- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Nippostrongylus brasiliensis Larvae for the Study of Host Skin Response
Published: Vol 10, Iss 24, Dec 20, 2020 DOI: 10.21769/BioProtoc.3849 Views: 2904
Reviewed by: Kristin L. ShinglerKara FilbeyAyush Ranawade

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bacterial Lawn Avoidance and Bacterial Two Choice Preference Assays in Caenorhabditis elegans
Jogender Singh and Alejandro Aballay
May 20, 2020 5240 Views

Parasitemia Evaluation in Mice Infected with Schistosoma mansoni
Naiara Clemente Tavares and Marina Moraes Mourão
May 20, 2021 4529 Views

Quantification of Bacterial Loads in Caenorhabditis elegans
Alyssa C. Walker [...] Daniel M. Czyz
Jan 20, 2022 4231 Views
Abstract
Hookworms are skin penetrating parasites, however in the laboratory the hookworm model Nippostrongylus brasiliensis, the parasite is traditionally administered subcutaneously bypassing the skin (epidermis and dermis). Here, we describe two complementary approaches for infecting mice with N. brasiliensis in order to study the skin immune responses. The first approach employs a skin percutaneous injection that is poorly efficient with the laboratory strain of the parasite in mice, but represents a natural infection. The second approach employs an intradermal injection of the parasite, allowing the controlled delivery of the parasitic larvae and leads to an infection that closely mimics the natural kinetics of parasite migration and development. Both of those infection models allow the investigator to study the skin immune response mounted against the parasite, in addition to detailed investigations of the early immunomodulatory strategies employed by the parasite during skin invasion.
Keywords: Nippostrongylus brasiliensisBackground
Hookworm larvae migrate from the skin to the lungs, before finally reaching the intestine, where they complete their maturation into adult worms and start to reproduce. Immunity against hookworms arise during each of these developmental phases and following both primary and secondary infections (Bouchery et al., 2017). Whilst the host immune responses against the lung and intestinal phases of the parasite infection are well characterised, the skin response has only been partially characterised as current models that utilize the rodent hookworm, Nippostrongylus brasiliensis (Nb), traditionally deliver the infectious larvae via the subcutaneous route of infection bypasses the skin de-facto. To counteract this problem, we developed two methods of infection that allow investigation of the skin-penetrating phase. These models utilize an intradermal infection of infective larvae, allowing the delivery of a controlled dose of the parasite, or the topical application of larvae mimicking the natural skin penetration of hookworms (Gharib, 1955).
Hookworms undergo their transition from a free-living organism to an infective parasite as they migrate through the skin and as such this is the first site where the parasite needs to express products aimed at the modulation of the host immune response. Indeed, we have recently used the protocols described herein to demonstrate the production of a DNAse II by skin penetrating hookworm larvae which acts to degrade neutrophil extracellular traps and thus evade killing by host neutrophils (Bouchery et al., 2020). It is likely that many additional interactions occur between the host and the parasite within the skin but that these remain to be characterised. Further study of such interactions could provide an important avenue for the identification of new vaccine targets against hookworms. Furthermore, the study of immunomodulatory events that occur early after infection could provide potential new treatments against inflammatory diseases.
The skin penetration route is already widely employed in experimental studies of Schistosomiasis infection (Winkel et al., 2018; Haas and Haeberlein, 2009), and we expect our methodology could easily be adapted to other skin penetrating helminths, such as Strongyloides spp (Jannasch et al., 2015).
Materials and Reagents
10 μl XL Freload Reload System pipette tips, low retention, sterile (Interpath Services, catalog number: 33410 )
1.5 ml microcentrifuge tube, graduated, boil proof, sterile (DNAase & RNAase free) (SSI, sold by Interpath Services, catalog number: MCT-175-C )
Insulin syringe 29 G, 12.7 cm, 0.5 ml ultrafine (BD, catalog number: BDM00183 )
15 ml Conical Centrifuge Tubes (BD FalconTM, catalog number: BD352096 )
Tape (generic medical tape, for example Transpore surgical tape, or Nexcare medical tape strong hold, 3M Health care, Australia)
Tissue Kimwipes (Kimberly-Clark, catalog number: 18821277 )
SuperFrost Slides (Menzel, sold by Thermofisher, catalog number: MENSF21201/40NC )
3.5 ml flexible plastic pasteur pipette (Samco, sold by Thermofisher, catalog number: BER225-1S[EA]
N. brasiliensis L3 infective larvae (iL3) (Camberis et al., 2003)
Note: Camberis et al. (2003) provides a detailed review on the preparation of N. brasiliensis. Store at 26 °C.
PBS (DPBS, no calcium, no magnesium) (GibcoTM, sold by ThermoFisher Scientific, catalog number: 14190144 )
Penicillin/Streptomycin 10,000 U/ml (GibcoTM, sold by ThermoFisher Scientific, catalog number: 15140122 , store at 4 °C)
Gentamicin solution 10 mg/ml (Sigma-Aldrich, catalog number: G1272 , store at 4 °C)
Virkon 5-gram tablet (DuPont, catalog number: 500607 )
Antibiotic wash solution (see Recipes)
Equipment
Stereomicroscope (Nikon, SMZ1000)
Tweezers (Student Dumont #5 Forceps, Fine Science Tools, sold by DANIELS HEALTH LABORATORY PRODUCTS, catalog number: 91150-20)
Procedure
Part I: Infection by percutaneous administration
Skin preparation
Note: This step does not require anaesthesia of the mouse, however the experimenter may wish to utilise anaesthesia prior to the described skin preparation in order to increase the ease of the procedure, as well as ensure optimal welfare of the animal.
Depilation of the infection site
One day prior to infection, prepare the mouse skin for injection by depilation. For this purpose, hold the mouse by the scruff of the neck. Using tweezers, take out hairs that surround the infection site, around ~1.5 cm2 (typically on the abdomen or the back, see Video 1 and Figure 1).
Video 1. The mouse is hold by the scruff of the neck, while using tweezers the hairs are removed on the belly skin. This video was made at Monash University according to guidelines from the AEC committee of the Alfred campus, Melbourne Australia on Animal Care with authorization number E/1846/2018/M.
Figure 1. Typical surface area obtained after depilation and tape stripping (here, the skin has been excised one day after infection to perform imaging). Scale bar = 0.4 cm.Note: Do not use depilatory cream or a razor, as they are toxic for larvae, and may injure the skin resulting in suboptimal larval penetration or alterations to the host immune response. We found that the parasite does not naturally penetrate the ear skin, but prefers to enter the skin in areas of the body that are warmer.
Tape stripping of infection site
Apply a tape band of 1.5 cm2 on the previously depilated skin area. Pull the tape off the skin. Repeat the application and the removal motion with a new tape strip for a total of ten times to the same skin area.
Note: This step allows the breakage of the stratum corneum to facilitate entry of the parasite. The skin usually appears shiny after tape stripping.
Larval preparation
On the day of infection, prepare sufficient Nb larvae (L3) for the planned experiment by washing larvae two times with 10 ml of PBS in a 15 ml tube. For each wash allow the larvae to settle to the bottom of the tube by gravity at room temperature. This should take approximately 10-15 min and the larvae should form a pellet at the bottom of the tube. Carefully remove the supernatant using a flexible plastic pasteur pipette, discard the supernatant and replace it with 10 ml of fresh PBS.
Note: 100 Nb L3 are used per application, with a total of 3 applications (300 Nb L3) per mouse. The waste generated from washing Nb L3 needs to be treated with virkon or bleach for 30 min before disposal.
Prepare a suspension of Nb L3 containing 100 L3 per 10 μl of PBS. Calculate the dilution required to generate this suspension by quantifying the larvae generated in step one using a stereomicroscope. Always thoroughly mix the worm solution prior to counting by inverting the tube several times before placing a drop of 2 μl of the worm solution onto a slide, or into a Petri-dish, and counting all of the larvae present in the drop. Adjust the larval concentration by adding more PBS or by removing PBS from the tube containing larvae that have settled to the bottom by gravity (as described in Step B1).
Note: A minimum of three counts should be performed and averaged to optimally estimate the larval concentration.
Topical application and infection
Humidify the treated area of the skin by applying a wet piece of tissue paper.
Note: This step does not require anaesthesia of the mouse, however the experimenter may wish to utilise anaesthesia prior to the described skin preparation in order to increase the ease of the procedure, as well as ensure the optimal welfare of the animal.
Remove the tissue and apply 10 μl of larval solution to the skin, then spread the solution evenly using a manually bent 10 μl tip to create a thin layer of liquid (see Video 2).
Video 2. The skin of the mouse is prepared as reported in Video 1 and humidified using a wet piece of tissue paper. The larval solution is then pipetted using a 10 μl tip. The tip is manually bent and the drop of larvae is applied and evenly spread onto the skin.Repeat three times every 15 min.
Notes:
Ensure thorough mixing of the larval suspension between each application to avoid settling of larvae.
To check for efficacy of infection, mice can be sacrificed one or two days post-infection and the worm burden assessed in the lungs (please see bioprotocol Camberis et al., 2016). Alternatively, live imaging can be conducted following infection (see Figure 2).
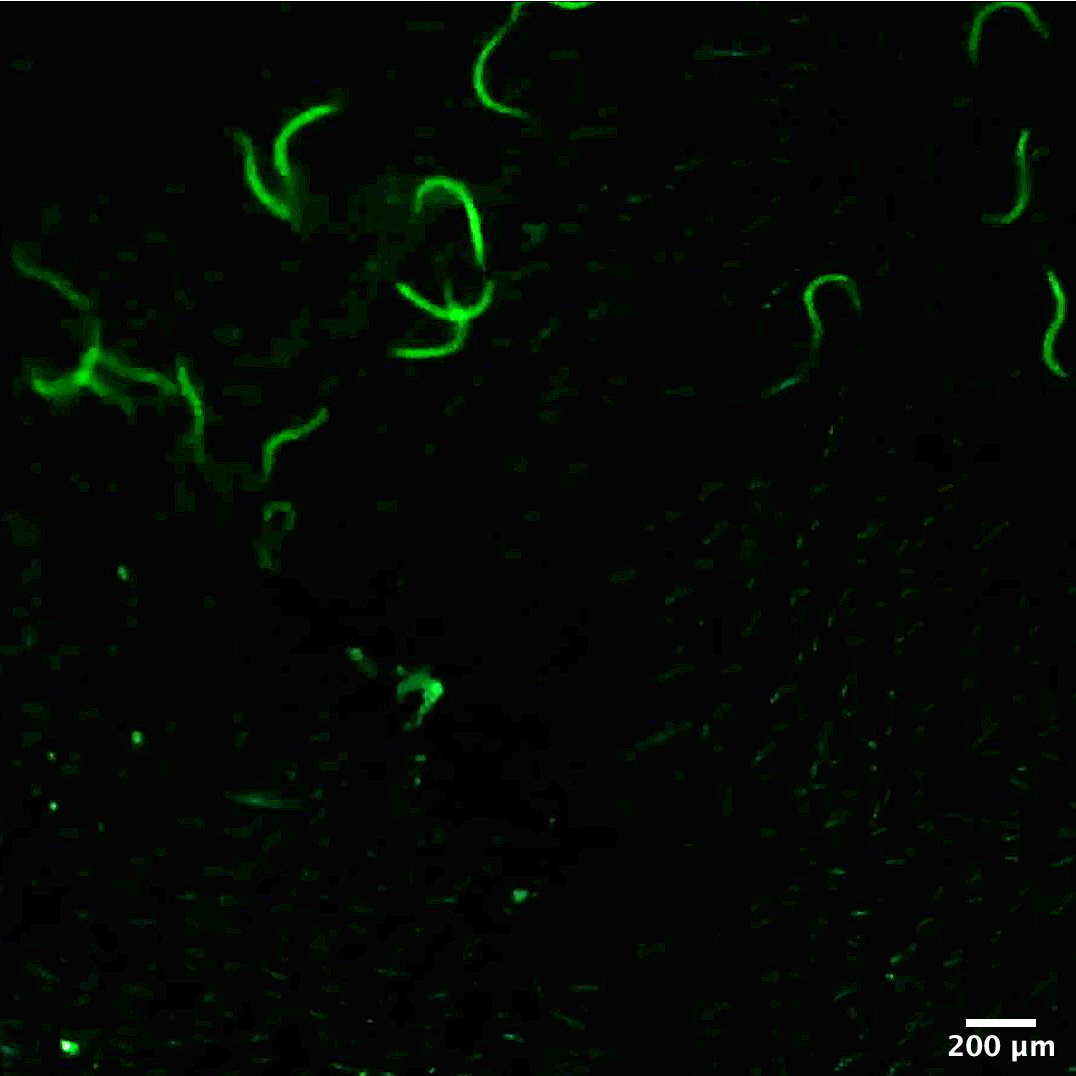
Figure 2. Typical image of fluorescently-labelled larvae applied on the skin using the method described herein and imaged with a stereomicroscope. The protocol for fluorescent labelling of the larvae is available in Bouchery et al., 2017.
Part II: Infection by intradermal injection
Depilation of the infection site
One day prior to infection, prepare the mouse skin for injection by depilation. For this purpose, hold the mouse by the scruff of the neck. Using tweezers, take out hairs surrounding the infection site around ~1.5 cm2 (typically on the abdomen or the back).
Larval preparation
On the day of infection, prepare sufficient Nb larvae (L3) for the planned experiment by washing larvae two times with 10 ml of PBS in a 15 ml tube. For each wash allow the larvae to settle to the bottom of the tube by gravity at room temperature, this should take approximately 10-15 min and the larvae should form a pellet at the bottom of the tube. Carefully remove the supernatant using a flexible plastic pasteur pipette, discard the supernatant and replace it with 10 ml of fresh PBS.
Note: 250 Nb L3 in 10 μl PBS are used per injection.
Transfer the pellet of L3 into a 1.7 ml micro-centrifuge tube using a 1 ml pipette, allow the larvae to form a pellet at the bottom of the tube by gravity for approximately 15 min, and then discard any remaining PBS.
Note: The larvae usually harbour bacteria at their surface. To avoid bacterial contamination during infection, it is possible to wash the larvae in antibiotic solution. To perform this wash, add 500 ul of PBS containing Penicillin/Streptomycin 1,000 μg/ml (Gibco), Gentamicin 1.6 μg/ml (Sigma) to the pelleted larvae. Incubate at room temperature for 30 min and then rinse in sterile PBS. This step can also be used for the percutaneous infection. However, after skin penetration, we have observed less bacterial contamination, potentially as the bacteria are naturally “cleaned” from the parasite surface during the entry into the skin.
Prepare a suspension containing 250 Nb L3 per 10 μl PBS by quantification using a stereomicroscope. Always thoroughly mix the worm solution prior to counting by inverting the tube several times before placing a 2 μl drop of the worm solution onto a slide, or into a Petri-dish, and counting all of the larvae present in the drop. Adjust the larval concentration by adding more PBS or by removing PBS from the tube containing larvae that have settled to the bottom by gravity.
Note: A minimum of three counts should be performed and averaged to estimate optimally the larval concentration.
Mix worm solution thoroughly prior to infecting each mouse. Draw 10 μl of larval solution in an insulin syringe. Inject intradermally.
Note: To check for efficacy of infection, mice can be sacrificed one or two days post-infection and the worm burden assessed in the lungs (please see bioprotocol Camberis et al., 2016). Do not inject more than 10 μl of solution, as this will damage the skin architecture.
Recipes
Antibiotic wash solution
100 ml PBS
5 ml Penicillin/Streptomycin
1.5 ml Gentamycin solution
Can be made in advance and stored for up to one month at 4 °C
Acknowledgments
This protocol is derived from Bouchery et al., 2020, Cell Host and Microbes.
Competing interests
The authors declare no competing interest.
Ethics
All animal experiments were approved by the Service de la consommation et des affaires vétérinaires (1066 Épalinges, Canton of Vaud, Switzerland) with the authorization number VD 3001, or by the AEC committee of the Alfred campus, Melbourne Australia with authorization number E/1846/2018/M
References
- Bouchery, T., Volpe, B., Shah, K., Lebon, L., Filbey, K., LeGros, G. and Harris, N. (2017). The study of host immune responses elicited by the model murine hookworms Nippostrongylus brasiliensis and heligmosomoides polygyrus. Curr Protoc Mouse Biol 7(4): 236-286.
- Bouchery, T., Moyat, M., Sotillo, J., Silverstein, S., Volpe, B., Coakley, G., Tsourouktsoglou, T. D., Becker, L., Shah, K., Kulagin, M., Guiet, R., Camberis, M., Schmidt, A., Seitz, A., Giacomin, P., Le Gros, G., Papayannopoulos, V., Loukas, A. and Harris, N. L. (2020). Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe 27(2): 277-289 e276.
- Camberis, M., Le Gros, G. and Urban, J. (2003). Animal Model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol 55: 19.12.1-19.12.27.
- Camberis, M., Bouchery, T. and Gros, G. L. (2016). Isolation of Nippostrongylus brasiliensis larvae from mouse lungs. Bio-protocol 6(4): e1736.
- Gharib, H. M. (1955). Observations on skin penetration by the infective larvae of Nippostrongylus brasiliensis. J Helminthol 29(1-2): 33-36.
- Haas, W. and Haeberlein, S. (2009). Penetration of cercariae into the living human skin: Schistosoma mansoni vs. Trichobilharzia szidati. Parasitol Res 105(4): 1061-1066.
- Jannasch, M., Groeber, F., Brattig, N. W., Unger, C., Walles, H. and Hansmann, J. (2015). Development and application of three-dimensional skin equivalents for the investigation of percutaneous worm invasion. Exp Parasitol 150: 22-30.
- Winkel, B. M. F., Dalenberg, M. R., de Korne, C. M., Feijt, C., Langenberg, M. C. C., Pelgrom, L., Ganesh, M. S., Yazdanbakhsh, M., Smits, H. H., de Jong, E. C., Everts, B., van Leeuwen, F. W. B., Hokke, C. H. and Roestenberg, M. (2018). Early Induction of Human Regulatory Dermal Antigen Presenting Cells by Skin-Penetrating Schistosoma mansoni Cercariae. Front Immunol 92510.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
bouchery, T., Coakley, G. and Harris, N. L. (2020). Preparation of Nippostrongylus brasiliensis Larvae for the Study of Host Skin Response. Bio-protocol 10(24): e3849. DOI: 10.21769/BioProtoc.3849.
Category
Microbiology > Microbe-host interactions > Nematode
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











