- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Whole-mount Immunohistochemistry of Adult Zebrafish Retina for Advanced Imaging
Published: Vol 10, Iss 24, Dec 20, 2020 DOI: 10.21769/BioProtoc.3848 Views: 5423
Reviewed by: Subhra Prakash HuiVictor Steven Van LaarMatthew Swire

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Whole-mount Senescence-Associated Beta-Galactosidase (SA-β-GAL) Activity Detection Protocol for Adult Zebrafish
Marta Marzullo [...] Miguel Godinho Ferreira
Jul 5, 2022 5606 Views

Long-term in toto Imaging of Cellular Behavior during Nerve Injury and Regeneration
Weili Tian [...] Hernán López-Schier
May 5, 2023 2400 Views

Quantifying Cell Proliferation Through Immunofluorescence on Whole-Mount and Cryosectioned Regenerating Caudal Fins in African Killifish
Augusto Ortega Granillo [...] Alejandro Sánchez Alvarado
Dec 20, 2023 2644 Views
Abstract
Immunohistochemistry is a widely used technique to examine the expression and subcellular localization of proteins. This technique relies on the specificity of antibodies and requires adequate penetration of antibodies into tissues. The latter is especially challenging for thick specimens, such as embryos and other whole-mount preparations. Here we describe an improved method of immunohistochemistry for retinal whole-mount preparations. We report that a cocktail of three reagents, Triton X-100, Tween-20, and DMSO, in blocking and antibody dilution buffers strongly enhances immunolabeling in whole-mount retinas from adult zebrafish. In addition, we establish that in whole retinal tissues, a classic epitope retrieval method, based on citrate buffer, is effective for immunolabeling membrane-associated proteins. Overall, this simple modification allows precise and reproducible immunolabeling of proteins in retinal whole-mounts.
Keywords: PhotoreceptorBackground
To understand complex biological processes, morphological and histological analyses allow for practical qualitative and quantitative approaches. Immunohistochemistry is a robust technique used to visualize the expression and localization of intra- and extra-cellular proteins. Although conventional histological sectioning offers high resolution images of immunostained proteins, sectioning 3-dimensional tissues results in poor preservation of tissue architecture. In sections, complete cellular structures and a complete understanding of the 3-dimentional distribution of proteins are not readily appreciated. In contrast, whole-mount preparations provide large volume 3D information, including intact cellular structures and the spatial relationships of cells and molecules in a complex tissue. However, adapting immunohistochemistry for use with intact tissues often results in poor permeability of antibodies, resulting in incomplete labeling, and a high degree of non-specific background.
In the retina, the cell body of the radial Müller glia spans the entire thickness of the retina (Bringmann et al., 2006). Microglia, the innate immune cells in the central nervous system, with a small cell body and ramified processes, are distributed throughout the retinal parenchyma (Li et al., 2015; Silverman and Wong, 2018). In response to neuronal damage or death, Müller glia and microglia undergo significant structural remodelling. Characterizing the morphology of these two cell types provides a readout the local environment surrounding each cell type and the overall health of the retina (Nagashima et al., 2020; Silva et al., 2020).
Detergents and organic solvents are common reagents that efficiently break down the permeability barriers of cells and nuclei (Jamur and Oliver, 2010). Triton X-100 and Tween-20 are widely used non-ionic detergents, which perturb the phospholipid bilayer structure of biological membranes (Kalipatnapu and Chattopadhyay, 2005; Koley and Bard, 2010; Cheng et al., 2019). Although dimethyl sulfoxide (DMSO), a polar organic solvent, or acetone, a common fixative,has been shown to enhance permeability of cell membranes (de Ménorval et al., 2012), its utility in immunohistochemical procedures is less common. Most procedures select a single reagent of detergents or solvents for permeabilization, and the efficiency of combining more than two chemicals is not well documented.
Here we describe an improved method of whole-mount immunohistochemistry. We report that a cocktail of three reagents, Triton X-100, Tween-20, and DMSO diluted in blocking and antibody dilution buffers dramatically enhances immunolabeling in whole-mount retinas. We also report that a classic citrate buffer based epitope retrieval method (Shi et al., 1993) is effective for immunolabeling membrane associate proteins. For completeness, we also include a detailed protocol for isolating whole retinas from zebrafish.
Materials and Reagents
Animals
Adult Zebrafish (Danio Rerio, Zebrafish International Resource Center, Eugene, OR, 4 to 12 months)
Materials
Colorfrost Plus Microscope Slides (Fisher Scientific, catalog number: 12-550-18 )
Cover Glass, Rectangle No. 1½, 24 x 50 mm (Corning, catalog number: 2980-245 )
1.5 ml Microcentrifuge Tubes, Free of detectable RNase, DNase, DNA & pyrogens (USA Scientific, catalog number: 1615-5500 )
1 ml Syringe without needle (BD, REF 309659 )
ProLong Gold Antifade Mountant (hard-set; ThermoFisher Scientific, catalog number: P36930 )
Reagents
Tissue preparation and fixationTricaine-S/MS-222 (Syndel, Ferndale, WA)
Sodium Bicarbonate (Sigma-Aldrich, catalog number: S5761-500G )
Paraformaldehyde (SPI Supplies, catalog number: 02615-AB )
Sodium Hydroxide/NaOH pellets (Sigma-Aldrich, catalog number: S-0899 )
Sodium Phosphate Monobasic Monohydrate/NaH2PO4·H2O (Millipore Sigma, catalog number: SX0710-3 )
Sodium Phosphate Dibasic/Na2HPO4 (Sigma-Aldrich, catalog number: S7907-1KG )
Sucrose (Sigma-Aldrich, catalog number: S9378-1KG )
10x Anesthesia/Tricaine stock solution (see Recipes)
Anesthesia/Tricaine working solution (see Recipes)
1.0% Sodium Bicarbonate solution (see Recipes)
40% paraformaldehyde stock (see Recipes)
4% paraformaldehyde in 0.1 M Phosphate buffer with 5% sucrose (see Recipes)
10x Phosphate Buffer stock (1.0 M, pH 7.4) (see Recipes)
0.1 M Phosphate buffer with 5% sucrose (see Recipes)
Sodium Citrate Dihydrate/C6H5Na3O7·2H2O (Fisher Scientific, catalog number: BP327-500 )
Tween 20 (Fisher Scientific, catalog number: BP337-500 )
Hydrochloric Acid/HCl (Fisher Scientific, catalog number: A144-500 )
Sodium Phosphate Monobasic Monohydrate/NaH2PO4·H2O (Millipore Sigma, catalog number: SX0710-3 )
Sodium Phosphate Dibasic/Na2HPO4 (Sigma-Aldrich, catalog number: S7907-1KG )
Sodium Chloride/NaCl (Sigma-Aldrich, catalog number: S7653 )
Potassium Chloride/KCl (Sigma-Aldrich, catalog number: P3911-500G )
Triton X-100 (Sigma-Aldrich, catalog number: T9284-500ML )
Sodium Azide (Sigma-Aldrich, catalog number: S2002-100G )
Dimethyl Sulfoxide (Sigma-Aldrich, catalog number: D8418-100ML )
Goat Serum Donor Herd (Sigma-Aldrich, catalog number: G6767-500mL )
Anti-mCherry antibody (rabbit polyclonal, Abcam, catalog number: 167453 )
Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 (Invitrogen, catalog number: A21245 )
Mouse monoclonal anti-ZO1 (ThermoFisher Scientific, ZO1-1A12, catalog number: 33-9100 )
Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 (Invitrogen, catalog number: A21422 )
Sodium Citrate Buffer (see Recipes)
10x Phosphate Buffered Saline (PBS, pH 7.4) stock (see Recipes)
PBS (see Recipes)
PBS with 0.5% Triton X-100 (see Recipes)
10x PBS with 1% sodium azide (see Recipes)
Whole-mount IHC blocking buffer (see Recipes)
Whole-mount IHC dilution buffer (see Recipes)
Whole-mount IHC washing buffer (see Recipes)
Equipment
Scissors, VANNAS, 8 cm STR (G) (World Precision Instruments, model: 14003-G )
Tweezers Dumont #5 INOX (World Precision Instruments, model: 501985 )
Leica 205FA Stereomicroscope (Leica Microsystems)
Fisher brand Isotemp Stirring Hotplate (ThermoFisher Scientific, model: SP88857200 )
Rocker
1,500 ml beaker (PYREX, model: CE-BEAK1L )
LidLocks Microcentrifuge Tube Locks (VWR International, model: 14229-941 )
Round Microcentrifuge Floating Bubble Rack (VWR International, model: 60-86-342 )
Spoon Knife, Double Bevel, Angled 3.3 Blade (Hilcovision, model: 625-0743061-06 )
15 ml Conical Tube (FALCON, model: 352097 )
Transfer Pipets (Fisher Scientific, model: 13-711-7M )
Injection Needle with LuerLok Syringe 1 ml 30G ½ (BD, model: 305778 )
Zeiss AxioImage ZI Epifluorescent Microscope (Carl Zeiss MicroImaging LLC)
Fisherbrand Nutating Mixers-Fixed speed (Fisher Scientific, model: 88-861-041)
Software
ImageJ
Adobe Photoshop CC 2019 (Adobe Systems)
Procedure
Retinal dissection and fixation
Dark adapt animals for at least 3 h prior to dissecting retinas.
Note: Dark adaptation is crucial to separate neural retina from the retinal pigment epithelium. However, this step can be omitted when staining synaptic components, as dark adaptation can induce rapid changes in synaptic structure.
In dark room under red illumination, sacrifice fish by immersion in ice cold water and cervical transection.
Using stereomicroscope, microscissors and tweezers, carefully insert microscissors into the back of the globe to visualize optic nerve (Figure 1, arrows). Cut the optic nerve, gently remove the globe, and place it on paper towel (Figure 2).
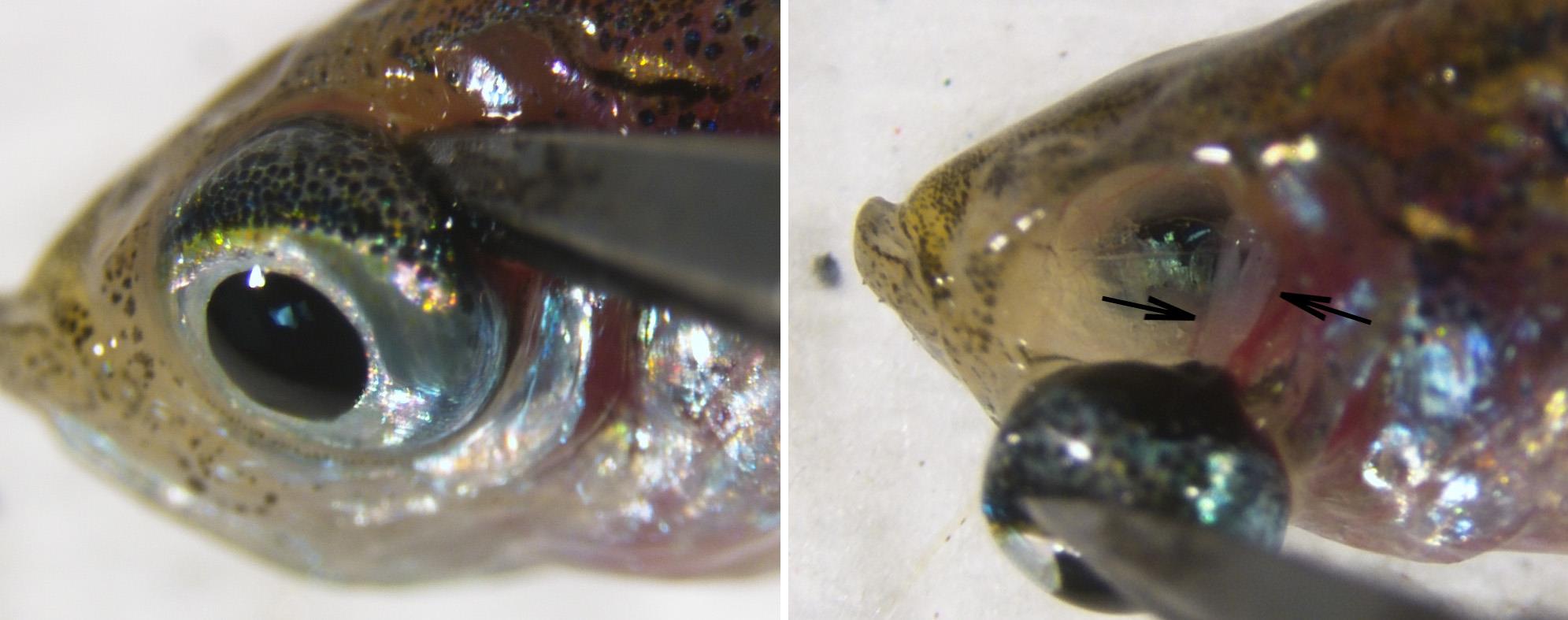
Figure 1. Enucleating the eye globe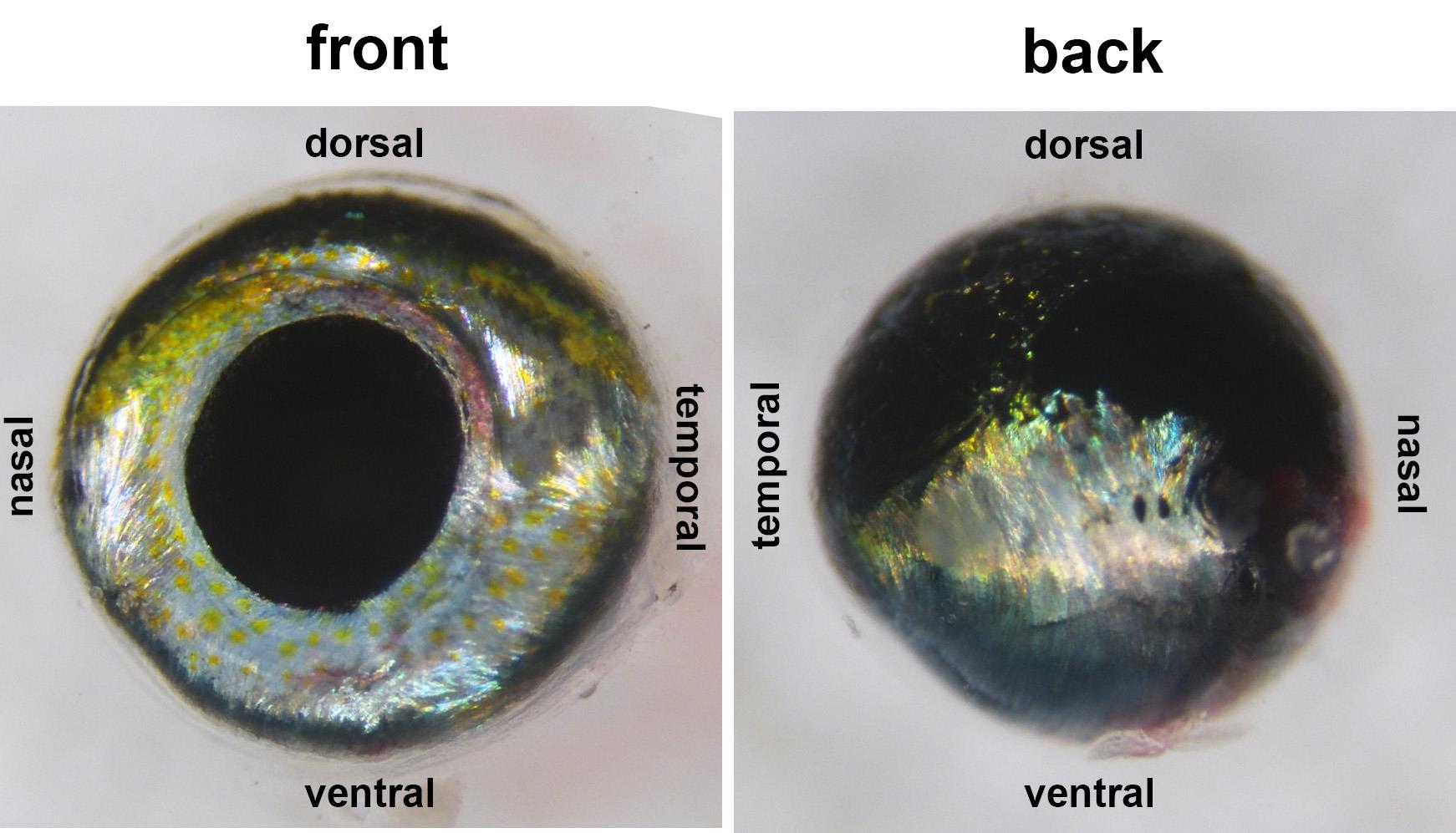
Figure 2. Front and back views of dissected globeNote: For better separation of neural retina and the retinal pigment epithelium, avoid bright lights. Dim illumination is acceptable.
Using 30 G ½ Injection needle, make a small hole ventrally at the choroid fissure (Figure 3, red circle).
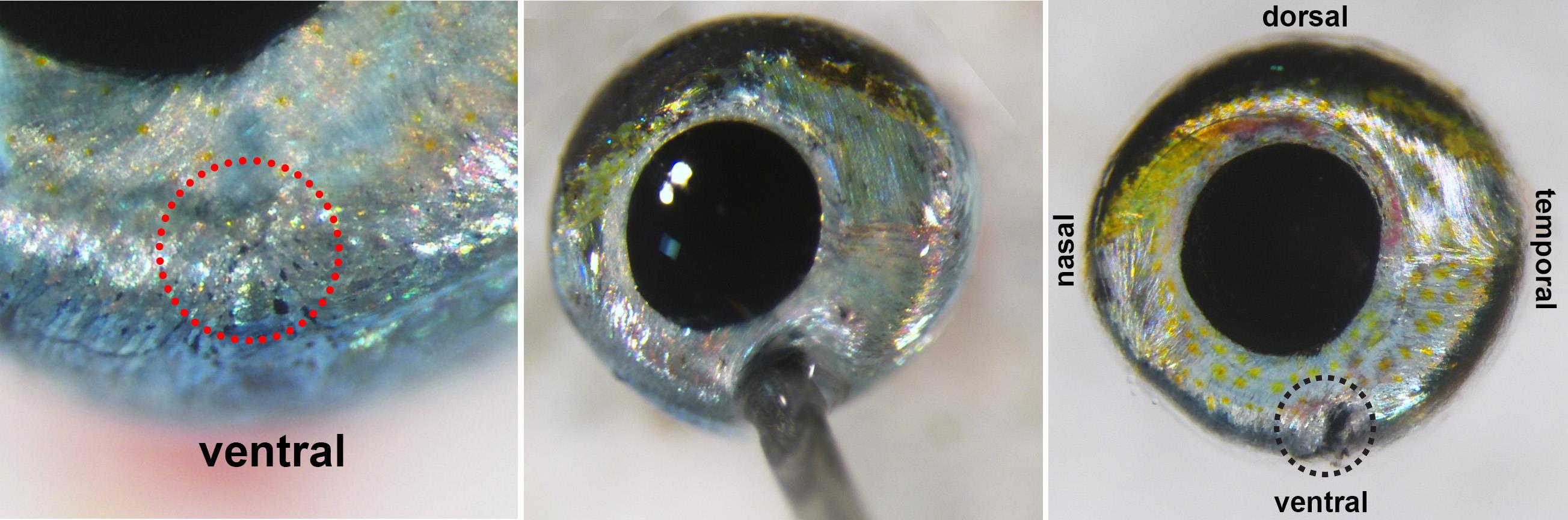
Figure 3. Globe with ventral holeNote: If the choroid fissure is not visible, use other anatomical landmarks to distinguish the dorsal-ventral axis. The dorsal aspect of the eye has a greater amount of melanin/black pigments and fewer iridophores/shiny, silvery pigments, which are enriched ventrally (Figure 2).
With microscissors, extend the ventral hole about 5 mm toward the optic nerve head for orientation.
Cut circumferentially at the junction of the cornea and iris (Figure 4 left, red dashed line) with microscissors. Remove the entire anterior segment of the eye and lens (Figure 4).
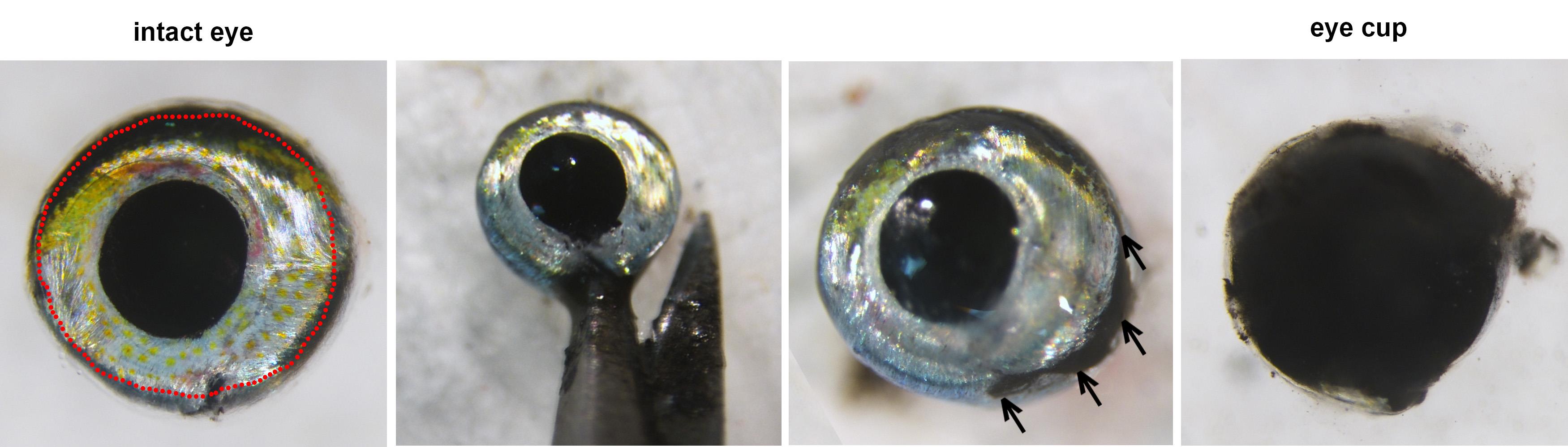
Figure 4. Removal of the anterior segmentPlace the eye cup into PBS and gently separate the neural retina from retinal pigment epithelium by inserting micro tweezers between two layers. The neural retina is faintly pink and contrasts with the underlying retinal pigmented epithelium (Figure 5).
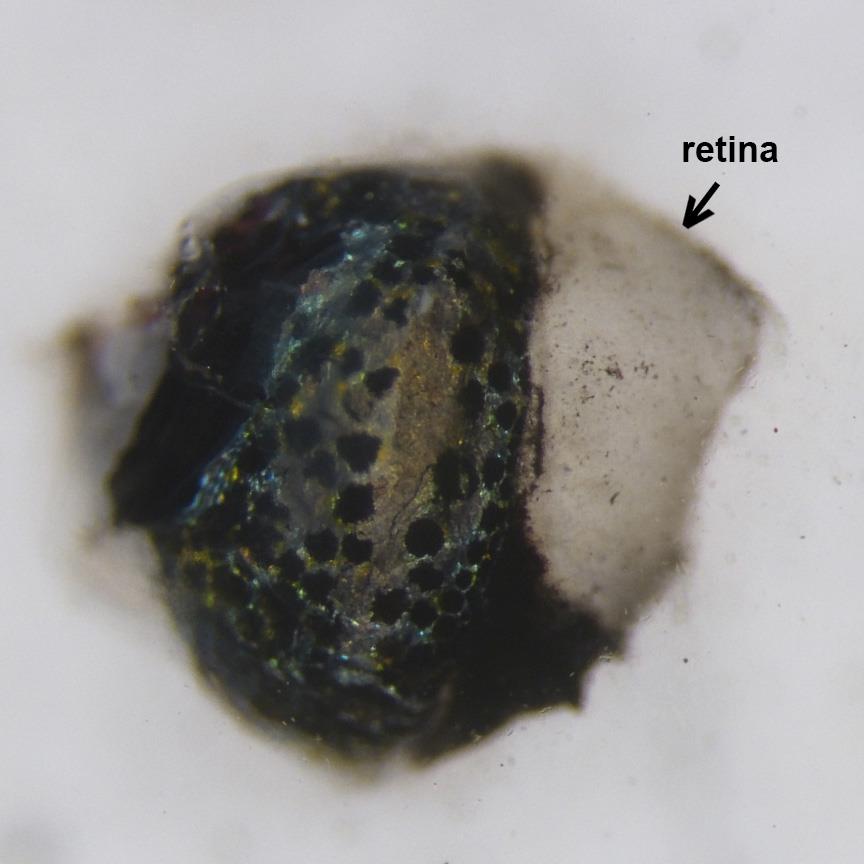
Figure 5. Lateral view of the eye cup in PBSUsing microsurgical blade, make short relaxation cuts from the margin toward the center of the retina at the nasal, temporal and dorsal quadrants (Figure 6). These cuts help to flatten retinas during mounting on the glass slides.
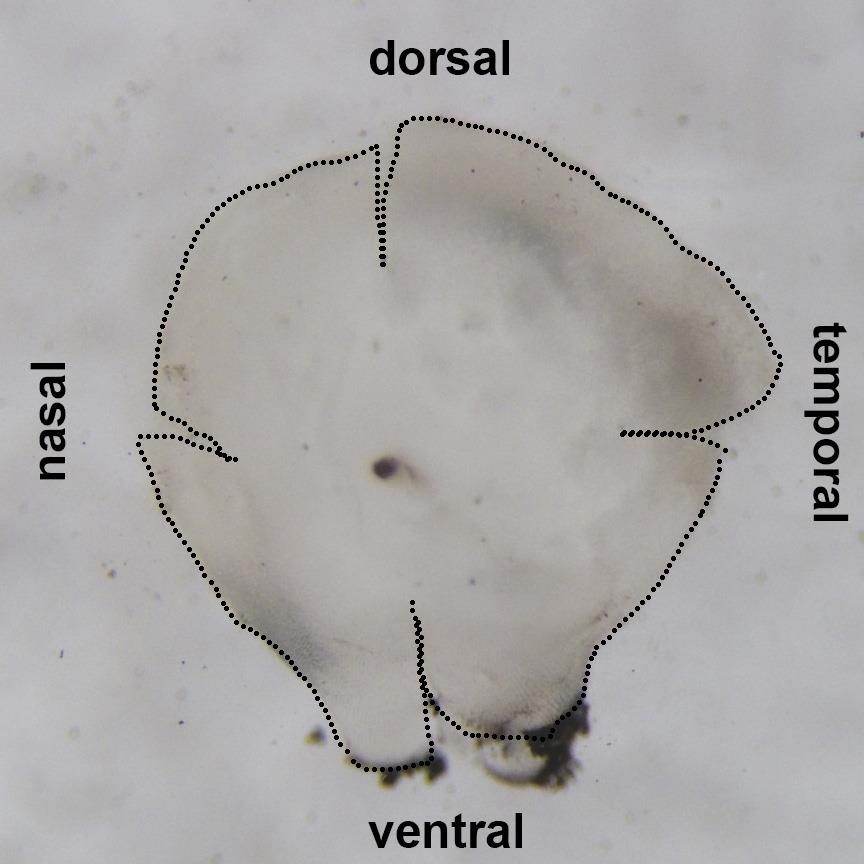
Figure 6. Dissected retinaPlace individual retinas in 1.5 ml microcentrifuge tube containing 1 ml of 4% paraformaldehyde in 0.1 M Phosphate buffer (pH 7.4) with 5% sucrose, and fix overnight at 4 °C on rotator (Figure 7).

Figure 7. Fixation of retinasThe next day, rinse retinas with 1 ml of 5% sucrose in 0.1 M Phosphate buffer (pH 7.4) 3 times for 20 min each on rotator at room temperature.
Epitope retrieval (optional)
Note: This procedure is necessary if your antibody recognizes membrane-associated proteins, such as Zonula Occludens (Figure 13; Nagashima et al., 2020) and N-Cadherin (Nagashima et al., 2017).
Using hot plate, prepare a boiling water bath.
Exchange 5% sucrose in 0.1 M Phosphate buffer (pH 7.4) with 500 μl of Sodium Citrate buffer.
Immerse centrifuge tubes in boiling water bath for 5 to 10 min (Figure 8).
Note: Install a LidLock on each microcentrifuge tube and use a floating rack.

Figure 8. Epitope retrievalRemove beaker from hot plate and let it cool down for 5 min. Do not remove microcentrifuge tubes from the beaker.
After 5 min, remove microcentrifuge tubes from the beaker and rinse retinas with 1 ml of PBS with 0.5% Triton X-100 for 10 min on rotator.
Immunohistochemistry
Remove buffer from the microcentrifuge tubes and add 500 μl of Whole-mount IHC blocking buffer. Incubate retinas with Whole-mount IHC blocking buffer at room temperature for 2 h on rotator.
Dilute antibodies with Whole-mount IHC dilution buffer into optimized dilution (if unknown, start with 1:200). Make sure to centrifuge for 30 s before use.
Note: If you need double or triple labeling, mix all primary antibodies together. If your sample has transgenes, such as GFP or mCherry, we recommend using anti-GFP, anti-dsRed or anti-mCherry antibodies to amplify signals. Optimal dilutions for anti-GFP, anti-dsRed or anti-mCherry vary depending on the type of transgene, cell types transgenes are expressed, and the expression level. We recommend starting with 1:200 if optimized dilution is unknown. Anti-GFP, anti-dsRed, or anti-Cherry is necessary for retinas following epitope retrieval.
Incubate retinas with primary antibodies at room temperature overnight (NO agitation).
Note: Add at least 100 μl of dilution buffer containing desired antibodies.
Rinse retinas with 1 ml of Whole-mount IHC washing buffer 20 min three times at room temperature.
Incubate retinas with secondary antibodies at room temperature overnight in the dark.
Note: Add at least 100 μl of dilution buffer containing desired antibodies with optimized dilution (if unknown, start with 1:200). If you need double or triple labeling, mix all secondary antibodies together. If possible, avoid green-fluorescent conjugate, such as Alexa Fluor 488, since green channel often shows higher background, especially after antigen retrieval.
Rinse retinas with 1 ml of Whole-mount IHC washing buffer 20 min three times at room temperature.
Mounting on slide glass
Using transfer pipette, transfer retina on a slide glass (Figure 9).

Figure 9. Transferred retina on a slide glassRemove excess buffer with Kimwipe (DO NOT let retinas completely dry).
Using tweezer, clean up and orient retinas in photoreceptor side down. A large ventral cut (see Step A8) serves as a landmark for orientating the retina. Add 1 to 2 drops of Prolong Gold onto cover glass. Gently place the cover glass on the retina for mounting (Figure 10).
Note: Cure at least for 1 week at room temperature in the dark.
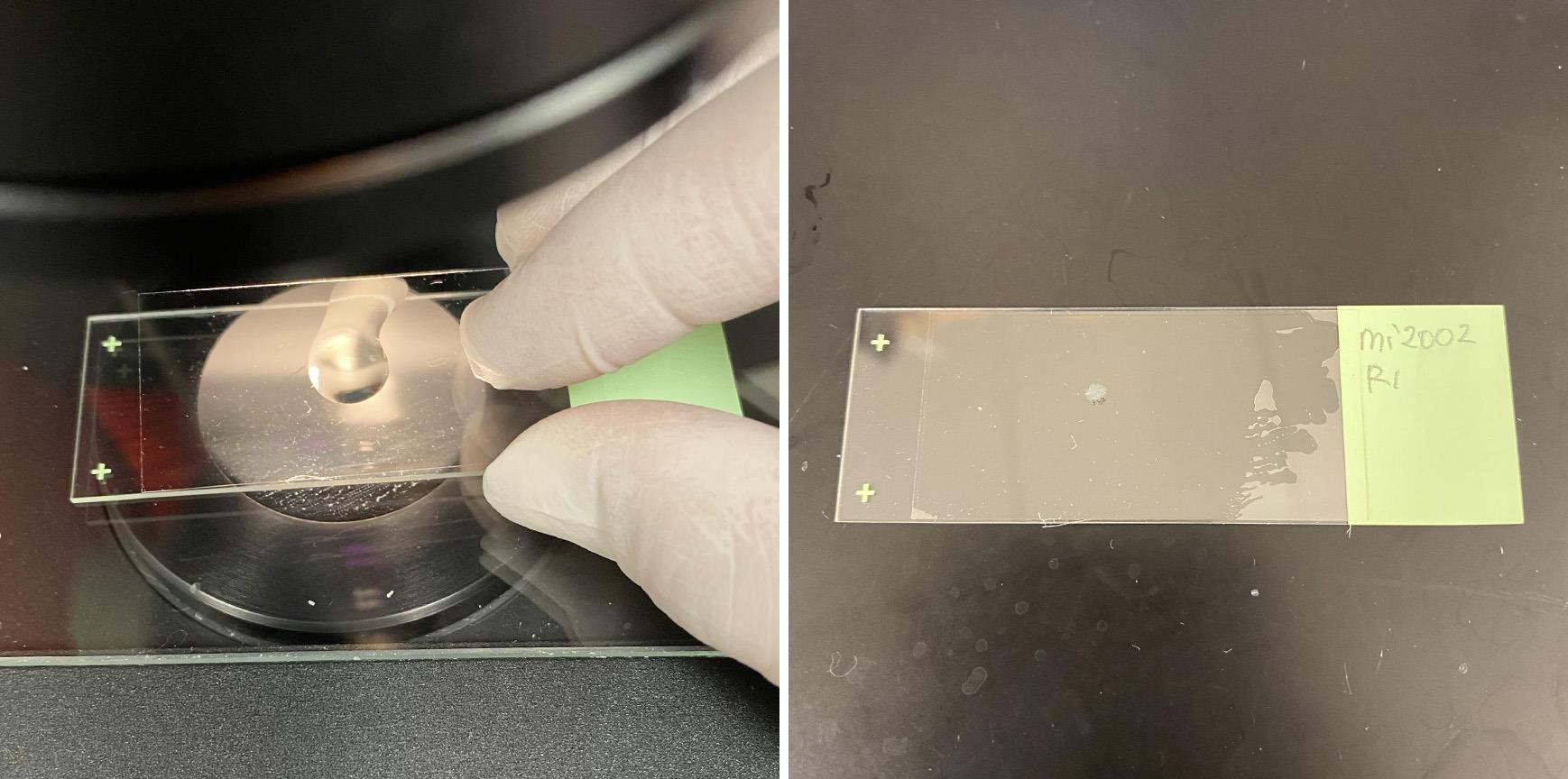
Figure 10. Mounting retina on a slide glass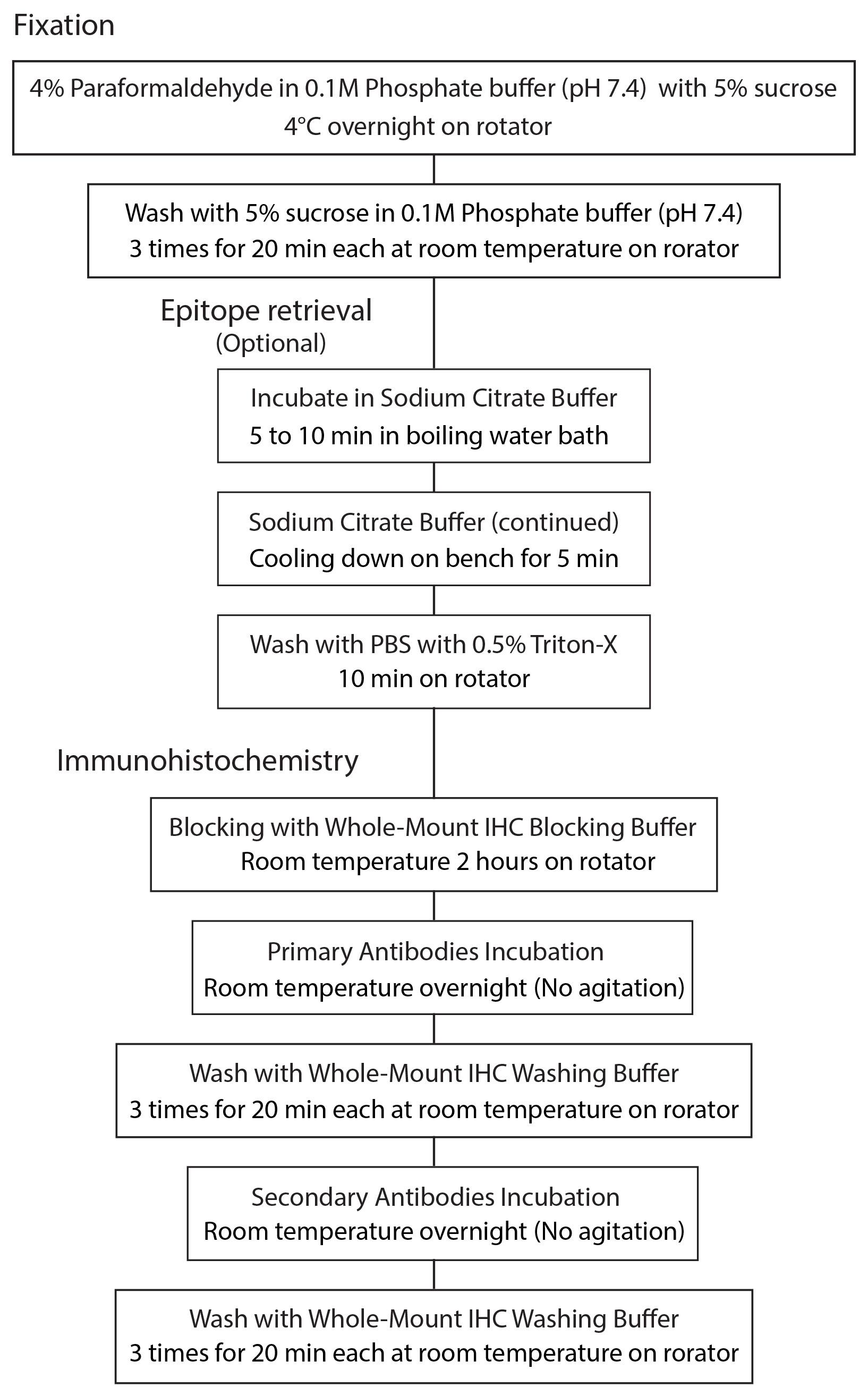
Figure 11. Procedure of fixation and immunohistochemistry
Data analysis
Results and Discussions
Using the protocol described above (Figure 11), we successfully imaged Tg(gfap:egfp)mi2002 labeled Müller glia (Bernardos and Raymond, 2006; Nagashima et al., 2017 and 2020), Tg(mpeg1:mCherry)gl23-labeled microglia (Figure 12; Ellett et al., 2011 : Silva et al., 2020), Tg(-5.5sws1:egfp)kj9- or Tg(trb2:tdTomato)-labeled cone photoreceptors (Nagashima et al., 2017 ; Nunley et al., 2019), and Tg(-3.7rho:egfp)kj2-labeled rod photoreceptors (Raymond et al., 2014).
Optical sectioning is essential for imaging immunofluorescence in thick specimens. Confocal microscopy eliminates out-of-focus signals and aids rapid three-dimentional reconstructions. Alternatively, we recommend epifluorescence microscopes equipped with structured illumination technology (Wu and Shroff, 2018) or computational clearing systems, which mathematically eliminate out-of-focus blur.
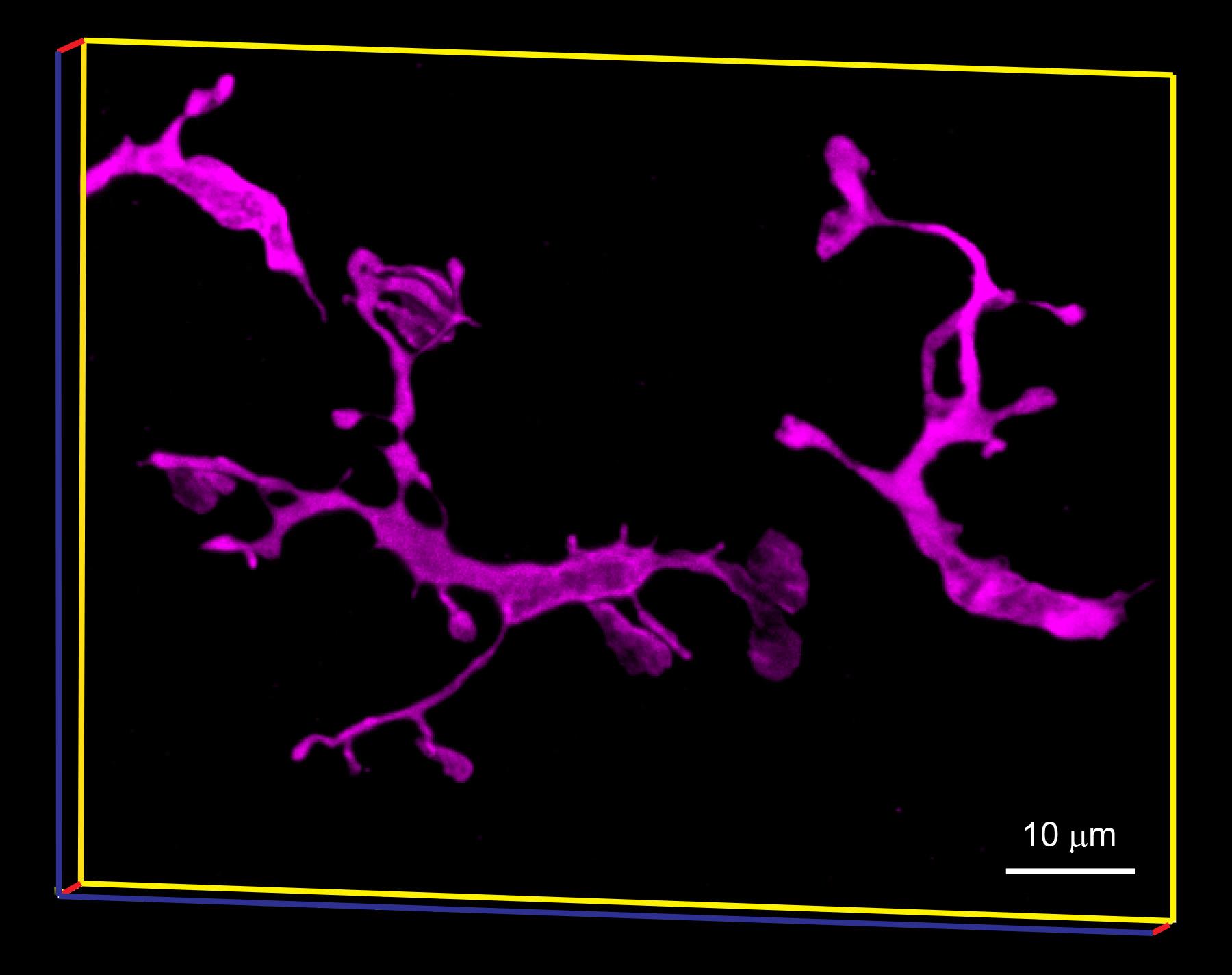
Figure 12. Tg(mpeg1:mCherry)gl23-labeled microglia in zebrafish retina
The transgene, Tg(mpeg1:mCherry)gl23 (Ellett et al., 2011), was immuno-labeled with rabbit polyclonal anti-mCherry (1:200) and Alexa Fluor 647 Goat anti-Rabbit antibodies (1:200). Image was captured using a Leica TCS SP5 confocal microscope (Leica Microsystems). ImageJ software (https://imagej.nih.gov/ij/) was used for 3D rendering.
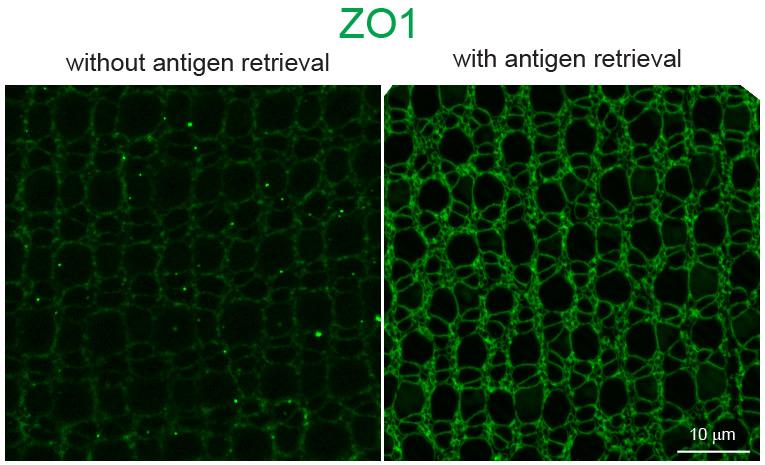
Figure 13. Comparison of zebrafish retinas immuno-labeled with ZO-1 without (left) and with (right) antigen retrieval
An integral membrane protein, ZO1, is immuno-labeled with mouse monoclonal anti-ZO1 (1:200) and Alexa Fluor 555 Goat anti-Mouse IgG antibodies (1:200) without (left) or with (right) antigen retrieval (Figure 13). Images were captured with a Zeiss AxioImage ZI Epifluorescent Microscope. To create maximum projection of the Z-stack series, Adobe Photoshop CC 2019 (Adobe Systems) was used.
Recipes
10x Anesthesia/Tricaine stock solution (10 mg/ml Tricaine methanesulfonate)
Add 1.0 g of Tricaine methanesulfonate (MS-222) to 100 ml of Mili-Q water
Adjust pH to 7.4 using 1.0% Sodium Bicarbonate solution
Make 2 ml aliquots and store frozen at -20 °C
Anesthesia/Tricaine working solution
Add 2 ml of Anesthesia/Tricaine stock solution to 100 ml zebrafish system water
1.0% Sodium Bicarbonate solution
Add 1 g of Sodium Bicarbonate into 100 ml of Mili-Q water
40% paraformaldehyde stock
Preheat 200 ml of Mili-Q water to 50-60 °C
Add 200 g of paraformaldehyde to preheated water
Stir well and add concentrated NaOH (NaOH pellets) until solution is clear
Bring up volume to 500 ml (adjustment of pH is not necessary)
Make 2 ml aliquots and store frozen
4% paraformaldehyde in 0.1 M Phosphate buffer with 5% sucrose
Thaw 2 ml of 40% paraformaldehyde stock in/under hot water
Add 1 g of sucrose and 2 ml of 10x Phosphate Buffer stock to 16 ml of Mili-Q water
Add 2 ml of 40% paraformaldehyde stock
Store at 4 °C, good for 2 weeks
10x Phosphate Buffer stock (1.0 M, pH 7.4)
Dissolve 13 g of NaH2PO4·H2O and 55.75 g of Na2HPO4 in 350 ml of Mili-Q water
Adjust pH to 7.4 and bring volume to 500 ml with Mili-Q water
Autoclave for a long time storage
0.1 M Phosphate buffer with 5% sucrose
Dissolve 25 g of sucrose in 400 ml of H2O
Add 50 ml of 10x Phosphate buffer
Adjust pH to 7.4 if needed
Bring up volume to 500 ml with Mili-Q water
Sodium Citrate Buffer (10 mM Sodium Citrate with 0.05% Tween 20, pH 6.0)
Add 0.294 g of C6H5Na3O7·2H2O to 100 ml of Mili-Q water
Adjust pH to 6.0 using 1 N HCl
Add 0.05 ml of Tween 20 and mix well
Store at room temperature for 3 months, or at 4 °C for longer storage
10x Phosphate Buffered Saline (PBS, pH 7.4) stock
Add 2.76 g of NaH2PO4·H2O, 11.36 g of Na2HPO4, 87.6 g of NaCl, 1.87 g of KCl to 850 ml of Mili-Q water
Adjust pH to 7.4
Bring to volume to 1 L
Each must be dissolved in solution before the next salt is added
PBS
Add 100 ml of 10x PBS stock to 900 ml of Mili-Q water
Adjust pH to 7.4
PBS with 0.5% Triton X-100
Add 0.5 ml of Triton X-100 into 100 ml of PBS
Mix well until Triton X-100 is completely dissolved
10x PBS with 1% sodium azide
Add 0.5 g of sodium azide into 50 ml of 1x PBS
Whole-mount IHC blocking buffer (10% Normal goat serum, 1% Tween-20, 1% Triton X-100, 1% DMSO in 0.1 M PBS with 0.1% sodium azide)
Add 1.0 ml of 10x PBS with 1% sodium azide to 7.7 ml of Mili-Q water
Using disposable syringe, add 0.1 ml of Tween-20 and 0.1 ml of Triton X-100 into PBS containing sodium azide (a)
Add 100 μl of DMSO
Mix well until Tween-20 and Triton X-100 are completely dissolved
Add 1 ml of Normal goat serum
Store at 4 °C, good for 1 week
Whole-mount IHC dilution buffer (2% Normal goat serum, 1% Tween-20, 1% Triton X-100, 1% DMSO in 0.1M PBS with 0.1% sodium azide)
Add 1.0 ml of 10x PBS with 1% sodium azide to 8.65 ml of Mili-Q water
Using disposable syringe, add 0.1 ml of Tween-20 and 0.1 ml of Triton X-100 into PBS containing sodium azide (a)
Add 100 μl of DMSO
Mix well until Tween-20 and Triton X-100 are completely dissolved
Add 50 μl of Normal goat serum
Store at 4 °C, good for 1 week
Whole-mount IHC washing buffer (PBS with 1% Tween-20, 1% Triton X-100, 1% DMSO)
Add 10 ml of 10X PBS to 87 ml of Mili-Q water
Using disposable syringe, add 1 ml of Tween-20 and 1 ml of Triton X-100 into PBS
Add 1 ml of DMSO
Stir well until Tween-20 and Triton X-100 are completely dissolved
Store at 4 °C, good for 1 week
Acknowledgments
This work was supported by grants from the National Institutes of Health (NEI) - R01EY07060 and P30EYO7003 (PFH) and an unrestricted grant from the Research to Prevent Blindness, New York. Fish lines and reagents provided by ZIRC were supported by NIH-NCRR Grant P40 RR01. The authors thank Dilip Pawar for zebrafish maintenance and image acquisition.
Competing interests
The authors declare no competing interests.
Ethics
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan.
References
- Bernardos, R. L. and Raymond, P. A. (2006). GFAP transgenic zebrafish. Gene Expr Patterns 6(8): 1007-1013.
- Bringmann, A., Pannicke, T., Grosche, J., Francke, M., Wiedemann, P., Skatchkov, S. N., Osborne, N. N. and Reichenbach, A. (2006). Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25(4): 397-424.
- Cheng, R., Zhang, F., Li, M., Wo, X., Su, Y. W. and Wang, W. (2019). Influence of fixation and permeabilization on the mass density of single cells: A surface plasmon resonance imaging study. Front Chem 7: 588.
- de Ménorval, M. A., Mir, L. M., Fernandez, M. L. and Reigada, R. (2012). Effects of dimethyl sulfoxide in cholesterol-containing lipid membranes: a comparative study of experiments in silico and with cells. PLoS One 7(7): e41733.
- Ellett, F., Pase, L., Hayman, J. W., Andrianopoulos, A. and Lieschke, G. J. (2011). mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117(4): e49-e56.
- Jamur, M. C. and Oliver, C. (2010). Permeabilization of cell membranes. Methods Mol Biol 588: 63-66.
- Kalipatnapu, S. and Chattopadhyay, A. (2005). Membrane protein solubilization: recent advances and challenges in solubilization of serotonin1A receptors. IUBMB Life 57(7): 505-512.
- Koley, D. and Bard, A. J. (2010). Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy(SECM). Proc Natl Acad Sci U S A 107(39): 16783-16787.
- Li, L., Eter, N. and Heiduschka, P. (2015). The microglia in healthy and diseased retina. Exp Eye Res 136: 116-130.
- Nagashima, M., Hadidjojo, J., Barthel, L. K., Lubensky, D. K. and Raymond, P. A. (2017). Anisotropic Müller glial scaffolding supports a multiplex lettice mosaic of photoreceptors in zebrafish retina. Neural Dev 12(1): 20.
- Nagashima, M., D’Cruz, T. S., Danku, A. E., Hesse, D., Sifuentes, C., Raymond, P. A. and Hitchcock, P. F. (2020). Midkine-a is required for cell cycle progression of müller glia during neuronal regeneration. in the vertebrate retina. J Neurosci 40(6): 1232-1247.
- Nunley, H., Nagashima, M., Martin, K., Gonzalez, A. L., Suzuki, S. C., Norton, D., Wong, R. O. L., Raymond, P. A. and Lubensky, D. K. (2019). Defect patterns on the curved surface of fish retinae suggest mechanism of cone mosaic formation. https://doi.org/10.1101/806679
- Raymond, P. A., Colvin, S. M., Jabeen, Z., Nagashima, M., Barthel, L. K., Hadidjojo, J., Popova, L., Pejaver, V. R. and Lubensky, D. K. (2014). Patterning the cone mosaic array in zebrafish retina requires specification of ultraviolet-sensitive cones. PloS One 9(1): e85325.
- Shi, S. R., Chaiwun, B., Young, L., Cote, R. J. and Taylor, C. R. (1993). Antigen retrieval technique utilizing citrate buffer or urea solution for immunohistochemical demonstration of androgen receptor in formalin-fixed paraffin sections. J Histochem Cytochem 41(11): 1599-1604.
- Silva, N. J., Nagashima, M., Li, J., Kakuk-Atkins, L., Ashrafzadeh, M., Hyde, D. R. and Hitchcock, P. F. (2020). Inflammation and matrix metalloproteinase 9(Mmp-9) regulate photoreceptor regeneration in adult zebrafish. Glia 68(7): 1445-1465.
- Silverman, S. M. and Wong, W. T. (2018). Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu Rev Vis Sci 4: 45-77.
- Wu, Y. and Shroff, H. (2018). Faster, sharper, and deeper: structured illumination microscopy for biological imaging. Nat Methods 15(12): 1011-1019.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Nagashima, M. and Hitchcock, P. F. (2020). Whole-mount Immunohistochemistry of Adult Zebrafish Retina for Advanced Imaging. Bio-protocol 10(24): e3848. DOI: 10.21769/BioProtoc.3848.
- Nagashima, M., D’Cruz, T. S., Danku, A. E., Hesse, D., Sifuentes, C., Raymond, P. A. and Hitchcock, P. F. (2020). Midkine-a is required for cell cycle progression of müller glia during neuronal regeneration. in the vertebrate retina. J Neurosci 40(6): 1232-1247.
Category
Developmental Biology > Cell growth and fate > Regeneration
Neuroscience > Development > Histological staining
Cell Biology > Cell imaging > Fixed-tissue imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link