- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Super-resolution Imaging of the T cell Central Supramolecular Signaling Cluster Using Stimulated Emission Depletion Microscopy
Published: Vol 10, Iss 21, Nov 5, 2020 DOI: 10.21769/BioProtoc.3806 Views: 4065
Reviewed by: Chiara AmbrogioSneha RayAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Iterative Immunostaining and NEDD Denoising for Improved Signal-To-Noise Ratio in ExM-LSCM
Lucio Azzari [...] Elina Mäntylä
Sep 20, 2024 2605 Views

Measuring Piezo1 and Actin Polarity in Chemokine-Stimulated Jurkat Cells During Live-Cell Imaging
Chinky Shiu Chen Liu [...] Dipyaman Ganguly
Oct 5, 2024 1797 Views

Using HBmito Crimson to Observe Mitochondrial Cristae Through STED Microscopy
Xichuan Ge [...] Baoxiang Gao
Jan 5, 2025 1641 Views
Abstract
Supramolecular signaling assemblies are of interest for their unique signaling properties. A µm scale signaling assembly, the central supramolecular signaling cluster (cSMAC), forms at the center interface of T cells activated by antigen presenting cells (APC). The adaptor protein linker for activation of T cells (LAT) is a key cSMAC component. The cSMAC has widely been studied using total internal reflection fluorescence microscopy of CD4+ T cells activated by planar APC substitutes. Here we provide a protocol to image the cSMAC in its cellular context at the interface between a T cell and an APC. Super resolution stimulated emission depletion microscopy (STED) was utilized to determine the localization of LAT, that of its active, phosphorylated form and its entire pool. Agonist peptide-loaded APCs were incubated with TCR transgenic CD4+ T cells for 4.5 min before fixation and antibody staining. Fixed cell couples were imaged using a 100x 1.4 NA objective on a Leica SP8 AOBS confocal laser scanning microscope. LAT clustered in multiple supramolecular complexes and their number and size distributions were determined. Using this protocol, cSMAC properties in its cellular context at the interface between a T cell and an APC could be quantified.
Keywords: Immune SynapseBackground
T cell activation in response to antigen presenting cells (APCs) is controlled by engagement of the T cell receptor (TCR) in combination with co-inhibitory and co-stimulatory receptors and orchestrated by the integrated function of multiple signaling intermediates. Dynamic recruitment of signaling intermediates into microscopically detectable structures at T cell interface is an important element of signal integration. µm scale signaling assemblies were first described at the center and periphery of T cells activated by antigen presenting cells (APC) for the TCR, PKCθ and LFA-1, talin, respectively, as central and peripheral supramolecular activation clusters (cSMAC and pSMAC) (Monks et al., 1997 and 1998; Grakoui et al., 1999). µm scale of assemblies, in particular in the form of supramolecular protein complexes, provides unique biophysical and signaling properties (Li et al., 2012; Banani et al., 2016; Shin and Brangwynne, 2017). Such complexes are readily observed by fluorescence microscopy, held together by a network of multivalent protein interactions and often have distinct phase properties (Li et al., 2012; Banani et al., 2016; Shin and Brangwynne, 2017). The cSMAC has many properties of such supramolecular protein complexes: It contains various multivalent signaling intermediates (Balagopalan et al., 2015), prominently LAT (linker for activation of T cells), components of this complex including LAT and PKCθ exchange with the remainder of the cell to a moderate extent and slowly (Roybal et al., 2015), and components of this complex can be assembled into supramolecular structures in vitro (Su et al., 2016). Therefore, understanding biophysical properties of the cSMAC is of substantial importance.
The cSMAC has been imaged using confocal microscopy of fixed T cell APC couples (Monks et al., 1997 and 1998; Freiberg et al., 2002), spinning disk confocal live cell imaging of T cell APC couples (Singleton et al., 2009; Clark et al., 2019), and using total internal reflection (TIRF) imaging of T cells activated on planar APC substitutes (Yokosuka et al., 2005; Varma et al., 2006). Resolution in confocal imaging is limited by the Abbe diffraction limit. Therefore, only overall cSMAC formation can be detected but not details of cSMAC structure. The use of planar APC substitutes allows more efficient diffraction limited imaging but does not reflect the membrane topology of T cell APC couples well (Roybal et al., 2015) and, therefore, may result in altered structural details of µm scale signaling complexes (Clark et al., 2019). To overcome these experimental restrictions, we imaged single z planes of fixed T cell APC couples stained for the critical cSMAC component LAT with STED super-resolution microscopy on a microscope capable of 50 nm lateral resolution. In STED imaging, a doughnut shaped laser beam silences fluorescence around a central excitation spot and thereby diminishes the excitation volume of a confocal laser scanning microscopy system to below the conventional diffraction limit. Using automated object detection, we quantified structural properties of LAT signaling complexes.
This protocol provides access to structural features of supramolecular signaling complexes that form in T cells activated in a cellular interaction with antigen presenting cells.
Materials and Reagents
1 ml syringe plunger (such as VWR, catalog number: NSCAS7510-1 )
24-well plate (such as BD Biosciences, catalog number: 353047 )
50 ml Falcon tube
15 ml Falcon tube
1.5 ml Eppendorf tube
40 µm cell strainer
Petri dish
Coverslips (Carl ZeissTM, catalog number: 10474379 ), store at room temperature
PEP pen (ImmEdge, Vector Laboratories: H-4000 ), store at room temperature
Microscope Slide (VWR, SuperFrost Plus, catalog number: 631-0108 , store at room temperature)
5C.C7 TCR transgenic mice (Davis Lab, Stanford [Seder et al., 1992], RRID:MGI:3799371)
CH27 Cell Line (RRID:CVCL_7178)
Anti-LAT pY191 (Cell Signaling, catalog number: 3584 ), store at 4 °C
Anti-LAT (Cell Signaling, catalog number: 9166 ), store at 4 °C
Anti-rabbit IgG, Alexa Fluor 488 (Molecular Probes, catalog number: R37118 ), store at 4 °C
Anti-CD19 antibody (BD Biosciences, Biotin Rat Anti-mouse CD19, Clone 1D3, catalog number: 553784 ), store at 4 °C
Paraformaldehyde (Sigma-Aldrich, catalog number: P6148 ), store at 4 °C
Ammonium Chloride (Sigma-Aldrich, catalog number: 213330 ), store at room temperature
Triton X-100 (Sigma-Aldrich, catalog number: T8787 ), store at room temperature
PBS (Thermo Fisher, catalog number: 14200075 ), store at room temperature
Fc block, Rat Anti-Mouse CD16/CD32 (BD Bioscience, catalog number: 53141 ), store at 4 °C
ProLong Gold (Thermo Fisher, catalog number: P36930 ), store at room temperature
Moth cytochrome C (MCC) peptide, recombinant peptide (ANERADLIAYLKQATK), store at -20 °C
SDS (Sigma, catalog number: 71729 ), store at room temperature
Biotinylated BSA (Thermo Fisher, catalog number: 29130 ), store at -20 °C
T50 solution (10 mM Tris-Cl pH = 8.0, 50 mM NaCl), store at 4 °C
Neutravidin (0.2 mg/ml diluted in ddH2O, Thermo Scientific, catalog number: 31000 ), store at 4 °C
Sodium orthovanadate (Na3VO4) (20 mM working stock in ddH2O, Sigma-Aldrich, catalog number: S6508 ), store at -20 °C
Hydrogen Peroxide (H2O2) (30% solution, Sigma, catalog number: H1009 ), store at 4 °C
100% Ethanol (Sigma-Aldrich, catalog number: 459835 ), store at room temperature
RPMI with L-glutamine (Lonza, catalog number: BE12-702F )
- FBS (SLS LAB PRO, catalog number: S-001A-BR )
100 U/ml Penicillin plus 100 µg/ml streptomycin (Gibco, catalog number: 15140122 )
2-mercaptoethanol (Gibco, catalog number: 31350010 )
T cell Culture Components (see Recipes)
Complete medium
Interleukin-2 medium
Moth cytochrome C (MCC) agonist peptide for the stimulation of 5C.C7 TCR transgenic T cells
2x Pervanadate components (see Recipes)
Imaging buffer (see Recipes)
Equipment
P2, P20, P200 pipettes
Centrifuge
Incubator
Confocal laser scanning microscope (Leica, SP8 AOBS ) equipped with pulsed white light laser 470-670 nm with 2.5 mW/nm, 592 nm continuous wave STED depletion laser, Leica STED White 100x 1.4 NA objective and time gated Leica SMD HyD detectors
Software
Huygens Professional (SVI, www.svi.nl)
ImageJ plugin (Modular Image Analysis, version 0.5.22), available on GitHub as https://github.com/SJCross/MIA with .mia settings file provided as supplementary material.
Procedure
T cell and APC Culture (Ambler et al., 2017)
T cell culture: Cull a 5C.C7 T cell receptor transgenic mouse over 6-week-old using approved procedures such as those listed in Schedule One of the UK Animals (Scientific Procedures) Act 1986. All animal breeding, maintenance and handling needs to be approved by the appropriate authorities.
Dissect lymph nodes and collect them in a 15 ml Falcon tube containing 5 ml of complete medium.
Place a 40 µm cell strainer over a 50 ml Falcon tube. Pour all lymph nodes onto the cell strainer and mechanically dissociate lymph nodes by gently forcing through the filter once using a 1 ml syringe plunger. Wash the plunger and cell strainer using 5 ml of complete medium.
Centrifuge the cells for 3 min at 200 x g. Remove supernatant, resuspend the cell pellet in 1 ml of complete medium and count the cells.
Dilute cells to a density of 4 x 106 cells/ml.
Culture 1 ml of cell suspension in one well of a 24-well plate and add 0.65 µl of 5 mM MCC peptide to achieve a final MCC concentration of 3 µM. Incubate at 37 °C, 6% CO2 overnight.
Split 5C.C7 T cells with interleukin-2 medium as necessary, usually 1:1 every day. The MCC peptide is only used to prime the culture. T cell maintenance is achieved using interleukin-2.
Experiments need to be repeated with at least two independent primary T cell cultures.
APC culture: CH27 B cell lymphoma cells are used as antigen presenting cells and are maintained in complete medium by splitting 1:10 every two days.
T cell stimulation using Pervanadate
As a positive control, T cells can be stimulated with the phosphatase inhibitor Pervanadate to induce generalized and extensive tyrosine phosphorylation.
On day 5 of T cell culture, collect T cells from as many wells of the 24-well plate as needed (4 x 106 cells/well) into a 15 ml Falcon tube, centrifuge at 200 x g for 3 min. Resuspend T cell pellet in 150 µl of imaging buffer per well.
Count T cells and collect required cell number (40,000 per experimental replicate) in a 1.5 ml Eppendorf tube.
Add freshly prepared 2x pervanadate at a ratio of 1:1 to cells with complete medium in a 1.5 ml Eppendorf tube. Incubate for 5 min at 37 °C and stain (Procedure D).
Coating of STED compatible coverslips
Wash STED compatible coverslips (high performance, thickness no. 1.5, 0.170 ± 0.005 mm, Zeiss) with 10% SDS for 10 min followed by one wash with ddH2O.
Submerge the washed coverslips in 100% ethanol for 30 min in a Petri dish.
Wash the coverslips once with ddH2O and bake on a heat block at 60 °C for 30 min until dry.
When dry, draw a circular boundary (~10 mm diameter) using a PEP pen and allow it to dry.
Resuspend biotinylated BSA in T50 solution at a concentration of 1 mg/ml.
Add 50 µl of biotinylated BSA on the middle of a coverslip and incubate at 37 °C for 7 min.
Wash the coverslip once with 50 µl T50 solution.
Add 50 µl of Neutravidin solution (final concentration of 0.2 mg/ml in ddH2O) onto the coverslip for 1 min at 37 °C.
Wash once with T50 solution followed by two washes with PBS.
Dilute anti-CD19 antibody to a final concentration of 10 µg/ml in imaging buffer.
Add 50 µl of diluted anti-CD19 onto the coverslip and incubate at 37 °C for 10 min.
Wash the coverslip twice with 50 µl of imaging buffer and add a further 50 µl of imaging buffer to keep the coverslip moist.
Store the coated coverslips in a parafilm sealed dish at 4 °C until use.
ΔCRITICAL STEP Use the coated coverslips within 3 days of coating.
Cell staining
Primary 5C.C7 T cells are activated by interaction with CH27 APCs presenting the MCC agonist peptide recognized by the 5C.C7 T cell receptor. As a control, T cells are additionally activated with the phosphatase inhibitor Pervanadate.
Remove the imaging buffer that is used to keep the cover slide moist using a P200 pipette.
Prime APCs, CH27 cells (from Step A9), with 10 µM MCC peptide by incubation in complete medium for at least 4 h (Ambler et al., 2017) and resuspend to 40,000 cells/50 µl of imaging buffer. Resuspend 5C.C7 TCR transgenic T cells (from Step A7) (Ambler et al., 2017) to 40,000 cells per 5 µl of imaging buffer.
Seed 50 µl of APCs (40,000 cells) onto the coated cover slide and allow to settle for 10 min at room temperature.
Add 5 µl of T cells (40,000 cells) or 10 µl of Pervanadate-treated T cells (40,000 cells) for 4.5min at room temperature.
ΔCRITICAL STEP Gently remove the volume using a P200 pipette and add 50 µl of 4% PFA for 20 min at 4 °C.
Gently wash the cover slide once with 50 µl PBS using a P200 pipette.
Quench by adding 50 µl of 50 mM of NH4Cl for 10 min at 4 °C and wash the cover slide three times with 50 µl of PBS.
Permeabilize the cells using 50 µl of 0.02% Triton X-100 in PBS for 20 min at 4 °C.
Wash the coverslip three times with 50 µl of PBS followed by incubation with 50 µl of blocking solution (1% BSA in PBS) for 30 min at room temperature.
Remove the solution and add 50 µl of primary antibody, anti-phospho-LAT (Tyr 191) at a dilution of 1:50 or anti-LAT at a dilution of 1:100, in 1% BSA in PBS with Fc Block (Rat Anti-Mouse CD16/CD32) at the same dilution and incubate overnight at 4 °C.
Remove primary antibodies and wash the cover slide three times with 50 µl of PBS.
Incubate the cover slide with 50 µl of secondary antibody (Donkey anti-Rabbit IgG, Alexa Fluor 488) diluted 1:1,000 in 1% BSA in PBS with Fc Block at 1:500 for 1 h at room temperature.
Wash the cover slide with 50 µl of PBS for three times and remove the PBS from the coverslip.
After adding one drop of ProLong Gold mount a coverslip on the slide (VWR, SuperFrost Plus) and allow to cure for 24 h.
STED microscopy
First, image the slide with the Leica SP8X STED microscope in confocal mode using the following settings:
100x 1.4 NA HC PL APO CS2 STED white oil objective.
512 x 512 pixels.
400 Hz scan speed.
488 nm excitation selected on white light laser with corresponding NF488 filter.
Default 70% white laser power is to be adjusted with the acousto-optic tunable filter to as low as 10%.
HyD1 hybrid detector collection range 498-520 nm.
Hybrid detector gain 100%.
Hybrid detector gating 0.3-8 ns.
Line average 2.
Output image bit size 8 bit.
Whilst in confocal mode, place your sample and select a region of interest.
To image your region of interest with STED, duplicate sequence 1, i.e., the sequence of imaging steps used to choose the region of interest, and modify the duplicated sequence as follows:
Switch 592 nm STED laser ON. The 592 nm depletion laser generates a donut shaped depletion area for fluorophore excitation at 488 nm. Set it to 30-50%, i.e., 50-70% depletion, using the acousto-optic tunable filter depending on your Alexa Fluor 488 staining brightness. Excessive depletion may photodamage the sample.
Increase 488 nm laser power as needed to maintain good signal.
Select optimized frame size for STED with optimized pixel size as calculated automatically by the software using the ‘optimized pixel size’ function.
Hybrid detector collection range 498-510 nm.
Hybrid detector gain 200%.
Hybrid detector gating 1.5-8 ns. A longer start gate time before acquisition ensures efficient depletion of the majority of molecules to improve resolution.
ΔCRITICAL STEP If the data are to be deconvolved after acquisition ensure no/minimal saturation in the acquired image and adjust laser power and gain as necessary.
ΔCRITICAL STEP Once the STED sequence has been set, go to Configuration to align STED beams.
STED beams need to be aligned every 15 min for the first hour to minimize temperature drift.
Start scan and acquire STED images.
Data analysis
For deconvolution open STED .lif images with the Huygens Professional software.
Select image series to be deconvolved.
Right click on image and select microscope parameters. Check the auto read metadata is correct, e.g., embedding media was ProLong Gold (1.47 RI).
Set all parameters as verified and accepted.
Right click on image, select deconvolution wizard.
Draw three lines across the background in each image for the background intensity.
Key in the average background intensity value.
Click go and then max iterations set to 40 as the default value. Verify convergence of the deconvolution routine and adjust the number of iterations is necessary.
Once the deconvolution is complete, save the image as .TIFF file and analyze deconvolved image in Fiji (NIH) (Schindelin et al., 2012; Rueden et al., 2017).
Automated puncta analysis with Fiji (NIH). LAT puncta were detected and measured using a Wolfson Bioimaging ImageJ-Fiji plugin (Modular Image Analysis, version 0.5.22). This can be installed and used with the following steps:
Download v0.5.22 of the plugin from https://github.com/sjcross/MIA/releases/tag/v0.5.22 and place the “Modular_Image_Analysis-0.5.22.jar” file in the Fiji “plugins” folder.
To install required third-party libraries, launch Fiji and go to “Help > Update...”, then select “Manage update sites” and add the “Biomedgroup” and “IJPB-plugins” update sites. Restart Fiji when prompted.
Start MIA within Fiji by going to “Plugins > Wolfson Bioimaging > Modular image analysis”.
Click “Load” and select the “MIA object detection workflow.mia” workflow file provided as a supplement to this protocol. This contains all the instructions for MIA to perform the analysis. There are a few user-accessible settings to select files to process and to control the analysis:
“Input control > Input mode”: In “batch” all files within a selected folder will be processed, whereas in “single file” only one image file will be processed.
“Input control > Single file path": Select the batch-processing folder or single file to be analyzed.
“Input control > Series mode”: For multi-series files (e.g., Leica LIFs), all series can be processed, or a sub-set of series can be selected.
“Input control > Series number”: If processing a subset of available series the series to process can be specified as a comma-separated list of series indices.
“Threshold image > Threshold multiplier”: Adjusts the automatically detected threshold prior to application. Values <1 will lower the threshold and values >1 will increase it.
“Remove objects smaller than…”: Applies an optional size filter to detected objects. Sizes are specified in µm3 units. Disable using the power icon.
“Remove objects darker than…”: Applies an optional object intensity filter. Intensities are measured from the raw image stack. Disable using the power icon.
“Remove blobs not in a cluster”: Applies an optional filter, which removes any objects not identified as part of a cluster using DBSCAN. Disable using the power icon.
“Add cluster outlines to overlay”: Render the cluster outlines on the output overlay images (showing what was detected).
“Show outlines image”: Display the outlines image as soon as it is generated.
“Save outlines image”: Save the outlines image to the same folder as the input files, but with the suffix “_outlinesNew”.
Click “Run” to start processing. When finished, the message window at the bottom of the plugin will display “Complete!”. Results will be saved in the same folder as the input file.
Puncta were identified using the Otsu algorithm with a threshold multiplier of 3.5 followed by a watershed 3D method to separate close objects. The volume of each punctum was determined.
Small puncta detected in the APCs that don’t express LAT were used to derive a detection size threshold for LAT complexes such that all puncta smaller than the 95 percentile of the APC puncta size distribution were excluded from the analysis (Figure 1). Thus, complexes smaller than 0.04/0.06 µm3 in the LAT/pLAT data were excluded from the analysis. Repeating the analysis with a 99-percentile cut-off didn’t change the conclusions reached.
The closest distance between adjacent puncta that could be resolved was 100 nm.
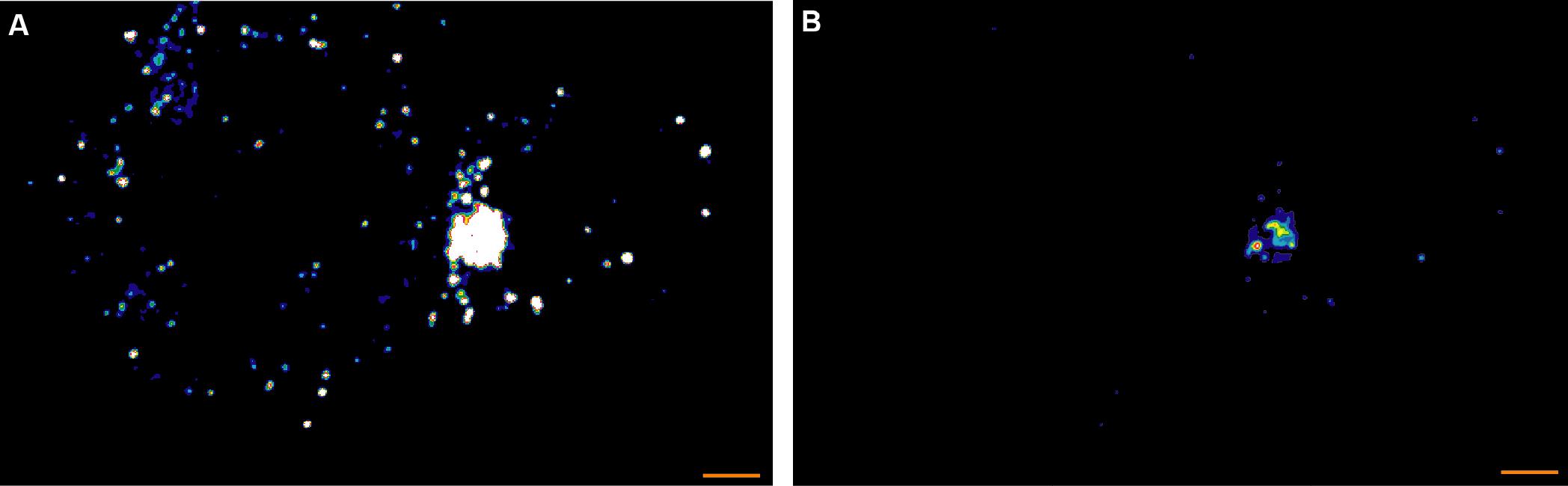
Figure 1.Two versions of one representative STED midplane image are given of 5C.C7 T cells activated by CH27 APCs (10 µM MCC agonist peptide) and stained with α-LAT pY191. Staining fluorescence intensity is given in rainbow-like false-color scale (increasing from blue to red). A. The Otsu algorithm with a threshold of 3.5 A.U. identifies large phospho-LAT clusters at the cellular interface and small, unspecific puncta in both APC and CD4+ T cell. B. The bright LAT cluster formed a ‘central’ pattern as more readily seen in B where the same data are displayed on a different intensity scale. Scale bars = 1 µm.LAT puncta could be clustered at the center of the T cell APC interface (Figures 1-2) or could be distributed across the entire interface (Figures 2-3). Such ‘central’ or ‘diffuse’ distributions correspond to the formation or lack of a formation of the cSMAC complex, respectively. Patterns were determined using established pattern classification methods (Singleton et al., 2009). Puncta numbers and sizes were quantified as a function of this pattern classification in Figure 3B of Clark et al. (2019).
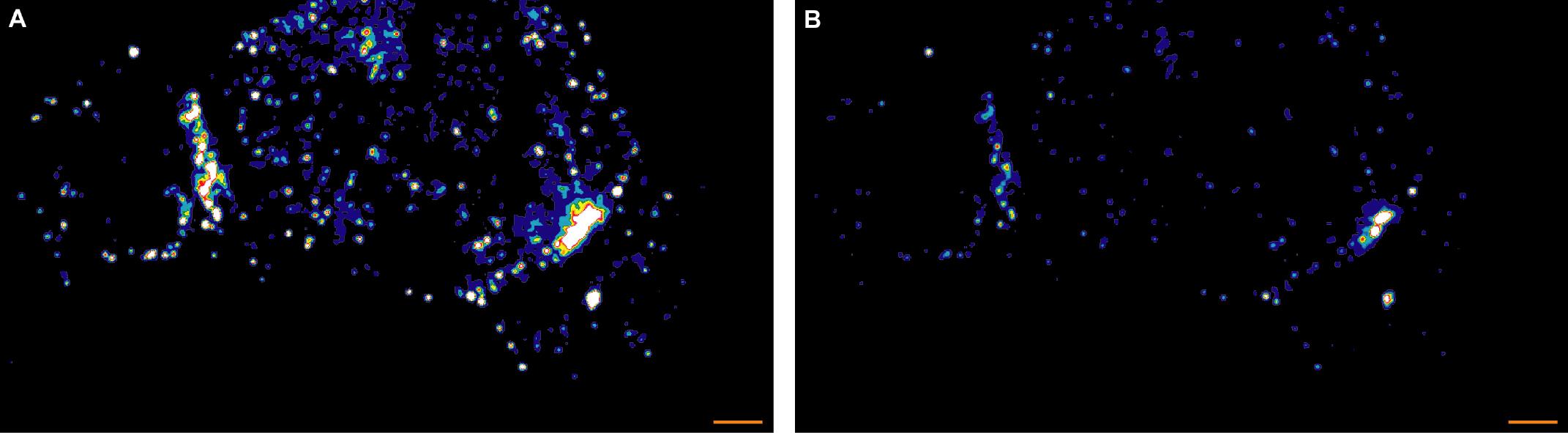
Figure 2. Two versions of a representative STED midplane image are given showing two different LAT patterns in 5C.C7 T cells activated by one CH27 APC (10 µM MCC, the middle cell) and stained with α-LAT pY191. Staining fluorescence intensity is given in rainbow-like false-color scale (increasing from blue to red). A. The 5C.C7 T cell on the left shows ‘diffuse’ patterning of phospho-LAT. The 5C.C7 T cell on the right show ‘central’ paterning. As in Figure 1 small, unspecific puncta in both APC and CD4+ T cells are visible. B. The patterns are more readily seen in B where the same data are displayed on a different intensity scale. Scale bars = 1 µm.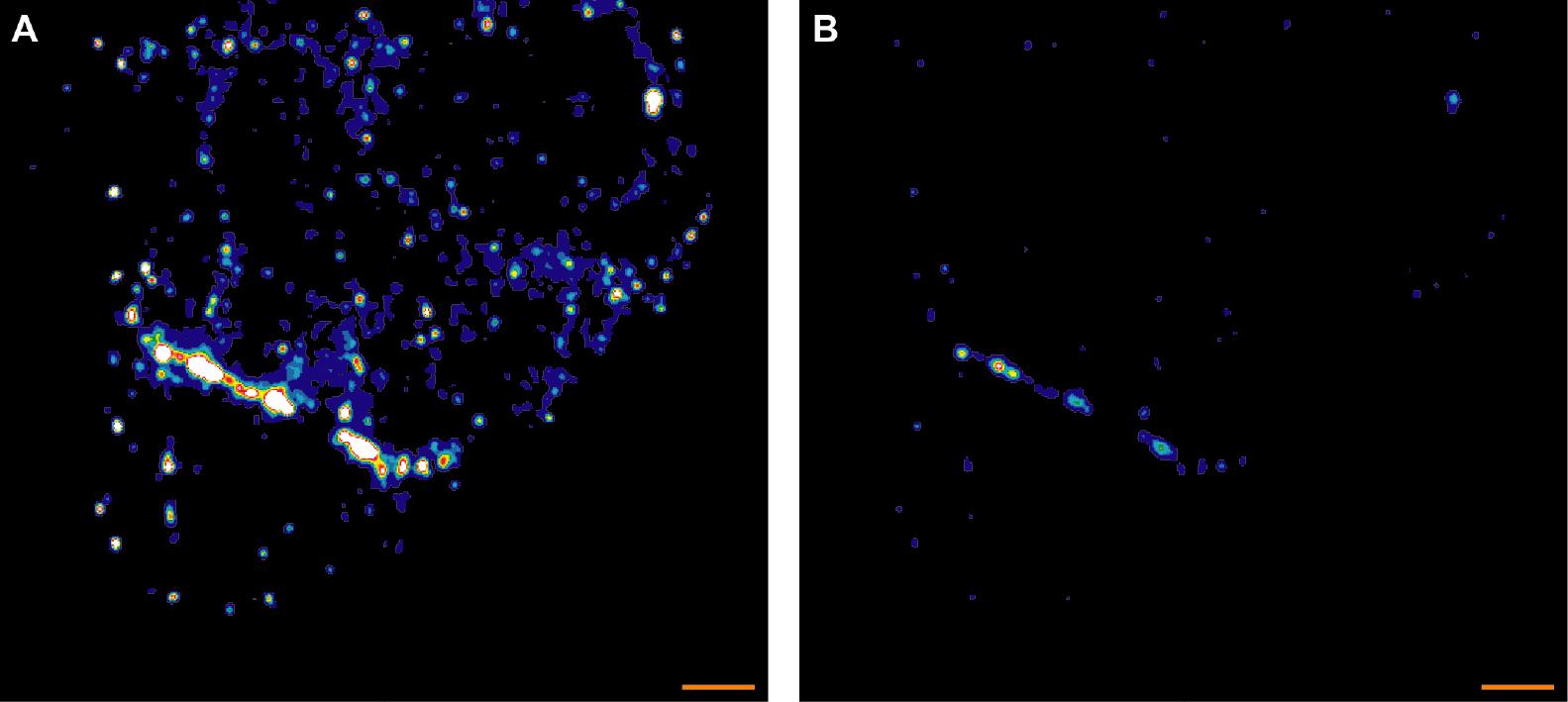
Figure 3. Two versions of a representative STED midplane image showing diffuse LAT protein patterning in a 5C.C7 T cells activated by one CH27 APC (10 µM MCC, the cell on the top). Staining fluorescence intensity is given in rainbow-like false-color scale (increasing from blue to red). A. The 5C.C7 T shows ‘diffuse’ patterning of phospho-LAT. As in Figure 1 small, unspecific puncta in both APC and CD4+ T cell are visible. B. The pattern is more readily seen in B where the same data are displayed on a different intensity scale. Scale bars = 1 µm.
Recipes
T cell Culture Components
Complete medium
RPMI with L-glutamine, 10% FBS, 100 U/ml Penicillin plus 100 µg/ml streptomycin, 50 µM 2-mercaptoethanol
Interleukin-2 medium
complete medium with 50 U/ml of rhIL-2
Moth cytochrome C (MCC) agonist peptide for the stimulation of 5C.C7 TCR transgenic T cells
5 mM solution in ddH2O
2x Pervanadate components
Dilute 30% H2O2 stock solution 1:10 in ddH2O to make up a 3% solution of H2O2
Make up 2x pervanadate stock by adding 4 µl of 3% H2O2 solution with 20 µl of 20 mM Na3VO4 in 976 µl of ddH2O in a 1.5 ml Eppendorf tube
Incubate 2x pervanadate for 10 min in the dark at RT and ready to use
Imaging buffer
PBS without calcium and magnesium, 10% FBS, 1 mM calcium chloride, 0.5 mM magnesium chloride
Acknowledgments
We acknowledge the University of Bristol FACS and Wolfson BioImaging facilities for providing equipment and technical support as well as BrisSynBio, a BBSRC/EPSRC-funded Synthetic Biology Research Centre (grant number: L01386X). The work was supported by a grant from the BBSRC (BB/P011578/1 to C.W.) and the EPSRC and BBSRC Centre for Doctoral Training in Synthetic Biology (EP/L016494/1 to H. T.). The procedure described in detail in this protocol paper was derived from Clark et al. (2019).
Competing interests
The authors declare no competing interests.
Ethics
All animals were maintained in pathogen-free animal facilities at the University of Bristol under the University mouse breeding Home Office License P10DC2972.
References
- Ambler, R., Ruan, X., Murphy, R. F. and Wulfing, C. (2017). Systems imaging of the immune synapse. Methods Mol Biol 1584409-421.
- Balagopalan, L., Kortum, R. L., Coussens, N. P., Barr, V. A. and Samelson, L. E. (2015). The linker for activation of T cells (LAT) signaling hub: from signaling complexes to microclusters. J Biol Chem 290(44): 26422-26429.
- Banani, S. F., Rice, A. M., Peeples, W. B., Lin, Y., Jain, S., Parker, R. and Rosen, M. K. (2016). Compositional control of phase-separated cellular bodies. Cell 166(3): 651-663.
- Clark, D. J., McMillan, L. E., Tan, S. L., Bellomo, G., Massoue, C., Thompson, H., Mykhaylechko, L., Alibhai, D., Ruan, X., Singleton, K. L., Du, M., Hedges, A., Schwartzberg, P. L., Verkade, P., Murphy, R. F. and Wulfing, C. (2019). Transient protein accumulation at the center of the T cell antigen-presenting cell interface drives efficient IL-2 secretion. Elife 8: e45789.
- Freiberg, B. A., Kupfer, H., Maslanik, W., Delli, J., Kappler, J., Zaller, D. M. and Kupfer, A. (2002). Staging and resetting T cell activation in SMACs. Nat Immunol 3(10): 911-917.
- Grakoui, A., Bromley, S. K., Sumen, C., Davis, M. M., Shaw, A. S., Allen, P. M. and Dustin, M. L. (1999). The immunological synapse: A molecular machinery controlling T cell activation. Science 285221-226.
- Li, P., Banjade, S., Cheng, H. C., Kim, S., Chen, B., Guo, L., Llaguno, M., Hollingsworth, J. V., King, D. S., Banani, S. F., Russo, P. S., Jiang, Q. X., Nixon, B. T. and Rosen, M. K. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483(7389): 336-340.
- Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. and Kupfer, A. (1998). Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395(6697): 82-86.
- Monks, C. R., Kupfer, H., Tamir, I., Barlow, A. and Kupfer, A. (1997). Selective modulation of protein kinase C-theta during T-cell activation. Nature 385(6611): 83-86.
- Roybal, K. T., Mace, E. M., Mantell, J. M., Verkade, P., Orange, J. S. and Wulfing, C. (2015). Early signaling in primary T cells activated by antigen presenting cells is associated with a deep and transient lamellal actin network. PLoS One 10(8): e0133299.
- Rueden, C. T., Schindelin, J., Hiner, M. C., DeZonia, B. E., Walter, A. E., Arena, E. T. and Eliceiri, K. W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18(1): 529.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J. Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P. and Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7): 676-682.
- Seder, R. A., Paul, W. E., Davis, M. M. and Fazekas de St. Groth, B. (1992). The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med 176(4): 1091-1098.
- Shin, Y. and Brangwynne, C. P. (2017). Liquid phase condensation in cell physiology and disease. Science 357(6357).
- Singleton, K. L., Roybal, K. T., Sun, Y., Fu, G., Gascoigne, N. R., van Oers, N. S. and Wülfing, C. (2009). Spatiotemporal patterning during T cell activation is highly diverse. Sci Signal 2(65): ra15.
- Su, X., Ditlev, J. A., Hui, E., Xing, W., Banjade, S., Okrut, J., King, D. S., Taunton, J., Rosen, M. K. and Vale, R. D. (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352(6285): 595-599.
- Varma, R., Campi, G., Yokosuka, T., Saito, T. and Dustin, M. L. (2006). T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 25(1): 117-127.
- Yokosuka, T., Sakata-Sogawa, K., Kobayashi, W., Hiroshima, M., Hashimoto-Tane, A., Tokunaga, M., Dustin, M. L. and Saito, T. (2005). Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP. Nat Immunol 6(12): 1253-1262.
Article Information
Copyright
Tan et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tan, S. L., Alibhai, D., Cross, S. J., Thompson, H. and Wülfing, C. (2020). Super-resolution Imaging of the T cell Central Supramolecular Signaling Cluster Using Stimulated Emission Depletion Microscopy. Bio-protocol 10(21): e3806. DOI: 10.21769/BioProtoc.3806.
- Clark, D. J., McMillan, L. E., Tan, S. L., Bellomo, G., Massoue, C., Thompson, H., Mykhaylechko, L., Alibhai, D., Ruan, X., Singleton, K. L., Du, M., Hedges, A., Schwartzberg, P. L., Verkade, P., Murphy, R. F. and Wulfing, C. (2019). Transient protein accumulation at the center of the T cell antigen-presenting cell interface drives efficient IL-2 secretion. Elife 8: e45789.
Category
Immunology > Immune cell imaging > Confocal microscopy
Cell Biology > Cell imaging > Super resolution imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









