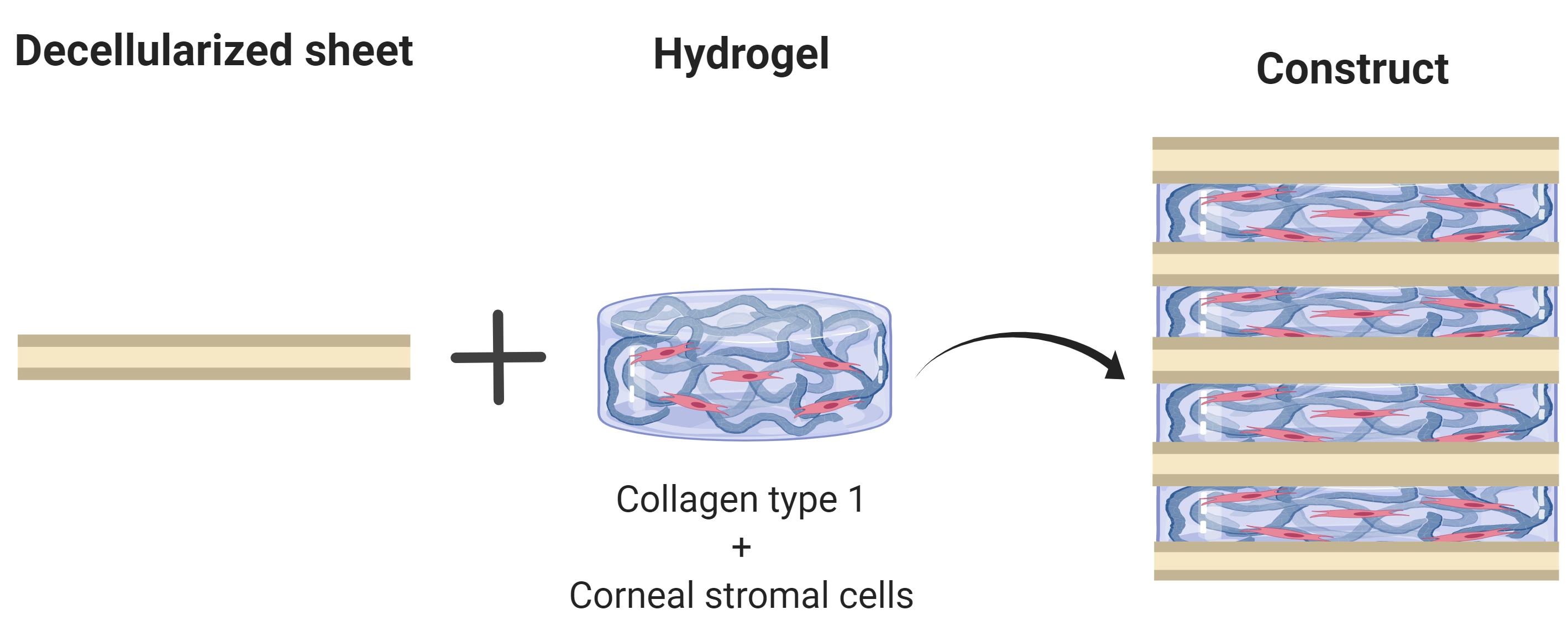
Overview of main process to obtain corneal stromal equivalents
Background
Corneal blindness affects millions of people worldwide and treatment primarily relies upon the transplantation of human donor corneas (Gain et al., 2016). Since these donations are scarce, alternatives based on the tissue engineering of biomaterials are desirable. A wide range of strategies and materials are being developed to engineer corneal tissue, and one promising approach is the use of decellularized animal corneas (Fernández-Pérez and Ahearne, 2020) to provide a collagen-proteoglycan scaffold upon which the cells can grow. Decellularized porcine corneas have been approved as implant material in trials for the treatment of corneal ulcers, with encouraging clinical outcomes (Zhang et al., 2015, Zheng et al., 2019). However, after implantation, repopulation of the acellular implant by the migration of cells from the recipient can result in corneal haze or permanent scar tissue (Li et al., 2020). To avoid this problem, the decellularized cornea can be repopulated in vitro with cells prior to implantation. To achieve this and ensure high cell density, cells can be injected into the decellularized cornea, but this can damage the collagen architecture. Alternatively, cells seeded directly onto the scaffolds are slow to migrate into the tissue and their phenotype can be adversely affected. Our tactic is therefore to combine the decellularized corneal sheets with a high density of keratocytes in a collagen hydrogel to engineer the corneal stroma. In this protocol, we describe a way of fabricating corneal equivalents by stacking thin sheets of decellularized porcine corneas interleaved with cell-laden collagen hydrogels. These constructs have high cell viability, display characteristic stromal markers and are robust enough to be sutured in an ex vivo model (Fernández-Pérez et al., 2020). Whilst this protocol has been developed for the cornea, other researchers have reported a similar approach to produce tissue engineered cartilage (Gong et al., 2010).
Materials and Reagents
Pipette tips
12 and 24-well cell culture plates (Greiner, Cellstar, catalog numbers: 665180 and 662160 )
Screw Cap Urine Cup (Sarsted, catalog number: 75.9922.721 )
Plastic base mould (Simport, catalog number: HIS0221 )
Parafilm M (Amcor, catalog number: PM996 )
2 ml tube (Sarstedt, catalog number: 72.693.105 ) and 5 ml tube (Sarstedt, catalog number: 62.558.201 )
Sterile filter 0.2 μm (Corning, catalog number: 431219 )
Petri dish 100 mm diameter (Corning, catalog number: CLS43016 )
Cotton swab (autoclaved) (purchased from local supermarket)
Porcine eye globes (Local abattoir or butcher)
Note: For this protocol, age and strain of animals could not be controlled. This may also vary between countries.
Corneal stromal cells from human donor tissue (described in Fernández-Pérez and Ahearne, 2019b)
Corneal epithelial cells from human limbal biopsies, or an immortalized human corneal epithelial cell line (EverCyte, catalog number: hTCEpi )
Povidone-Iodine antiseptic 10% w/w (Ecolab, Videne, catalog number: HAS051P )
OCT embedding cryoembedding matrix (Thermo Fisher Scientific, catalog number: 12678646 )
Liquid nitrogen held in small insulated container
Phosphate buffered saline (PBS) without CaCl2 and MgCl2 (Sigma-Aldrich, catalog number: D8537 )
Decellularization solution. Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L4390 ) and Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
Magnesium chloride (Sigma-Aldrich, catalog number: M0250 )
Calcium chloride solution (Sigma-Aldrich, catalog number: 21115 )
DNase (Sigma-Aldrich, catalog number: DN25 )
RNase (Sigma-Aldrich, catalog number: R5000 )
Autoclaved de-ionized water
Antimicrobials, 2% v/v Penicillin-streptomycin (Gibco, catalog number: 15140122 ) and 0.25 μg/ml amphotericin B (Sigma-Aldrich, Merck, catalog number: A2942 ) in PBS or cell culture medium.
Teflon (polytetrafluoroethylene) cut into 12 mm diameter discs (Chemours, 2 mm thick sheet)
Rat tail collagen ~10 mg/ml (Corning, catalog number 354249 )
Dulbecco’s modified Eagle media (DMEM) low glucose (Hyclone, catalog number: 10529702 )
DMEM/F12 1:1 (Hyclone, catalog number: SH30023.01 )
Foetal bovine serum (FBS) (Gibco, catalog number: 10270106 )
Ascorbic acid (Sigma-Aldrich, catalog number: A8960 )
ITS 100× (Gibco, catalog number: 41400045 )
Keratinocyte growth medium (PromoCell, catalog number: C20011 )
Manual cell counter and hemocytometer (Blaubrand, catalog number: BR717805 )
10× PBS without CaCl2 and MgCl2 (Corning, catalog number: 46-013-CM )
Phenol Red, sodium salt (Sigma-Aldrich, catalog number: P5530 )
1 M NaOH (Honeywell, catalog number: CCS8045 )
Decellularization solution (see Recipes)
MgCl2 Buffer (see Recipes)
DNase & RNase solutions (see Recipes)
10× PBS with phenol red (see Recipes)
Stromal cell media (see Recipes)
Keratocytic media (see Recipes)
Equipment
Dissection and manipulation tools (Sterile forceps, scissors, scalpels including a 2 mm wide spatula bent to a L-shape (Witig, catalog number: WITG7.412.051 )
Biopsy punch 10 mm diameter (Acuderm, Acu-Punch, catalog number: P1050 )
Cryostat (Leica, model number: CM1850UV )
-80 °C freezer (Thermo Fisher, Forma Model 906 )
Orbital shaker (Witeg SHO-1D)
Stirring plate (Henry Troemner, VWR, Advanced series 4x4)
Centrifuge (Hettich, model: 320R )
37 °C oven (Memmert, UF55 )
Biosafety cabinet (Faster S.r.l., SafeFAST Classic, 212 )
Humidified CO2 incubator (Thermo Scientific, Forma Steri-Cycle, i790 )
Vortexer (Fisherbrand, Mini-E, catalog number: 15212985 )
Benchtop mini centrifuge (StarLab, catalog number: SLN2631-0007 )
Pipettes (Brand, Transferpette, catalog number: 705891 )
Cooling tray. Expanded Polystyrene container with melting ice or pre-cooled metallic beads (Lab Armor Beads)
Procedure
This protocol is divided into 5 main procedures. Firstly, we describe how to excise the cornea from the porcine eye globes (A). After that, we explain how to obtain the decellularized sheets (B). Third, the process of preparing cell-laden collagen hydrogels is detailed (C). We then outline the procedure of assembling the constructs with the decellularized sheets and the hydrogels (D). Lastly, the process of adding epithelial cells to the construct is described (E).
Excision of cornea from eye globe (the overall process is depicted in Figure 1)
Obtain eye globes from a local source and transport to the laboratory in a sealed bag refrigerated on melting ice. Do not transport them immersed in PBS or similar. Use within 24 h.
Inspect for any damaged corneas and discard. For the undamaged globes, remove with scissors any remaining ancillary tissue, such as eyelids, muscle and tear glands.
Note: Superficial epithelial defects are not problematic, but deeper stromal abnormalities are. Discard if the stroma is not transparent and/or blood vessels are present in the cornea.
Perform all further manipulation stages in a biosafety cabinet using sterile technique.
Immerse the eye globes in povidone-iodine diluted 1:4 with PBS for 4 min, agitating the container gently. We recommend the use of sterile urine cups. 50 ml for each eye is sufficient. Solutions should be used at room temperature.
Wash the eye globes 3 times for 2 min each in PBS and keep moist during further stages. We recommend the use of sterile urine cups. 50 ml for each eye is sufficient. Solutions should be used at room temperature.
Dab gently the surface of the eye with a sterile cotton swab dipped in sterile 70% ethanol to promote the removal of the epithelium.
Use forceps around the circumference of the globe to increase intraocular pressure and cut through the central cornea with the circular biopsy punch.
Holding the cut corneal lenticule at its edge with fine tweezers, rinse in PBS (5 ml sterile PBS) and then transfer into a base mould filled with OCT matrix. Best results are obtained if the apical surface is facing down.
Place the histology mould onto liquid nitrogen, they do not need to be immersed; floating is acceptable. Leave for 2 min to ensure complete freezing; the transparent corneal lenticule and OCT matrix will turn white.
Transfer frozen lenticules into the -80 °C freezer. Store up to 12 months before use.
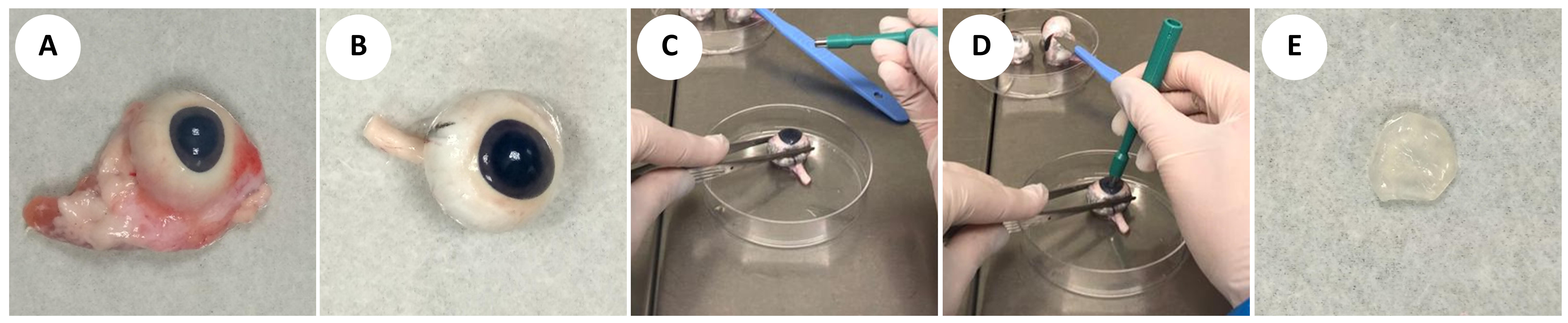
Figure 1. Main steps for excision of the cornea. A. The eye as received from the abattoir (Step A1). B. Eye after removing surrounding tissue and povidone-iodide washing (Steps A2-A5). C. Hold the eye with forceps to increase intraocular pressure (Step A7). D. Use a biopsy punch to remove the cornea (Step A7). E. The corneal lenticule after brief washing in PBS, before immersion in OCT and cryopreservation (Step A8).
Obtaining the decellularized tissue sheets
Put the base moulds with lenticules, a 24-well plate and the cutting blade inside the cryostat for temperature equilibration for at least 30 min.
Place some fresh OCT matrix onto the cryostat sample chuck and allow the sample to freeze onto it.
Set the thickness of sections to 60 µm.
Cut sections, discarding the first that will usually not be full sized. Collect each sheet, one to each well of the 24-well plate. One cornea will provide approximately 10 sections.
Notes:
Thicker sheets are easier to manipulate but will be slower to populate with cells. In a clinical setting, the number and thickness of corneal sheets can be changed to suit the full thickness of the corneal defect to be repaired.
When picking up the corneal sheets, or removing solutions, care needs to be taken to avoid damage or sucking up the sheet into the pipette tip. We do not recommend the use of vacuum aspirators, as the probability of aspirating the sheets is very high. When handling, pick up by grasping with fine forceps at the sheet edge; it is better to use in cooperation, two sets of forceps on opposite edges of the sheet. The L-shaped spatula can be used to gently lift the edge of sheets, or assist lifting the whole construct stack of sheets combined with one set of forceps. We do not recommend pooling slices and carrying out decellularization in 50 ml tubes as the sheets stick together.
Sheets can be stored below -20 °C for up to 3 months after sealing the plate with Parafilm.
Under sterile conditions, add 2 ml of sterile PBS into each well. Shake the plate gently to promote melting of the OCT matrix into the PBS. After 5 min remove the solution with a pipette.
Add 1 ml decellularization solution to each well. Place on an orbital shaker at 50 rpm for 24 h at room temperature.
Note: Here, decellularization is performed using two detergents SDS and Triton X-100. Other decellularization methods are available (Fernández-Pérez and Ahearne, 2020).
Remove the decellularization solution and then add the DNase & RNase solution, again 1 ml per well. Place the plate on an orbital shaker for 1 h, but this time in an oven at 37 °C. The sheets will have turned slightly white.
Remove the DNase & RNase solution and add 1 ml per well of PBS supplemented with antimicrobials. Place on orbital shaker for 24 h at room temperature.
Remove the antimicrobials and add 1 ml per well of sterile deionised water. Leave for at least 1 h to remove salts. Continue processing, or seal the plate with Parafilm and store refrigerated at 4 °C for up to 2 weeks.
Remove each sheet carefully, lifting it onto a Teflon disc. When sheets are removed from the water they often curl. Flatten any curled sheet with curved fine forceps.
Leave the sheets uncovered to dry in the biosafety cabinet for at least 1 h.
Carefully peel off each sheet, bending the Teflon disc to ease their detachment. Collect the sheets in a 5 ml tube. The sheets are lightweight and care should be taken that they are not drawn away by the air flow of the biosafety cabinet. Dried in this way, the sheets can be stored for 6 months in a sealed tube before use. Dried decellularized sheets can be seen in Figure 2A.
Note: The OCT matrix, base moulds, liquid nitrogen and cryostat blade were not sterile in this procedure and we relied upon antimicrobials after decellularization and in the culture media to avoid contamination. Alternatively, we expect that a femtosecond laser could be used to cut fresh tissue and avoid the use of OCT matrix and any microbial contamination from this stage of sheet manufacture. Additionally, if a shaped sheet is desirable to change refractive properties then the laser could also provide a suitable lenticule lens.
Preparing collagen hydrogel
Prepare a cooling tray with ice or beads.
Precool all solutions and pipette tips under sterile conditions and keep cool until constructs are assembled.
Calculate the required final volume and pipette the corresponding amount of rat tail collagen solution sufficient to obtain a final concentration of 3.5 mg/ml into a 2 ml tube. Allow for 120 μl total per construct of 5 sheets.
Add 5% of the final required volume of 10× PBS.
Mix using vortexer and the solution will become yellow (acidic).
Add 1 M NaOH, calculated on the volume of rat tail collagen solution used × 0.023.
Mix using vortexer. The solution should now have a red colour. Variation in batches of collagen solution may mean they need further adjustment of the NaOH concentration to achieve neutrality.
Add sterile deionized H2O.
Mix using vortexer and spin briefly using a benchtop mini centrifuge.
Notes:
Aspirate and dispense solutions slowly throughout. Use a positive displacement pipette to aliquot the rat tail collagen. Bubbles from rapid pipetting can reduce cohesion of the layers.
Example of calculation for hydrogel preparation, volume 1 ml (add in this order):
Calculate the needed volume of stock collagen as follows: final volume × final concentration/stock concentration. For example, if stock collagen is at 9 mg/ml, then use 388.9 µl of it when making 1 ml of solution (3.5 mg/ml × 1,000 µl/9 mg/ml).
50 µl 10× PBS
8.94 µl 1M NaOH. This volume is calculated by multiplying the volume of stock collagen used by 0.023 (in this example: 388.9 µl × 0.023 = 8.94 µl)
502.16 µl sterile deionized H2O. This is calculated by subtracting all the volumes used to the final volume [1,000 µl – (388.90 µl + 50 µl + 8.89 µl + 50 µl) = 502.16 µl]
Adjust cell density to have 10 × 106 cells in 50 µl 10× PBS. This is quite a high cell density so pipette carefully.
Perform cell trypsinization and cell suspension adjustment once everything else is ready to avoid having the cells at cold temperatures for long. As long as the collagen solution is on ice, it will not gelate for 4 h. Avoid keeping the cells in the 10× PBS for long, otherwise they can suffer an osmotic shock.
Collagen sources other than from rat tail might also be suitable, but have not been tested with our protocol. ECM-derived hydrogels can provide a more mimetic environment, as they contain ECM components other than collagen type I, and can be obtained from porcine corneas (Ahearne and Lynch, 2015; Fernández-Pérez and Ahearne, 2019a).
Assembling tissue constructs with corneal stromal cells in collagen hydrogel (the process is shown in Figures 2B-2E)
Harvest the corneal stromal cells using a standard procedure of trypsinization and washing.
Count the cells and adjust cell density to 200 × 106 cells/ml in 10× PBS. Add the cell suspension to the collagen mix. The volume of cell suspension will be 5% of total volume of solution. Mix gently using a pipette, but thoroughly, to ensure an even cell distribution.
Place a sterile Teflon disc in a Petri dish and place one of the dry sheets on top. Wet this first sheet with 30 µl of sterile deionized water and then remove the excess by pipette.
Add 25 µl of cell-collagen hydrogel as a drop to the centre of the sheet.
Carefully place a second dry sheet over the first. The second sheet will absorb much of the cell-collagen hydrogel mix. Add another 25 µl of cell-collagen hydrogel onto the second sheet and again cover with another collagen sheet. Repeat the process until there are five decellularized sheets and 4 layers of cell-collagen hydrogel mix.
Replace the Petri dish lid and incubate at 37 °C for 30 min to allow gelation of the collagen.
Add 2-3 ml of stromal cell medium to each well of a 12-well plate (Recipe 5), pre-warmed to 37 °C.
Lift the Teflon disc with the construct and carefully submerge the disc into a plate well. The Teflon disc is then removed with fine forceps. The construct will often immediately detach from the disc, but if this does not occur, it can be assisted to release using the L-shaped flat spatula. Otherwise, the sheet should have detached the following day, or it will need to be discarded. Constructs can be moved to a 24-well plate.
To promote a keratocytic phenotype, a keratocytic serum-free media should be used (Recipe 6). Refresh media three times per week.
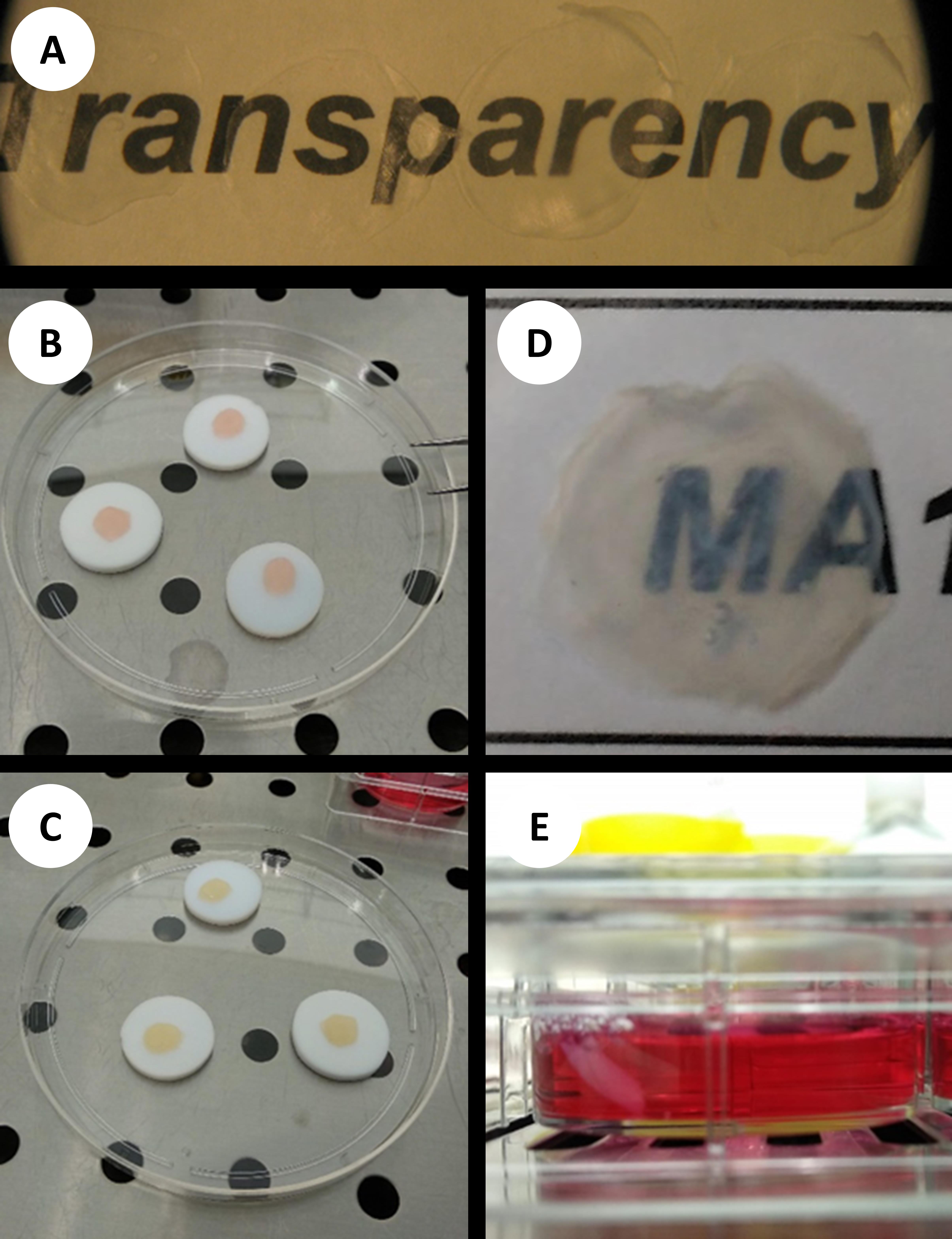
Figure 2. Overview of the protocol. A. Dried decellularized porcine corneal sheets show good optical properties. B. Constructs immediately after assembly of cornea sheets and hydrogel. C. Constructs after gelation process (note slight lightening in colour). D. Constructs present good optics. E. Construct in tissue culture.
Adding an epithelial cell layer
Allow the construct 2 weeks of culture for the corneal stromal cells to establish and the sheets to stabilize before adding epithelial cells.
Harvest the epithelial cells using a standard trypsinisation protocol and adjust the cell density to 3.33 × 106 cells/ml, to obtain a final seeding density of 50,000 cells per construct.
Remove the medium from the well, and carefully pipette 15 µl of well-mixed epithelial cell suspension onto the construct surface. Incubate for 30-45 min to allow cell attachment.
After cell attachment, add 1 ml of the keratinocyte medium. Culture for 1 week, changing the media every 2 days.
Recipes
Decellularization solution
To 100 ml of deionised water add 500 mg SDS and 1 ml Triton X-100.
To help drawing up the viscous Triton X-100, first cut the end off the pipette tip. Mix thoroughly on a stirring plate, at room temperature and then sterilize by 0.2 μm filter in a biosafety cabinet. Can normally be stored at room temperature for 6 months, but if there is a precipitate, discard.
MgCl2 Buffer
Prepare the buffer by adding 203.3 mg MgCl2 to 100 ml deionised water
Bring pH to 7.5 by carefully adding 1 M NaOH
Can be stored for 1 year at room temperature. Check pH before each use and filter sterilize the amount required.
DNase & RNase solutions
Under sterile conditions, prepare stock solutions of 400 U/ml DNase with 5 mM CaCl2 and 900 U/ml of RNase in de-ionised water.
Divide into 1 ml aliquots and store at -20 °C for up to 12 months for the DNase and 6 months for the RNase.
To prepare the decellularization solution, just before use, add 250 µl DNase and 110 µl RNase stock to 10 ml MgCl2 buffer to give a final concentration of 10 U/ml of each nuclease.
10× PBS with phenol red
Note: This solution is only used to prepare hydrogels and allows to monitor the pH of the solution.
Add 15.9 mg of phenol red sodium salt to 100 ml of 10× PBS
Sterile filter inside laminar flow hood
Stable for 1 year at room temperature
Stromal cell media
Media consisted of DMEM low-glucose supplemented with 10% v/v FBS and 100 U/ml Penicillin/Streptomycin.
Keratocytic media
DMEM-F12 (1:1) supplemented with 1× ITS and 100 µg/ml ascorbic acid and 100 U/ml Penicillin/Streptomycin.
Acknowledgments
The research leading to these results has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement no. 637460) and from Science Foundation Ireland (15/ERC/3269).
The protocol described here was used in the publication: Fernández-Pérez et al. (2020).
Graphical abstract created with BioRender.com.
Competing interests
The authors report no conflicts of interest.
Ethics
Porcine eye globes were provided postmortem as a byproduct of food supply. Human cells came from tissue remnants following corneal transplantation via the Eye Tissue Bank of the Irish Blood Transfusion Service, Dublin, after appropriate research consent of the donor family. Ethics approval for the use of human tissue to obtain cells was approved by the School of Medicine research ethics committee at Trinity College Dublin.
References
- Ahearne, M. and Lynch, A. P. (2015). Early observation of extracellular matrix-derived hydrogels for corneal stroma regeneration. Tissue Eng Part C Methods 21(10): 1059-1069.
- Fernández-Pérez, J. and Ahearne, M. (2019a). The impact of decellularization methods on extracellular matrix derived hydrogels. Sci Rep 9(1): 14933.
- Fernández-Pérez, J. and Ahearne, M. (2019b). Influence of Biochemical Cues in Human Corneal Stromal Cell Phenotype. Curr Eye Res 44(2): 135-146
- Fernández-Pérez, J. and Ahearne, M. (2020). Decellularization and recellularization of cornea: Progress towards a donor alternative. Methods 171: 86-96.
- Fernández-Pérez, J., Madden, P. W. and Ahearne, M. (2020). Engineering a corneal stromal equivalent using a novel multilayered fabrication assembly technique. Tissue Eng Part A 26(19-20): 1030-1041.
- Gain, P., Jullienne, R., He, Z., Aldossary, M., Acquart, S., Cognasse, F. and Thuret, G. (2016). Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 134(2): 167-173.
- Gong, Y. Y., Xue, J. X., Zhang, W. J., Zhou, G. D., Liu, W. and Cao, Y. (2011). A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials 32(9): 2265-2273.
- Li, S., Deng, Y., Tian, B., Huang, H., Zhang, H., Yang, R., Zhong, J., Wang, B., Peng, L. and Yuan, J. (2020). Healing characteristics of acellular porcine corneal stroma following therapeutic keratoplasty. Xenotransplantation 27(2): e12566.
- Zhang, M. C., Liu, X., Jin, Y., Jiang, D. L., Wei, X. S. and Xie, H. T. (2015). Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am J Transplant 15(4): 1068-1075.
- Zheng, J., Huang, X., Zhang, Y., Wang, Y., Qin, Q., Lin, L., Jin, X., Lam, C. and Zhang, J. (2019). Short-term results of acellular porcine corneal stroma keratoplasty for herpes simplex keratitis. Xenotransplantation 26(4): e12509.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Fernandez-Perez, J., Madden, P. W. and Ahearne, M. (2021). Multilayered Fabrication Assembly Technique to Engineer a Corneal Stromal Equivalent. Bio-protocol 11(6): e3963. DOI: 10.21769/BioProtoc.3963.
Category
Biological Engineering > Biomedical engineering
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










