- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Treadmill Running of Mouse as a Model for Studying Influence of Maternal Exercise on Offspring
Published: Vol 10, Iss 23, Dec 5, 2020 DOI: 10.21769/BioProtoc.3838 Views: 4173
Reviewed by: Miao HeXiaoyu LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assay for Site-Specific Homologous Recombination Activity in Adherent Cells, Suspension Cells, and Tumor Tissues
Yuki Yoshino [...] Natsuko Chiba
Apr 5, 2025 2482 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1851 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 173 Views
Abstract
Epidemiological studies robustly show the beneficial effects of maternal exercise in reducing maternal birth complications and improving neonatal outcomes, though underlying mechanisms remain poorly understood. To facilitate mechanistic exploration, a protocol for maternal exercise of mice is established, with the regimen following the exercise guidelines for pregnant women. Compared to volunteer wheel running, treadmill running allows precise control of exercise intensity and duration, dramatically reducing variations among individual mouse within treatments and facilitating translation into maternal exercise in humans. Based on the maximal oxygen consumption rate (VO2max) before pregnancy, the treadmill exercise protocol is separated into three stages: early stage (E1.5 to E7.5 at 40% VO2max), mid stage (E8.5 to E14.5 at 65% VO2max), and late stage of pregnancy (E15.5 to birth at 50% VO2max), which demonstrated persistent beneficial effects on maternal health and fetal development. This protocol can be useful for standardizing maternal treadmill exercise using mice as an experimental model.
Keywords: Maternal exerciseBackground
Maternal exposure to adverse environmental stimuli leads to various physiological changes in the offspring (Hay et al., 2016; Walejko et al., 2019; Battarbee et al., 2020), including predisposition of offspring to the development of obesity and type 2 diabetes (Godfrey et al., 2017; Wesolowski et al., 2018). Physical exercise is highly accessible and a prominent therapeutic tool to combat diet-induced obesity in adults (Peres Valgas da Silva et al., 2019). Epidemiological studies robustly show the beneficial effects of maternal exercise in improving maternal health and neonatal outcomes (Hopkins and Cutfield, 2011; Nascimento et al., 2012; Moyer et al., 2016; da Silva et al., 2017; Rogozinska et al., 2017; Davenport et al., 2018a and 2018b; Ming et al., 2018; Beetham et al., 2019; Hoover and Louis, 2019; Pastorino et al., 2019; Wang et al., 2019). But surprisingly, the biological effects of maternal exercise on offspring health have only been sparsely examined in the context of maternal obesity and high fat diet intake (Laker et al., 2014; Stanford et al., 2015; Stanford et al., 2017; Musial et al., 2019; Uddin et al., 2019), as well as normal chow diet (Raipuria et al., 2015), the effects of maternal exercise of healthy mothers on fetal development remain largely unexplored. Mice is the most commonly used model for biomedical studies; to facilitate mechanistic exploration, an exercise protocol for pregnant mice which can be easily translated into exercise in pregnant women is needed.
Both treadmill exercise and voluntary wheel running are commonly used for exercise training in rodents. We recommend to use treadmill exercise because the exercise intensity and length can be precisely controlled, which is very similar to treadmill training in humans, increasing translational value. On the other hand, large variations in exercise intensity and duration among individual mouse exist in voluntary wheel running, making data interpretation difficult (Goh and Ladiges, 2015). Also, the exercise intensity and duration cannot be controlled in voluntary wheel running and thus difficult to be translated into human exercise. Nonetheless, treadmill exercise training induces low levels of acute stress to mice, but this concern can be alleviated by applying the same procedure to both control and exercised mice. Actually, regular treadmill exercise at moderate intensity has protective effects in stress-related symptoms (Patki et al., 2014; Seo, 2018; Loprinzi and Frith, 2019). Based on exercise guidelines published by American College of Obstetricians and Gynecologists (Zavorsky and Longo, 2011), we propose the following protocol for treadmill exercise, which composes of three stages with exercise intensity calculated based on the maximal oxygen intake (VO2max) before pregnancy. The initial stage is from embryonic day 1.5 (E1.5) to E7.5, mid-stage from E8.5 to E14.5, and late-stage from E15.5 to birth, with the intensity of exercise at 40%, 65%, and 50% of VO2max, respectively. Using this protocol, we found that maternal exercise slightly reduced weight gain during pregnancy, but had no effect on birth weight; in addition, offspring metabolic health was improved due to maternal exercise and detailed changes were reported previously (Son et al., 2019 and 2020). Thus, this protocol fits for studying maternal impacts on fetal development and long-term health of the next generation.
Materials and Reagents
Adult C57BL/6J pregnant mice
High-fat diet (HFD), 60% energy from fat (Research Diets, D12492 )
Equipment
Oxymax Fast 4 lane modular treadmill system (Columbus Instruments, Columbus, OH, USA)
Indirect open circuit calorimetry system (Comprehensive Lab Animal Monitoring System, CLAMS; Columbus Instruments, Columbus, OH, USA)
Software
Oxymax for Windows v4.93 (Columbus Instruments, Columbus, OH, USA) with listing parameters: VO2, O2 in&out, DO2, ACCO2, VCO2, CO2 in&out, DCO2, ACCCO2, RER, HEAT, Flow (Figure 1)
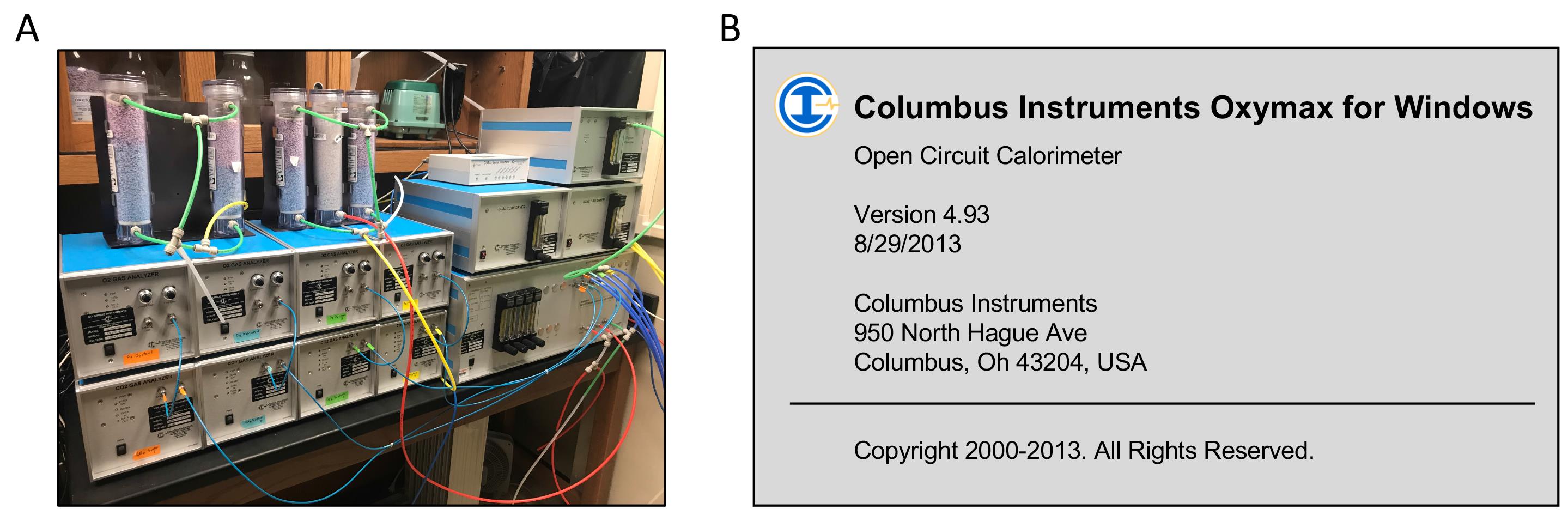
Figure 1. Calorimeter and software for measuring oxygen consumption. A. Open circuit calorimeter. B. Software for open circuit calorimeter.
Procedure
Setting target intensities
Before mating and initiating exercise training, female mice need to be accustomed with treadmill running by either training daily or every alternative days for one week (10 m/min for 10 min once)
Measuring the maximal oxygen consumption rates (VO2max)
Before mating, female mice are subjected to VO2max measurement. The body weight (g) and age of mice need to be recorded, which are needed for normalization of oxygen consumption rate by the software.
Before treadmill running, mice are put on the treadmill with indirect open circuit calorimetry system for 5 min to calm down.
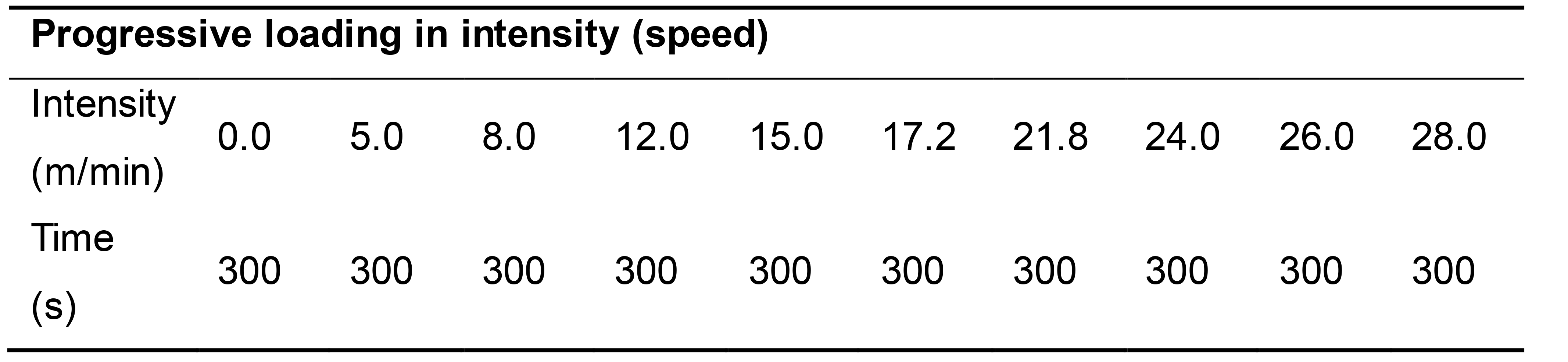
Treadmill running (without incline) is initiated following the progressive loading in intensity shown in Table 1.
Running is stopped when mice exhibit exhaustion: When mice touch the electric shock bar three times within five seconds, those mice are regarded as exhaustion.
The target intensities (40%, 65%, and 50% of VO2max) will be calculated (Zavorsky and Longo, 2011).
Table 1. The intensity of single bout of exhaustive treadmill exercise for maximal oxygen consumption rates
Exercise during pregnancy
After mating which is confirmed in the morning by the presence of vaginal smear and designated as embryonic day 0.5 (E0.5), pregnant mice are subjected to treadmill exercise training without incline every morning starting at E1.5.
Based on the guideline for pregnancy (Zavorsky and Longo, 2011), a three stage exercise is used: the early stage (E1.5 to E7.5), mid-stage (E8.5 to E14.5), and late stage (E15.5 to birth) with target intensity of 40%, 65%, and 50% VO2max, respectively.
As an example, the target intensities of healthy or obese adult C57BL/6J female mice (16-week-old) are shown in Table 2 (Son et al., 2019 and 2020).
During exercise training, electric shocks are used to motivate individual mouse which may be reluctant to run, but the usage for pregnant mice should be avoided in order to minimize stress. Instead, tail touching is recommended to encourage run.
In a single training session, individual mice will only be allowed to be tail-touched twice without being stopped. If mice refuse to run upon 2nd touch, mice will be allowed to rest for 5 min before resuming the training/testing protocol. Mice will be removed from the trial if a 4th touch occurs.
To encourage mice to rum, a black cover in the front end of treadmill can be used, which creates a dark area (see Video 1).
Table 2. The target intensities for adult C57BL/6J pregnant mice
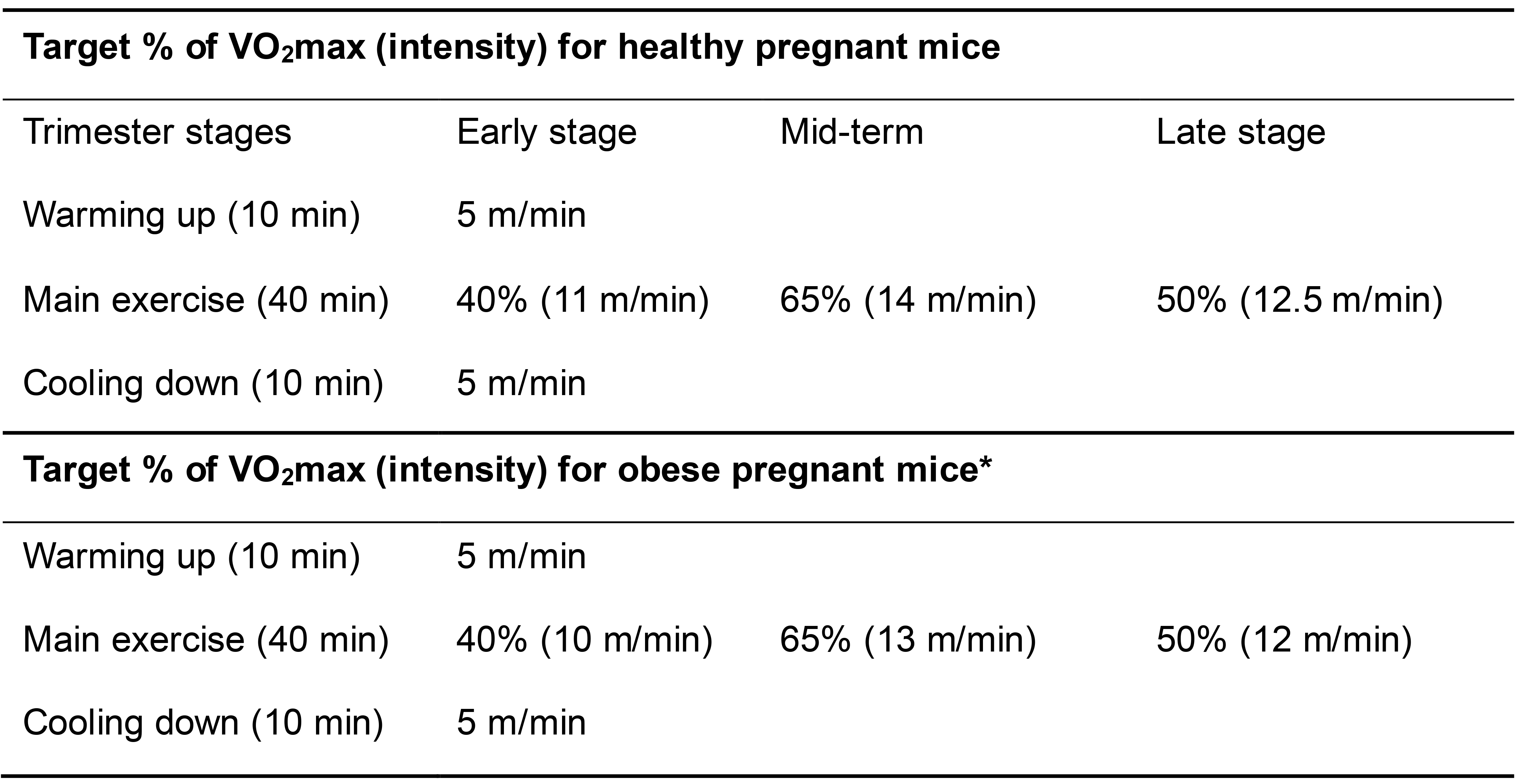
*Obese adult mice are fed a high-fat diet (HFD, 60% energy from fat; D12492 , Research Diets) for 8 weeks. The mice had gained over 25% of initial body weight due to HFD.
Video 1. Mice during treadmill exercise training
Data analysis
Indirect open circuit calorimetry system is utilized to measure oxygen consumption (VO2), carbon dioxide production (VCO2), and respiratory exchange ratio (RER). For setting target intensity, VO2max is determined as the highest value with RER over 1.0. Then, the target percentages (40, 65, and 50%) of intensities (speeds) are calculated to be proportional to the speed at VO2max. For example, if the VO2max is 10,000 ml/kg/hr when the speed is 20 m/min, the 50% of exercise intensity is the speed when the oxygen consumption rate is:
(10,000 – resting value) x 0.5 + resting value.
Notes
To set the target intensities, heart rate and total calorie use are alternative endpoints. We recommend to use the target intensities (speeds) which proportionally match with target percentages of VO2max and can be easily performed.
Acknowledgments
This work was supported by the National Institutes of Health (NIH R01-HD067449).
Competing interests
The authors declare that they have no competing interests.
Ethics
All animal experiments were approved by the Institute of Animal Care and Use Committees (IACUC) at the Washington State University (Protocol number: 6704; valid between Sept. 25, 2020-Sept. 24, 2023).
References
- Battarbee, A. N., Venkatesh, K. K., Aliaga, S. and Boggess, K. A. (2020). The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. J Perinatol 40(2): 232-239.
- Beetham, K. S., Giles, C., Noetel, M., Clifton, V., Jones, J. C. and Naughton, G. (2019). The effects of vigorous intensity exercise in the third trimester of pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth 19(1): 281.
- da Silva, S. G., Hallal, P. C., Domingues, M. R., Bertoldi, A. D., Silveira, M. F. D., Bassani, D., da Silva, I. C. M., da Silva, B. G. C., Coll, C. V. N. and Evenson, K. (2017). A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act 14: 175.
- Davenport, M. H., Meah, V. L., Ruchat, S. M., Davies, G. A., Skow, R. J., Barrowman, N., Adamo, K. B., Poitras, V. J., Gray, C. E., Jaramillo Garcia, A., Sobierajski, F., Riske, L., James, M., Kathol, A. J., Nuspl, M., Marchand, A. A., Nagpal, T. S., Slater, L. G., Weeks, A., Barakat, R. and Mottola, M. F. (2018). Impact of prenatal exercise on neonatal and childhood outcomes: a systematic review and meta-analysis. Br J Sports Med 52(21): 1386-1396.
- Davenport, M. H., Ruchat, S. M., Poitras, V. J., Jaramillo Garcia, A., Gray, C. E., Barrowman, N., Skow, R. J., Meah, V. L., Riske, L., Sobierajski, F., James, M., Kathol, A. J., Nuspl, M., Marchand, A. A., Nagpal, T. S., Slater, L. G., Weeks, A., Adamo, K. B., Davies, G. A., Barakat, R. and Mottola, M. F. (2018). Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med 52(21): 1367-1375.
- Godfrey, K. M., Reynolds, R. M., Prescott, S. L., Nyirenda, M., Jaddoe, V. W., Eriksson, J. G. and Broekman, B. F. (2017). Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 5(1): 53-64.
- Goh, J. and Ladiges, W. (2015). Voluntary Wheel Running in Mice. Curr Protoc Mouse Biol 5(4): 283-290.
- Hay, W. W., Brown, L. D., Rozance, P. J., Wesolowski, S. R. and Limesand, S. W. (2016). Challenges in nourishing the intrauterine growth-restricted foetus- Lessons learned from studies in the intrauterine growth-restricted foetal sheep. Acta Paediatr 105(8): 881-889.
- Hoover, E. A. and Louis, J. M. (2019). Optimizing Health: Weight, Exercise, and Nutrition in Pregnancy and Beyond. Obstet Gynecol Clin North Am 46(3): 431-440.
- Hopkins, S. A. and Cutfield, W. S. (2011). Exercise in pregnancy: weighing up the long-term impact on the next generation. Exerc Sport Sci Rev 39(3): 120-127.
- Laker, R. C., Lillard, T. S., Okutsu, M., Zhang, M., Hoehn, K. L., Connelly, J. J. and Yan, Z. (2014). Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 63(5): 1605-1611.
- Loprinzi, P. D. and Frith, E. (2019). Protective and therapeutic effects of exercise on stress-induced memory impairment. J Physiol Sci 69(1): 1-12.
- Ming, W. K., Ding, W., Zhang, C. J. P., Zhong, L., Long, Y., Li, Z., Sun, C., Wu, Y., Chen, H., Chen, H. and Wang, Z. (2018). The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy Childbirth 18(1): 440.
- Moyer, C., Reoyo, O. R. and May, L. (2016). The Influence of Prenatal Exercise on Offspring Health: A Review. Clin Med Insights Womens Health 9: 37-42.
- Musial, B., Fernandez-Twinn, D. S., Duque-Guimaraes, D., Carr, S. K., Fowden, A. L., Ozanne, S. E. and Sferruzzi-Perri, A. N. (2019). Exercise alters the molecular pathways of insulin signaling and lipid handling in maternal tissues of obese pregnant mice. Physiol Rep 7(16): e14202.
- Nascimento, S. L., Surita, F. G. and Cecatti, J. G. (2012). Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol 24(6): 387-394.
- Pastorino, S., Bishop, T., Crozier, S. R., Granstrom, C., Kordas, K., Kupers, L. K., O'Brien, E. C., Polanska, K., Sauder, K. A. and Zafarmand, M. H., et al. (2019). Associations between maternal physical activity in early and late pregnancy and offspring birth size: remote federated individual level meta-analysis from eight cohort studies. BJOG 126(4): 459-470.
- Patki, G., Li, L., Allam, F., Solanki, N., Dao, A. T., Alkadhi, K. and Salim, S. (2014). Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav 130: 47-53.
- Peres Valgas da Silva, C., Peres Valgas da Silva, Hernandez-Saavedra, D., White, J. D. and Stanford, K. I. (2019). Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 8.
- Raipuria, M., Bahari, H. and Morris, M. J. (2015). Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS One 10(4): e0120980.
- Rogozinska, E., Marlin, N., Jackson, L., Rayanagoudar, G., Ruifrok, A. E., Dodds, J., Molyneaux, E., van Poppel, M. N., Poston, L. and Vinter, C. A., et al. (2017). Effects of antenatal diet and physical activity on maternal and fetal outcomes: individual patient data meta-analysis and health economic evaluation. Health Technol Assess 21(41): 1-158.
- Seo, J. H., (2018). Treadmill exercise alleviates stress-induced anxiety-like behaviors in rats. J Exerc Rehabil 14(5): 724-730.
- Son, J. S., Liu, X., Tian, Q., Zhao, L., Chen, Y., Hu, Y., Chae, S. A., de Avila, J. M., Zhu, M. J. and Du, M. (2019). Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J Physiol 597(13): 3333-3347.
- Son, J. S., Zhao, L., Chen, Y., Chen, K., Chae, S. A., de Avila, J. M., Wang, H., Zhu, M. J., Jiang, Z. and Du, M. (2020). Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv 6(16): eaaz0359.
- Stanford, K. I., Takahashi, H., So, K., Alves-Wagner, A. B., Prince, N. B., Lehnig, A. C., Getchell, K. M., Lee, M. Y., Hirshman, M. F. and Goodyear, L. J. (2017). Maternal Exercise Improves Glucose Tolerance in Female Offspring. Diabetes 66(8): 2124-2136.
- Stanford, K.I., et al. (2015). Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 64: 427-433.
- Uddin, G. M., Youngson, N. A., Doyle, B. M., Sinclair, D. A. and Morris, M. J. (2017). Nicotinamide mononucleotide(NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise. Sci Rep 7(1): 15063.
- Walejko, J. M., Antolic, A., Koelmel, J. P., Garrett, T. J., Edison, A. S. and Keller-Wood, M. (2019). Chronic maternal cortisol excess during late gestation leads to metabolic alterations in the newborn heart. Am J Physiol Endocrinol Metab 316(3): E546-E556.
- Wang, J., Wen, D., Liu, X. and Liu, Y. (2019). Impact of exercise on maternal gestational weight gain: An updated meta-analysis of randomized controlled trials. Medicine(Baltimore) 98(27): e16199.
- Wesolowski, S. R., Mulligan, C. M., Janssen, R. C., Baker, P. R., 2nd, Bergman, B. C., D'Alessandro, A., Nemkov, T., Maclean, K. N., Jiang, H., Dean, T. A., Takahashi, D. L., Kievit, P., McCurdy, C. E., Aagaard, K. M. and Friedman, J. E. (2018). Switching obese mothers to a healthy diet improves fetal hypoxemia, hepatic metabolites, and lipotoxicity in non-human primates. Mol Metab 18: 25-41.
- Zavorsky, G. S. and Longo, L. D. (2011). Exercise guidelines in pregnancy: new perspectives. Sports Med 41(5): 345-360.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chae, S. A., Son, J. S., Zhu, M., de Avila, J. M. and Du, M. (2020). Treadmill Running of Mouse as a Model for Studying Influence of Maternal Exercise on Offspring. Bio-protocol 10(23): e3838. DOI: 10.21769/BioProtoc.3838.
- Son, J. S., Zhao, L., Chen, Y., Chen, K., Chae, S. A., de Avila, J. M., Wang, H., Zhu, M. J., Jiang, Z. and Du, M. (2020). Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv 6(16): eaaz0359.
Category
Developmental Biology > Reproduction
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










