- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Reverse Phase-high-performance Liquid Chromatography (RP-HPLC) Analysis of Globin Chains from Human Erythroid Cells
Published: Vol 11, Iss 2, Jan 20, 2021 DOI: 10.21769/BioProtoc.3899 Views: 4071
Reviewed by: Annamaria AprileFuming LiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

ZnCl2 Precipitation-Assisted Sample Preparation for Proteomic Analysis
Qiqing He [...] Fuchu He
Jul 20, 2025 2726 Views

Protocol for the Preparation of a Recombinant Treacle Fragment for Liquid–Liquid Phase Separation (LLPS) Assays
Nadezhda V. Petrova [...] Artem K. Velichko
Sep 20, 2025 1814 Views

Optimized Secretome Sample Preparation From High Volume Cell Culture Media for LC–MS/MS Proteomic Analysis
Basil Baby Mattamana [...] Peter Allen Faull
Dec 20, 2025 1229 Views
Abstract
β-hemoglobinopathies are severe genetic disorders characterized either by the abnormal synthesis of the adult β-globin chains of the hemoglobin (Hb) tetramer (βS-globin chains) in sickle cell disease (SCD) or by the reduced β-globin production in β-thalassemia. The identification and quantification of globin chains are crucial for the diagnosis of these diseases and for testing new therapeutic approaches aimed at correcting the β-hemoglobinopathy phenotype. Conventional techniques to detect the different Hb molecules include cellulose-acetate electrophoresis (CEA), capillary electrophoresis (CE), isoelectric focusing (IEF), and cation-exchange-HPLC (CE-HPLC). However, these methods cannot distinguish the different globin chains and precisely determine their relative expression. We have set up a high-resolution and reproducible reverse phase-HPLC (RP-HPLC) to detect and identify the globin chains composing the hemoglobin tetramers based on their different hydrophobic properties. RP-HPLC mobile phases are composed of acetonitrile (ACN) that creates a hydrophobic environment and trifluoroacetic acid (TFA), which breaks the heme group within the Hb tetramers releasing individual globin chains. Hb-containing lysates are loaded onto the AerisTM 3.6-µm WIDEPORE C4 200 Å LC Column and a gradient of increasing hydrophobicity of the mobile phase over time allows globin chain separation. The relative amount of globin chains is measured at a wavelength (λ) of 220 nm. This protocol is designed for evaluating globin chains in (i) red blood cells (RBCs) obtained from human peripheral blood, (ii) RBCs in vitro differentiated from hematopoietic stem/progenitor cells (HSPCs), and (iii) burst-forming unit-erythroid (BFU-E), i.e., erythroid progenitors obtained in vitro from human peripheral blood or in vitro cultured HSPCs. This technique allows to precisely identify the different globin chains and obtain a relative quantification. RP-HPLC can be used to confirm the diagnosis of β-hemoglobinopathies, to evaluate the disease severity and validate novel approaches for the treatment of these diseases.
Keywords: HPLCBackground
Hemoglobin (Hb) is a tetrameric polypeptide composed of two α-globin and two β-like globin chains (ϵ, Gγ, Aγ, δ, or β). Hb is expressed in the erythrocytes and carries the oxygen from the lung to the tissues. Globin genes are differentially expressed throughout the development. For instance, during fetal life, the most abundant Hb is composed of two α- and two γ-globin chains (HbF, fetal Hb), while in human adults HbA, consisting of two α- and two β-globin chains, is predominantly expressed. Disorders caused by mutations in the β-like globin genes (β-hemoglobinopathies) represent the most frequent inherited diseases worldwide. In particular, β-hemoglobinopathies are caused by mutations that reduce adult β-globin production (β-thalassemia) or generate a mutant β-globin (sickle cell disease, SCD).
β-hemoglobinopathies are the most common monogenic disorders, therefore they represent ideal candidates for gene therapy approaches. A key parameter for determining the severity of the disease and the curative potential of treatment is the abundance of the different Hbs and globin chains. Indeed, techniques evaluating this parameter give insights into the degree of anemia (e.g., the α-/non-α-globin ratio, which is altered in β-thalassemia), highlight the presence of aberrant or uncoupled, toxic globins (e.g., α-globin aggregates in β-thalassemia) and evaluate the therapeutic expression of endogenous or exogenous β-like globins (e.g., fetal γ-globin, which is beneficial for both β-thalassemia and SCD).
Different methods can be used for the characterization and the quantification of Hbs and globin chains for the diagnosis and prognosis of Hb disorders, and for evaluating the therapeutic potential of gene therapy approaches.
Electrophoresis-based methods were firstly used to analyze Hb tetramers and globin chains. Erythroid cell lysates are commonly submitted to a cellulose-acetate electrophoresis (CEA) at alkaline pH. In this method, Hb tetramers migrate differently depending on their charge and are identified based on known Hb references (Schneider and Barwick, 1978; Wajcman, 2003).
More recently, capillary electrophoresis (CE) has been adapted to the analysis of Hb molecules. The separation of Hb molecules is based on their electrophoretic mobility at alkaline pH and is directed by pH and endosmosis.
Another conventional technique is the isoelectric focusing (IEF) that allows the separation of Hb molecules based on their different isoelectric point. Hb molecules migrate across a gradient until they reach a position where the net charge is zero. This method can distinguish some Hb variants that cannot be separated by CEA.
Finally, cation exchange-HPLC (CE-HPLC) can distinguish the different Hb molecules by chromatography using a salt gradient to elute them at a specific retention time.
All these methods give limited information on the amount of individual globin chains, in particular the γ-globin chains, Aγ and Gγ, which differ by a single amino acid. Moreover, in partially differentiated cell culture samples or chimeric models, these limitations are exacerbated by the presence of additional proteins or hybrid hemoglobin species. Finally, they often require a high amount of globin protein, which is difficult to obtain in in vitro generated RBCs or BFU-Es (Aprile et al., 2016).
Robust detection and relative quantification of individual globin chains can be achieved by reversed-phase HPLC (RP-HPLC), which separates proteins according to their hydrophobicity. RP-HPLC can be used for measuring the α-/non-α-globin ratio that is altered in β-thalassemia because of the reduced β-globin expression. Furthermore, this technique allows the measurement of both types of fetal γ-globin chains (Aγ and Gγ). These globins are highly expressed in adult life in genetic conditions known as hereditary persistence of fetal hemoglobin (HPFH). Fetal γ-globin chain expression can also be induced by targeting known γ-globin silencers to alleviate the clinical severity of both β-thalassemia and SCD (Cavazzana et al., 2017). Importantly, this method can be applied to in vitro generated erythroid cells as it requires a relatively low amount of globin chains to evaluate the therapeutic potential of gene therapy approaches (Antoniani et al., 2018; Weber et al., 2020). As for the above-mentioned techniques, RP-HPLC does not allow an absolute quantification of globin chains.
Materials and Reagents
P1000, P200, P20, P10 sterile tips
1.5-ml Eppendorf tubes
FisherbrandTM 9 mm Short thread Plastic vial, Wide Opening (Fisher Scientific, FisherbrandTM, catalog number: 11707597 )
FisherbrandTM 9 mm PP short Thread seal, Blue, Center Hole, Assembled Septum (Fisher Scientific, FisherbrandTM, catalog number: 11797567 )
AerisTM 3.6 µm WIDEPORE C4 200 Å, LC Column 250 x 4.6 mm (Phenomenex, catalog number: 00G-4486-E0 ), storage: 4 °C for long-term use
Acetonitrile for HPLC, gradient grade, ≥ 99.9% (Sigma-Aldrich, catalog number: 34851 ), storage: room temperature (RT)
Trifluoroacetic acid reagent grade > 99% (Sigma-Aldrich, catalog number: 76508 ), storage: RT
MilliQ deionized water, storage: RT
Lyphochek Hemoglobin A2 control (Bio-Rad, catalog number: 553 ), storage: 4 °C for short-term use or -80 °C for long-term use
Mobile phase (see Recipes)
Equipment
Laminar flow hood
Chemical fume hood
NexeraX2 chromatograph (Shimadzu)
HPLC UV-Vis detector SPD-20AV (Shimadzu)
HPLC degassing unit DGU-20A3R (Shimadzu)
Solvent delivery unit LC-30AD (Shimadzu)
Autosampler SIL-30AC (Shimadzu)
Column oven CTO-20A (Shimadzu)
System Controller CBM-20A (Shimadzu)
Refrigerated microcentrifuge (Eppendorf 5424 R )
Software
LabSolutions WS-Single LC (version 5.51) (Shimadzu)
Procedure
Sample preparation
The preparation of samples can be performed in a BSL-1 (biosafety level 1) laboratory. However, we recommend to prepare potentially infectious samples under a laminar flow hood in a BSL2 lab. The quality of the chromatograph depends on sample preparation and storage. We recommend to use freshly prepared samples, when possible.
Lyphochek Hemoglobin A2 control (Bio-Rad)
This product is prepared from human whole blood and contains different forms of hemoglobin (A, A2, F, and S). It is used to generate quality and elution control profiles for each globin chain.
Reconstitute the lyophilized product in 500 µl of MilliQ deionized water and let it stand for 10 min, swirling every 3 min.
Gently invert the vial several times to obtain a homogeneous solution.
Prepare aliquots of 5 μl of the solution mixed with 575 μl of MilliQ deionized water in a 1.5-ml Eppendorf tube.
Store aliquots at -80 °C for long term-use.
Thaw at RT a new aliquot for each HPLC run. After ~10 min, vortex and keep the aliquot on ice.
Dilute 1:10 with ice-cold MilliQ deionized water.
Put 50 μl of the diluted control in an HPLC loading tube.
Storage: The solution is stable at -80 °C for long-term use and stable at 4 °C for 7 d.
Whole blood
Centrifuge 100 μl of blood 5 min at 300 x g in a 1.5-ml Eppendorf tube.
Discard the supernatant.
Add 900 μl of ice-cold MilliQ deionized water to the RBC pellet.
Vortex the samples in order to favor RBC lysis.
Centrifuge 10 min at 9,500 x g 4 °C.
Collect the supernatant and dilute 1:100 in ice-cold MilliQ deionized water in an HPLC loading tube.
Storage: RBC pellets can be stored at 4 °C for 7 d for short-term use or at -80 °C for long-term use.
In vitro differentiated RBCs (from 250,000 to 1,000,000 cells) and BFU-E (from 20 to 50 colonies). This protocol requires a minimal number of 250,000 in vitro differentiated RBCs or 20 BFU-E to obtain evaluable HPLC peaks.
Resuspend pellet in 50 μl of ice-cold MilliQ deionized water in a 1.5-ml Eppendorf tube.
Vortex samples in order to favor RBC lysis.
Centrifuge 10 min at 9,500 x g 4 °C.
Put 40 μl of the lysate in an HPLC loading tube.
Storage: Lysed differentiated RBCs or BFU-E can be stored at 4 °C for 7 d for short-term use or at -80 °C for long-term use.
Mobile phase preparation
The preparation of the mobile phases must be performed under a chemical fume hood. Mobile phases are composed by HPLC grade acetonitrile (ACN), creating a hydrophobic environment and Trifluoroacetic acid (TFA) that breaks the heme group within the Hb tetramers, releasing each globin chain:
Solution A: 5% ACN, 0.1% TFA in MilliQ deionized water
Solution B: 95% ACN, 0.1% TFA in MilliQ deionized water
Note: Percentage indicates the volume percent (v/v %). The protocol for the preparation of the mobile phase is reported in the paragraph “Recipes”.
HPLC: cleaning procedure
Before each run, rinse the LC column using the following procedure:
Put in the two solution reservoirs 100% MilliQ deionizedwater and 100% ACN, respectively.
Rinse the column using a slow gradient (1-2%/min) from 5% to 95% ACN and back to 5% ACN.
HPLC: acquisition procedure
Put solution A and solution B in the two solvent reservoirs.
Put the HPLC tubes samples in the tray of the sub-unit SIL-30AC.
Check that the four components of the HPLC machine (LC-30AD; SIL-30AC, CTO-20A, SPD-20AV) are correctly switched on.
Open the Labsolutions software, select “Instruments” from the menu bar, and double-click on the Instrument logo (Figure 1A).
Click on the Main assistant menu bar on the left and select “Realtime batch” to open the Realtime Batch window (Figure 1B).
Complete the Realtime Batch table by filling one line for each sample (Figure 1C):
Vial#: Select the number corresponding to the position of the sample in the tray of the HPLC sub-unit SIL30AC. The first 2 samples to be injected will be “blanks” (samples containing the solution without the analytes of interest) to place the chromatograph baseline. For blanks, there is no need to prepare HPLC tubes containing only the mobile phase: by selecting -1 as Vial#, only the mobile phase will be injected directly from the solution reservoir.
Tray name: Select 0 for blanks and 1 for the other samples.
Sample name: Write the name of each sample.
Sample type: Select “0:Unknown” for all the samples.
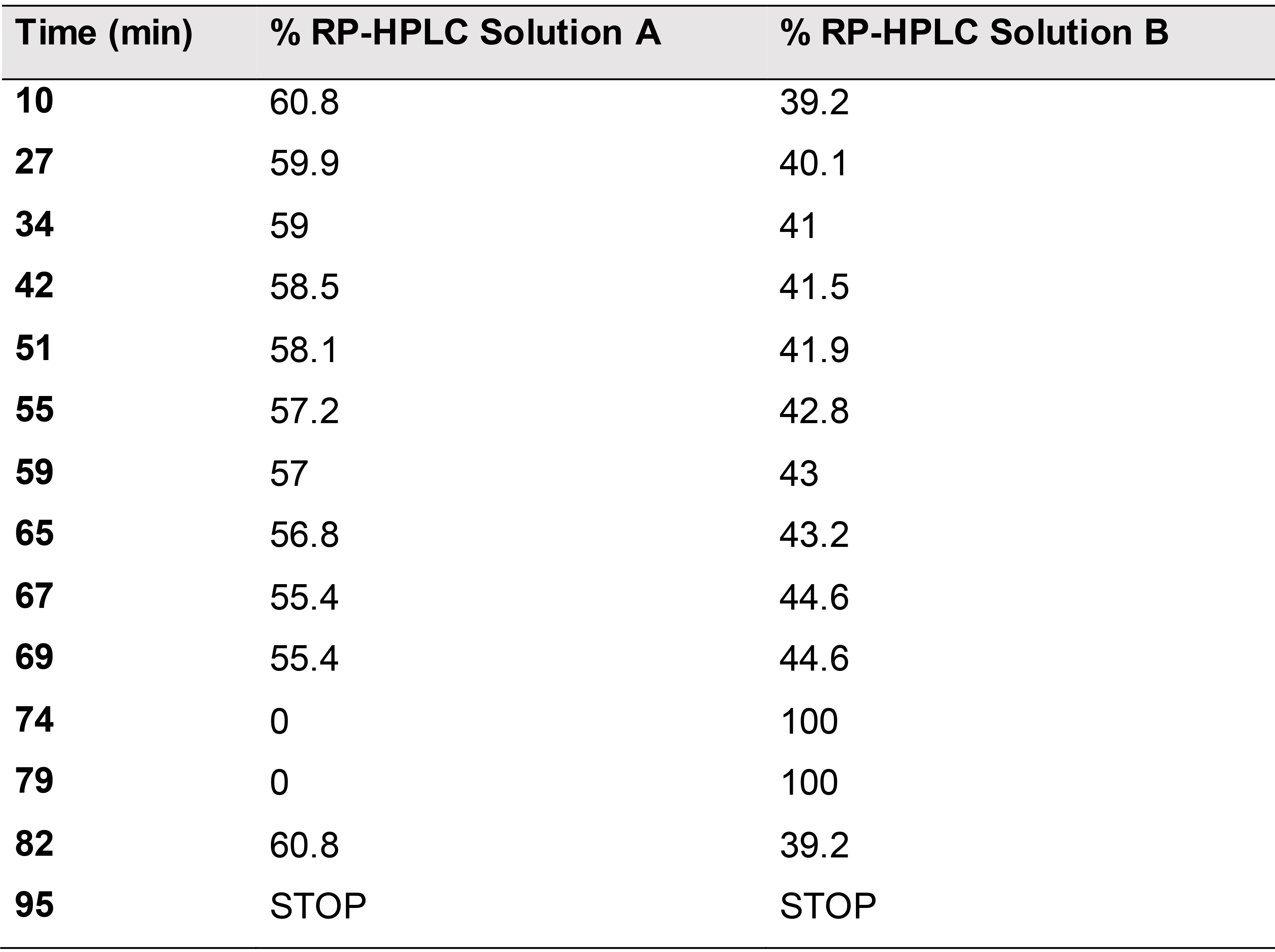
Method file: Select the .lcm file containing the method for globin chain detection. Apply a flow rate of 1.0 ml/min with a gradient of solution A and solution B (increasing over time the hydrophobicity) following the parameters reported in Table 1.
Table 1. RP-HPLC gradient scheme for globin chains analysis. The gradient is designed with a high percentage of solution A at the beginning and ends with 100% of solution B percentage (74-82 min) at a flow rate of 1.0 ml/min. The acquisition lasts 95 min per sample. 57.5 ml of solution A and 37 ml of solution B are used per sample, therefore a larger amount of solution A compared to solution B should be prepared.
Data file: Select (Auto Filename), the file containing the data will be automatically saved.
Level#: Select 0 for all the samples.
Injection volume: Write the volume (μl) that needs to be injected. Inject 5 μl for blanks and 35 μl for the remaining samples. Injection of 35 μl is optimal for the Lyphochek Hemoglobin A2 control and for extracts prepared as described above from whole blood, from 500,000 in vitro differentiated RBCs and from 25 BFU-E. If a different volume of whole blood or a different number of RBCs or BFU-E is used, reduce or increase the injection volume (from 1 µl to 45 µl).
Report output: tick the box to automatically print out the analysis report. It is not necessary to tick the box if you manually export the data (as described below).
Select from the menu bar “Save Batch File as” to save the file in a dedicated folder in the computer and enter the .lcb file name (Figure 1D).
Start the acquisition by selecting “Start Realtime Batch” from the Main assistant menu bar (Figure 1E). The acquisition lasts 95 min for each sample.
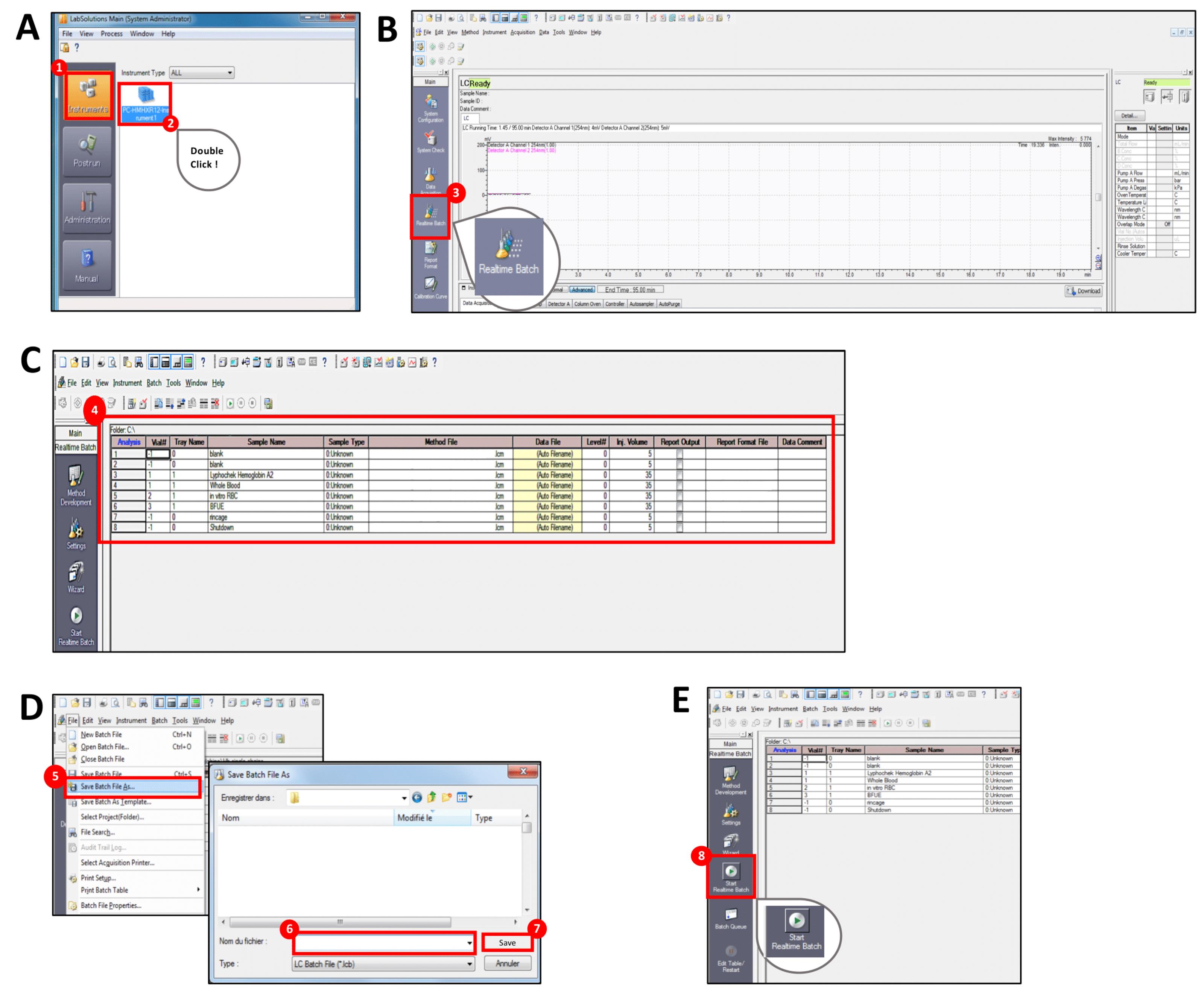
Figure 1. Flow chart describing the steps of the RP-HPLC acquisition procedure using the LabSolutions software
Data analysis
Open the LabSolutions software, select “Postrun session” and double-click on the “Postrun” (Figure 2A).
Select your file from the menu bar to open the window containing the chromatographic elution profile (Figure 2B).
Right-click on the chromatographic elution profile and select “Display settings” to set the Intensity Range for the y axis and the Time Range for the x axis (Figure 2C).
Right click on the chromatographic elution profile and select “Manual Integration Bar”. Use the different tools to define the baseline of each peak (Figure 2D).
Click on “Edit” in the Method View window, complete the table associating each peak to a specific globin chain and click on “View” (Figure 2E). The Area under the Curve (AUC, Area), the height, and the area % values will be reported for each peak in the table below the chromatographic elution profile (Figure 2E).
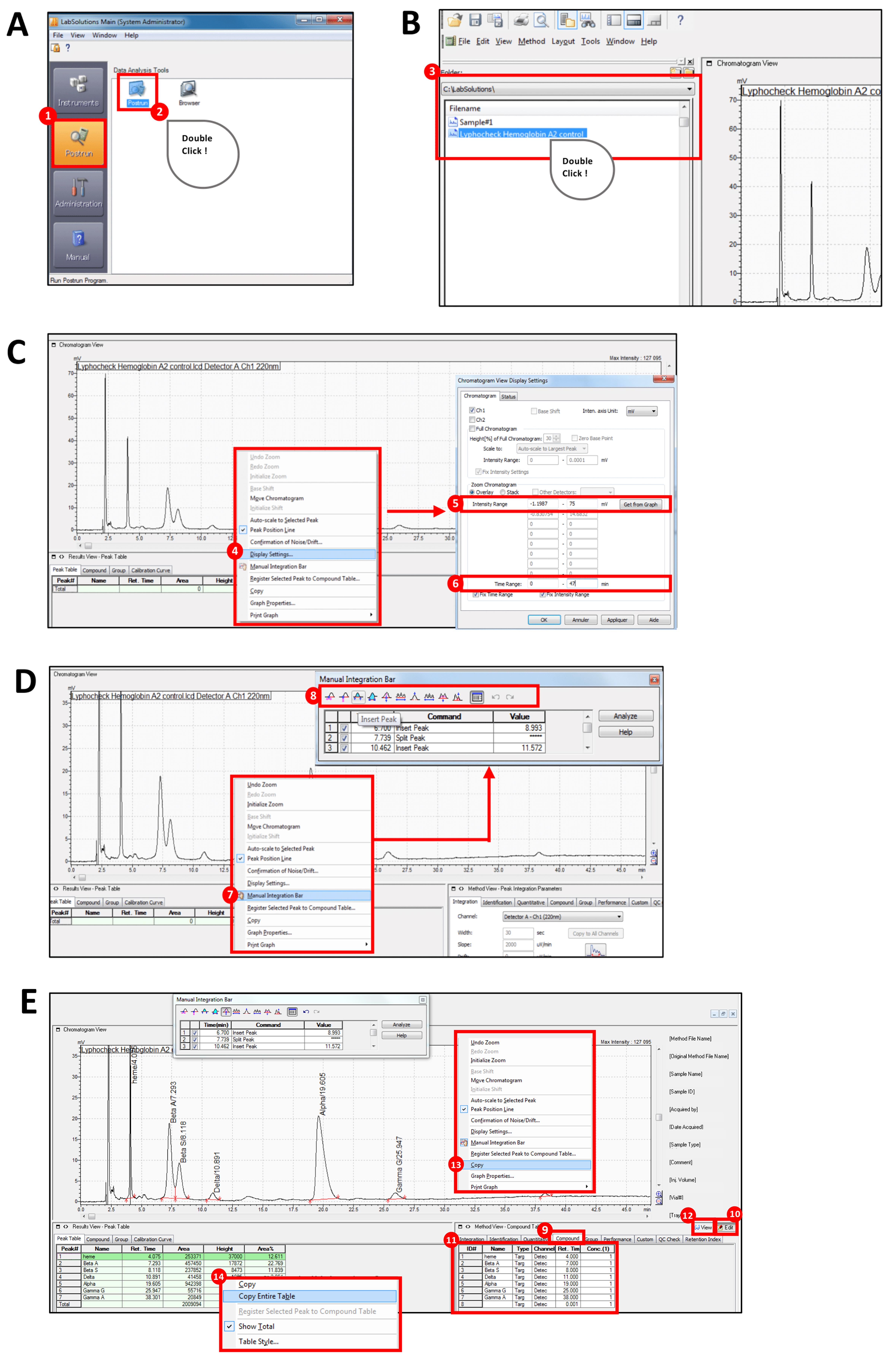
Figure 2. Flow chart describing the steps of the RP-HPLC data analysis using the LabSolutions softwareCopy and paste the chromatographic elution profile and the table below it in an Excel file.
Calculate the relative expression of each β-like globin chain over the total β-like globin chains or over the α-globin, and the ratio α/non- α globin chain using the AUC (area) values (Figure 3).
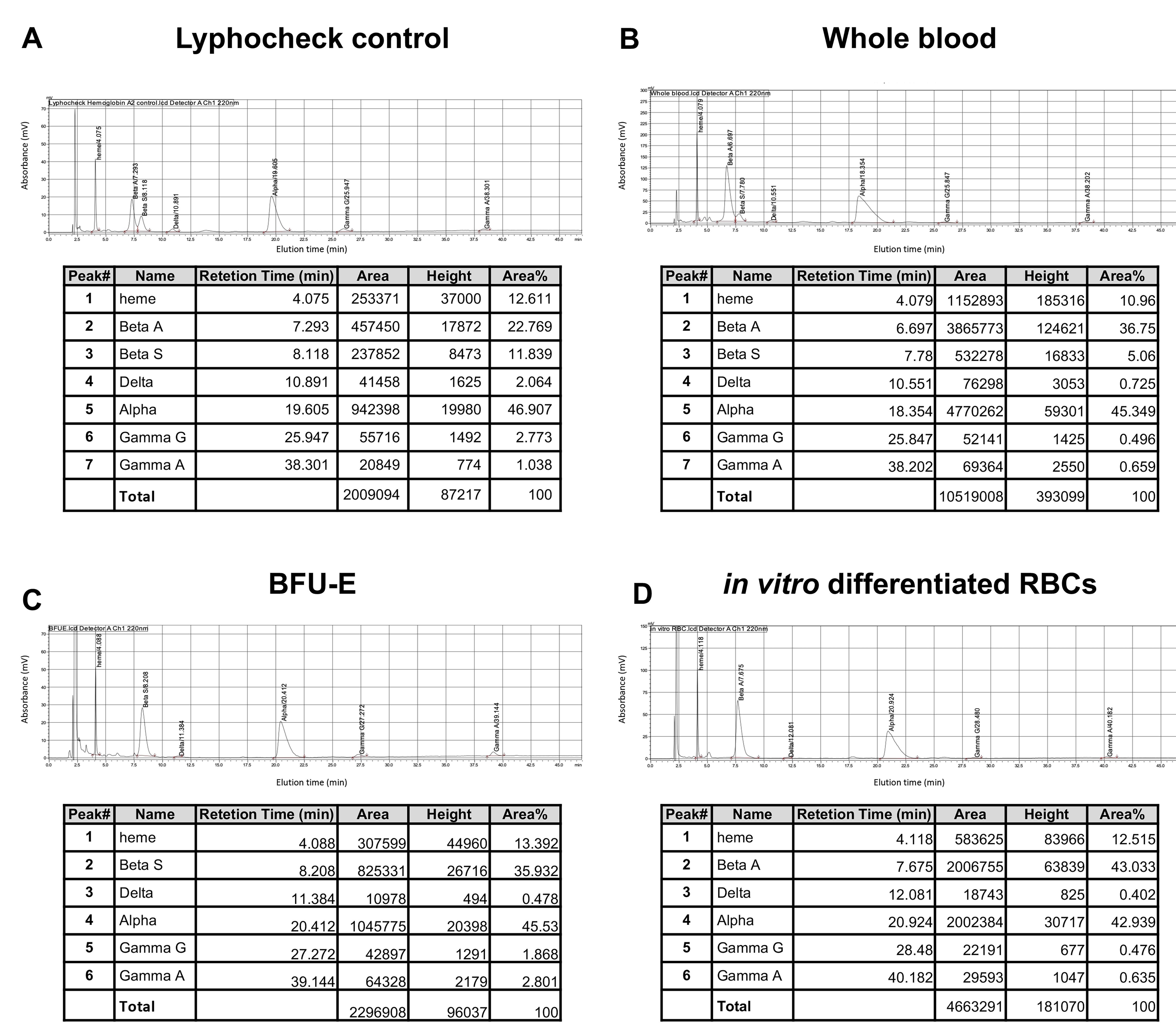
Figure 3. RP-HPLC analysis of Lyphochek Hemoglobin A2 control (A), Whole blood (B), BFU-E (C), and in vitro differentiated RBCs (D). Whole blood was obtained by an SCD patient transfused with healthy donor RBCs. BFU-E were obtained from an SCD patient and in vitro differentiated RBCs from a healthy donor. Globin chains are eluted in the following order from the hydrophilic to the hydrophobic globins: βA, βS, δ, α, Aγ, Gγ (A). The identification of globin chains is based on the elution profile of the Lyphochek Hemoglobin A2 control. Retention time, AUC (area), height, and Area % are reported for each peak.
Notes
Different runs may result in differences in retention times due to different reasons (e.g., the accuracy in the preparation of the mobile phase, the pressure stability of the machine). Therefore, we strongly recommend to use the Lyphochek Hemoglobin A2 control in each run.
The quality of the chromatograph depends on the sample lysis/preparation and its storage (if it is not freshly prepared). We recommend to use freshly prepared samples and mobile phases.
If peaks are not well-defined (e.g., because of the high background), it can be useful to perform an additional cleaning procedure.
Recipes
Mobile phase
Solution A: 5% ACN, 0.1% TFA in MilliQ deionized water
Solution B: 95% ACN, 0.1% TFA in MilliQ deionized water
Note: 57.5 ml of solution A and 37 ml of solution B are required for each sample. Determine the amount of mobile phase needed according to the number of samples. We recommend to use freshly prepared mobile phases.
Acknowledgments
This work was supported by State funding from the Agence Nationale de la Recherche under “Investissements d’avenir” program (ANR-10-IAHU-01) and the Paris Ile de France Region under “DIM Thérapie génique” initiative, by a grant from the European Research Council (865797 DITSB) and grants from the AFM-Telethon (22399 and 22206). This protocol was derived from Weber et al. (2020).
Competing interests
The authors declare no conflict of interest.
Ethics
All experiments were performed in accordance with the Declaration of Helsinki. The study was approved by the regional investigational review board (reference: DC 2014-2272, CPP Ile-de-France II “Hôpital Necker-Enfants malades”). Written informed consent was obtained from all adult subjects.
References
- Antoniani, C., Meneghini, V., Lattanzi, A., Felix, T., Romano, O., Magrin, E., Weber, L., Pavani, G., El Hoss, S., Kurita, R., Nakamura, Y., Cradick, T. J., Lundberg, A. S., Porteus, M., Amendola, M., El Nemer, W., Cavazzana, M., Mavilio, F. and Miccio, A. (2018). Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human β-globin locus. Blood 131(17): 1960-1973.
- Aprile, A., Passerini, G., Cappellini, M. D., Marktel, S., Ciceri, F., Ferrari, G. and Ceriotti, F. (2016). When diagnostics meets translational research: detection of hemoglobin fractions in cellular lysates from in vitro erythroid cultures by Capillarys 2 Flex Piercing analyzer(Sebia). Transl Res 169, 31-39 e31-34.
- Cavazzana, M., Antoniani, C. and Miccio, A. (2017). Gene Therapy for β-Hemoglobinopathies. Mol Ther 25(5): 1142-1154.
- Schneider, R. G. and Barwick, R. C. (1978). Measuring relative electrophoretic mobilities of mutant hemoglobins and globin chains. Hemoglobin 2(5): 417-435.
- Wajcman, H., (2003). Electrophoretic methods for study of hemoglobins. Methods Mol Med 82: 93-100.
- Weber, L., Frati, G., Felix, T., Hardouin, G., Casini, A., Wollenschlaeger, C., Meneghini, V., Masson, C., De Cian, A., Chalumeau, A., Mavilio, F., Amendola, M., Andre-Schmutz, I., Cereseto, A., El Nemer, W., Concordet, J. P., Giovannangeli, C., Cavazzana, M. and Miccio, A. (2020). Editing a γ-globin repressor binding site restores fetal hemoglobin synthesis and corrects the sickle cell disease phenotype. Sci Adv 6(7): eaay9392.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chalumeau, A., Frati, G., Magrin, E. and Miccio, A. (2021). Reverse Phase-high-performance Liquid Chromatography (RP-HPLC) Analysis of Globin Chains from Human Erythroid Cells. Bio-protocol 11(2): e3899. DOI: 10.21769/BioProtoc.3899.
- Weber, L., Frati, G., Felix, T., Hardouin, G., Casini, A., Wollenschlaeger, C., Meneghini, V., Masson, C., De Cian, A., Chalumeau, A., Mavilio, F., Amendola, M., Andre-Schmutz, I., Cereseto, A., El Nemer, W., Concordet, J. P., Giovannangeli, C., Cavazzana, M. and Miccio, A. (2020). Editing a γ-globin repressor binding site restores fetal hemoglobin synthesis and corrects the sickle cell disease phenotype. Sci Adv 6(7): eaay9392.
Category
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










