- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Design and Construction of Repacked Soil Columns for Measuring Solute Transport, Plant Growth and Soil Biota
Published: Vol 11, Iss 2, Jan 20, 2021 DOI: 10.21769/BioProtoc.3884 Views: 3785
Reviewed by: Nayidu NaghabushanaShahin S. AliAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1885 Views

Evaluating Arabidopsis Primary Root Growth in Response to Osmotic Stress Using an In Vitro Osmotic Gradient Experimental System
Selene Píriz-Pezzutto [...] Mariana Sotelo-Silveira
Jul 20, 2025 1911 Views

Synchronizing Germination Rates Across Plant Species for Fabricated Ecosystems EcoFAB 2.0
Romane S. F. Charbeaux [...] Michelle Watt
Dec 5, 2025 1469 Views
Abstract
Researchers face a number of challenges in the construction of soil columns which can affect the outcome of their experiments. The use of intact soil cores closely mimics actual field conditions. However, the excavation of large intact soil cores is a time-consuming, labor-intensive process and may lead to soil compaction that would influence the solute transport behavior of the soil column. Repacked soil columns are used as an option to circumvent these challenges of intact soil cores. However, repacked soil columns also have their limitations and introduce other challenges. Here, we present a step by step procedure for the design of repacked soil columns to achieve a realistic bulk density, prevent preferential flow paths, and ensure hydraulic connectivity between soil layers. This protocol will be beneficial to Soil Scientists, Hydrologists and other Environmental Scientists utilizing repacked soil columns.
Keywords: Soil columnsBackground
Soil columns play important roles in experimental set up for monitoring the fate and mobility of solutes such as contaminants and nutrients. They are also used in evaluating solute transport models. Soil columns are used widely in studies on the fate and transport of nutrients, pesticides, microbes, and heavy metals (Lewis and Sjöstrom, 2010).
Specifically, soil columns have been used in several studies including leaching of pharmaceuticals (Oppel et al., 2004), response of root growth to soil compaction levels (Tracy et al., 2012), dissolved organic carbon and nitrate fluxes (Eykelbosh et al., 2015), fate of organic micropollutants (Banzhaf and Hebig, 2016), and modelling of micropollutants to simulate riverbank filtration (Bertelkamp et al., 2016).
The experimental setup has a great influence on the outcome of soil column studies (Bromly et al., 2007). Therefore, it is very vital to design soil columns in such a way as to overcome associated challenges and minimize any potential source of bias (Weihermuller et al., 2007). The purpose of this protocol is to provide a step-by-step guidance in the construction and use of repacked soil columns to obtain reproducible experimental outcomes.
Materials and Reagents
- PVC pipes (e.g., sewer pipes having a thickness of 4.1 mm)
- Silicon sealant and gun
- Coarse textured Sandpaper
- 35-micron nylon mesh
- Measuring tape
- Syringe (10 ml)
- Measuring cylinder
- Collection trays
- Supporting trays
- Rhizon MOM (from Rhizosphere.com, catalogue number 19.21.21F )
- Buckets
- Ryegrass (or the plant of your interest)
- Soil
- 100 seeds of perennial ryegrass (Lolium perenne)
- Water
Equipment
- Hand drill with various size bits (e.g., 0.5 mm, 1 mm)
- Saw
- 3.35 mm mesh size sieve (depending on the purpose of the experiment)
- Shovel
Procedure
Cut the long PVC pipes having a diameter of 160 mm (Figure 1A) into 40 cm lengths (Figure 1B) to make the columns (adapt to your desired length of choice). This is because the PVC pipes comes in various lengths (2 m, 6 m, etc.).
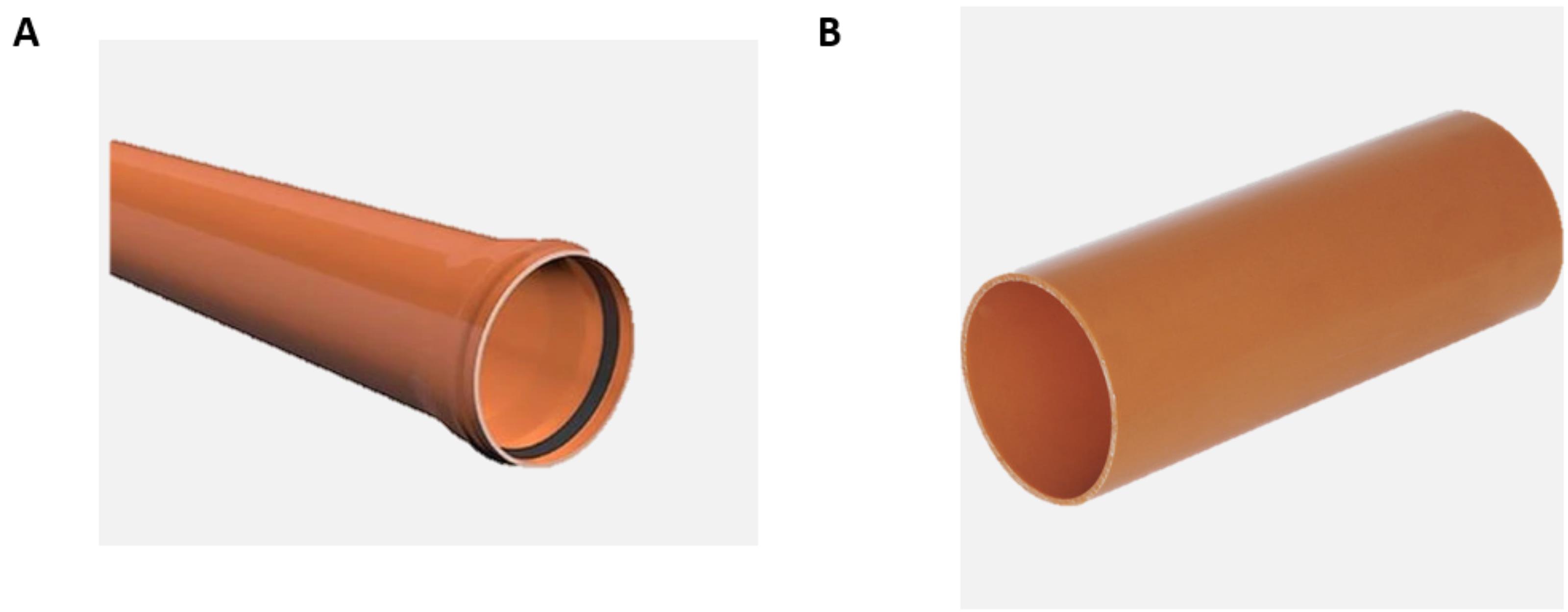
Figure 1. Photos of (A) a long PVC pipe and (B) a 40 cm long column cut from the pipeAt 10 cm height intervals, using the hand drill with the attached bit, drill holes (0.5 mm diameter) at the side so that rhizons can be fitted to collect soil solution samples. Also, at 5 cm from the bottom of the column, drill five holes (0.5 mm diameter) around the column at 5 cm distances. This is to allow free flow of water through the column and to avoid creating anaerobic conditions during the experiment. Moreover, the extra holes at the base of the soil columns will provide suction which is very important in obtaining realistic results for unsaturated soil columns.
Use the sandpaper to roughen the internal parts of the column to prevent preferential flow parts during water application to the columns.
Use water to wash off the internal parts of the columns and allow to dry for 5 min.
As both ends of the column are open (Figure 2A), cover one end (the end with five drilled holes used as the bottom of the column) with the 35 micron mesh and secure by sealing with the silicon sealant to hold the soil intact (Figure 2B). This will allow water to flow through and contain the root growth within the column. Hold the columns upside down while fixing the mesh and keep them in that position while the silicon hardens. Allow the sealing to dry for 36 h. Also, attach the rhizons at 10 cm height intervals into the holes drilled earlier (Figure 2C).
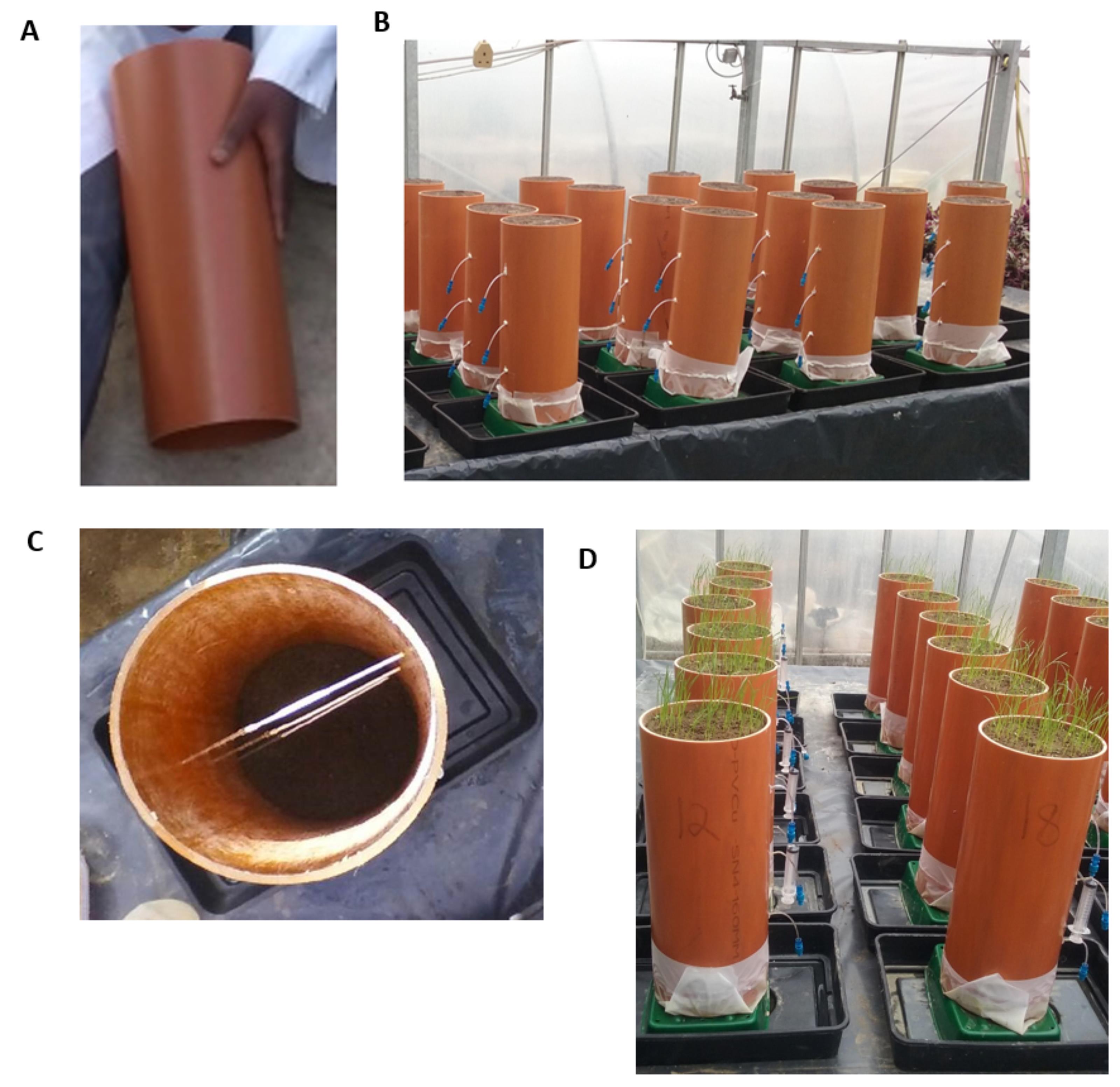
Figure 2. Photos of (A) soil column with open ends (B) columns secured with micron mesh attached at the base (C) soil column with attached rhizons and (D) columns with germinating ryegrassSieve the soils through the 3.35 mm mesh size sieve to keep out stones and other large particles.
Mix the soils thoroughly using shovel (hand mixing can be used depending on the scale of the experiment).
Stand the columns on the supporting tray (in the present case a green container in Figure 2D) to prevent the creation of anaerobic conditions during water application.
Place the column with the supporting tray into the larger collection tray (here a black container in Figure 2D) to collect the water flow through.
Pour water (rainwater or distilled water depending on the purpose of the study) to wet the soil (up to 70% field capacity, that is the amount of soil moisture or water content held by soil after excess water is drained so that the soil is just moist and not saturated), and fill the columns gradually with the wet soil, compressing at intervals to achieve a near-field bulk density.
Allow the columns to stand for three days (under the same conditions as the experimental conditions) and top up with soil as the soil levels decrease.
Sow 100 seeds of perennial ryegrass (Lolium perenne) 2 cm below the soil surface or use the recommended sowing rate of the test crop and allow the grass to grow (Figure 3A).
Water the columns with 200 ml of rainwater, three times every week for 14 weeks. This should be adjusted depending on the purpose of the study and available rainfall data.
Use the syringe to collect soil solution samples (1 ml or as much as required for the specific analysis) weekly (or as frequently as the experimental requirements) and analyze for macro and micro nutrients (or other parameters depending on the purpose of the study). When sampling, apply suction by pulling the plunger of the syringe. Use the cover of the rhizon to keep the suction for about 2-3 min or until the required volume of soil solution is collected (Figure 3B).
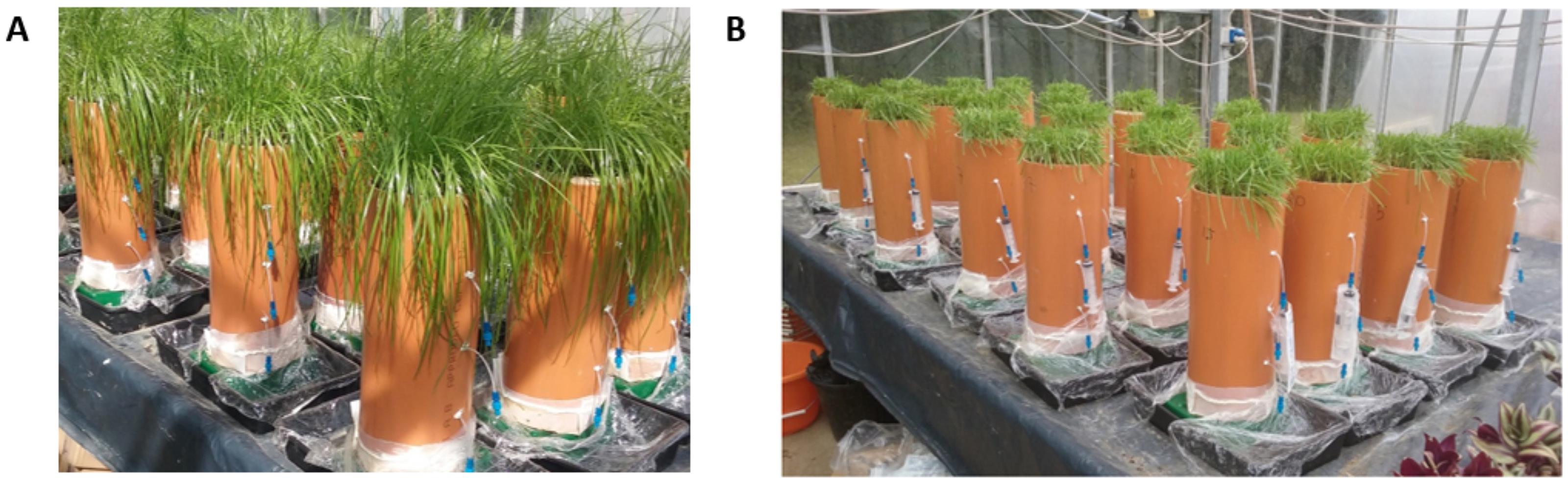
Figure 3. Photos of (A) soil columns with fully-grown ryegrass and (B) soil columns with syringe for sampling soil solutionFor analysis of plant growth and soil microbes, at the end of the 14 weeks (or end of study), harvest the grasses, use the saw to cut open the columns (Figure 4A) and take soil and/or root samples for analysis (see Figures 4B-4C). At the time of harvest, remove the rhizons carefully as they can be reused for about 2-3 more times.

Figure 4. Photos of (A) cutting the column open (B) deconstructed column and (C) sampling roots and soil from the columns
Notes
The results of using this method have been published in three peer-reviewed articles: Ikoyi et al. (2018, 2020a, 2020b).
Acknowledgments
This protocol was adapted from Ikoyi et al. (2018). Funding for this project was provided by Science Foundation Ireland Grant (Grant Number SFI/13/IA/1923). The authors wish to acknowledge Andrew Fowler as the overall grant holder for this project and Con Traas for providing soil for three consecutive years.
Competing interests
The authors declare no competing interests.
References
- Banzhaf, S. and Hebig, K. H. (2016). Use of column experiments to investigate the fate of organic micropollutants– a review. Hydrol Earth Syst Sci 20(9): 3719-3737.
- Bertelkamp, C., Verliefde, A. R., Reynisson, J., Singhal, N., Cabo, A. J., de Jonge, M. and van der Hoek, J. P. (2016). A predictive multi-linear regression model for organic micropollutants, based on a laboratory-scale column study simulating the river bank filtration process. J Hazard Mater 304: 502-511.
- Bromly, M., Hinz, C. and Aylmore, L. A. G. (2007). Relation of dispersivity to properties of homogeneous saturated repacked soil columns. Eur J Soil Sci 58(1): 293-301.
- Eykelbosh, A. J., Johnson, M. S. and Couto, E. G. (2015). Biochar decreases dissolved organic carbon but not nitrate leaching in relation to vinasse application in a Brazilian sugarcane soil. J Environ Manage 149: 9-16.
- Ikoyi, I., Egeter, B., Chaves, C., Ahmed, M., Fowler, A. and Schmalenberger, A. (2020). Responses of soil microbiota and nematodes to application of organic and inorganic fertilizers in grassland columns. Biol Fertil Soils 56: 647-662.
- Ikoyi, I., Fowler, A. and Schmalenberger, A. (2018). One-time phosphate fertilizer application to grassland columns modifies the soil microbiota and limits its role in ecosystem services. Sci Total Environ 630: 849-858.
- Ikoyi, I., Fowler, A., Storey, S., Doyle, E. and Schmalenberger, A. (2020). Sulfate fertilization supports growth of ryegrass in soil columns but changes microbial community structures and reduces abundances of nematodes and arbuscular mycorrhiza. Sci Total Environ 704: 135315.
- Lewis, J. and Sjöstrom, J. (2010). Optimizing the experimental design of soil columns in saturated and unsaturated transport experiments. J Contam Hydrol 115(1-4): 1-13.
- Oppel, J., Broll, G., Loffler, D., Meller, M., Rombke, J. and Ternes, T. (2004). Leaching behaviour of pharmaceuticals in soil-testing-systems: a part of an environmental risk assessment for groundwater protection. Sci Total Environ 328(1-3): 265-273.
- Tracy, S. R., Black, C. R., Roberts, J. A., McNeill, A., Davidson, R., Tester, M., Samec, M., Korošak, D., Sturrock, C. and Mooney, S. J. (2012). Quantifying the effect of soil compaction on three varieties of wheat(Triticum aestivum L.) using X-ray Micro Computed Tomography(CT). Plant Soil 353(1): 195-208.
- Weihermuller, L., Siemens, J., Deurer, M., Knoblauch, S., Rupp, H., Gottlein, A. and Putz, T. (2007). In situ soil water extraction: a review. J Environ Qual 36(6): 1735-1748.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ikoyi, I. O. and Schmalenberger, A. (2021). Design and Construction of Repacked Soil Columns for Measuring Solute Transport, Plant Growth and Soil Biota. Bio-protocol 11(2): e3884. DOI: 10.21769/BioProtoc.3884.
Category
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link


.jpg)







