- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Protocol for Flavonols, Kaempferol and Quercetin, Staining in Plant Root Tips
Published: Vol 10, Iss 19, Oct 5, 2020 DOI: 10.21769/BioProtoc.3781 Views: 6039
Reviewed by: María Victoria MartinShin‐nosuke HashidaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Vegetative Propagation of Cannabis sativa and Resin Obtained From its Female Inflorescences
Sebastián D´Ippolito [...] Silvana L. Colman
Feb 20, 2025 1735 Views

A New Approach to Detect and Semi-quantify All Molecular Species and Classes of Anionic Phospholipids Simultaneously in Plant Samples
Manon Genva [...] Laetitia Fouillen
Apr 20, 2025 1718 Views

ClearDepth Method for Evaluations of Root Depth in Soil-Filled Pots
Michel Ruiz Rosquete [...] Wolfgang Busch
Aug 20, 2025 2117 Views
Abstract
Flavonols are a subclass of flavonoids of the group of plant secondary metabolites. In planta, flavonols play various functions such as antioxidant and natural regulator of auxin polar transport. Many lines of evidence have shown that flavonols also contribute to human health in anti-oxidation, anti-inflammation, and even prevention some types of cancer. Several methods have been utilized to measure flavonols such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and diphenylboric acid-2-aminoethyl ester (DPBA) staining. While HPLC or LC-MS can quantitatively determine the level of flavonols, DPBA staining can provide an in-situ view of flavonols accumulation in the plants. In this protocol, a detailed procedure for staining the flavonols in Arabidopsis root tips is described. Five-day-old Arabidopsis seedlings are soaked in a solution containing DPBA and latterly the flavonols (kaempferol and quercetin) can be observed under a confocal microscope.
Keywords: DPBA stainingBackground
In Arabidopsis, flavonols are biosynthesized from a condensation reaction between one molecule of p-coumaroyl-CoA and three molecules of malonyl-CoA. Lewis et al. (2011) found that quercetin, but not kaempferol, is an inhibitor of root basipetal auxin transport. In addition, flavonols also function as an efficient antioxidant for plants and humans as well. A prior study investigated that DPBA can fluoresce when it interacts with flavonols (Sheahan and Rechnitz, 1992). Based on this, DPBA has been widely applied to detect the flavonols accumulation in the plants (Nguyen et al., 2013, 2015 and 2016; Vu et al., 2015). Here, a detailed protocol for DPBA-based detection of flavonols is described. Apart from Arabidopsis, this protocol can be also used for other plants such as Brassica napus L. (Vu et al., 2015). Since other plant species may have a thicker and larger tissue than Arabidopsis, a vacuum can be applied to facilitate the penetration of DPBA into the plant tissues.
Materials and Reagents
- Square dishes for tissue culture [External dimension (mm): 126.40 x 126.40 x 20.00] (SPL Life Sciences, catalog number: 10125)
- 1.5 ml micro-tubes (Eppendorf, catalog number: 0030121589)
- Microscope slides (dimensions: 76 x 26 mm; thickness: 1 mm) (Marienfeld, catalog number: 1000200)
- Transparent slides coverslips (dimensions: 60 x 24 mm; thickness: 0.170 mm ± 0.005 mm) (Marienfeld, catalog number: 0107242)
- Arabidopsis thaliana
- Diphenylboric acid-2-aminoethyl ester (DPBA) (Sigma-Aldrich, catalog number: D9754 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Murashige & Skoog medium including B5 vitamins (Duchefa Biochemie, catalog number: M0231 )
- Phyto Agar (Duchefa Biochemie, catalog number: P1003 )
- Sucrose (Duchefa Biochemie, catalog number: S0809 )
- DPBA staining solution (see Recipes)
Equipment
- Tweezers
- A plant growth chamber
- Micro-tubes rotator
- Pipette
- Confocal microscopy system (Leica, model: SP8 )
Procedure
- Plant growth and DPBA staining procedure
- Here, Arabidopsis thaliana is used. However, this protocol can be applied for other plants as well. Grow plants on MS medium supplemented with 2% sucrose and 1.2% phyto-agar for 5 days (Figure 1). The growth chamber conditions were 22 ± 1 °C, long-day (16 h light/8 h dark), and light intensity 100 μmol m−2 s−1.
Note: After seeding, remember to place the medium plates vertically in the growth chamber. Thereby, the roots grow on the surface of the medium and intact root tip samples can be obtained.

Figure 1. Five-day-old Arabidopsis seedlings. Scale bar = 5 mm. - Add 1 ml of DPBA staining solution (Recipe 1) to each micro-tube.
- Use a clean tweezers to carefully transfer the 5-day-old Arabidopsis seedlings to the micro-tube containing DPBA staining solution (5 seedlings/micro-tube).
- Place the micro-tube in the rotator and rotate for 5 min at room temperature.
- Stop the rotator, transfer the micro-tube to a rack and remove the DPBA staining solution.
Note: At this step, carefully use a pipette to remove the DPBA staining solution and try to not damage the plants, especially the root tips. - For washing, add 1.5 ml of distilled water to each micro-tube. Place the micro-tube in the rotator and rotate for 2 min at room temperature.
- Stop the rotator, transfer the micro-tube to a rack and remove water.
- Repeat Steps A6 and A7 two more times.
Note: After washing, can keep the plants in water and try to detect immediately. Do not leave the samples staying in water for longer than 30 min. - Transfer the seedlings to a microscope slide, cover it and detect the flavonols accumulation in the root tips by a confocal microscope.
- Here, Arabidopsis thaliana is used. However, this protocol can be applied for other plants as well. Grow plants on MS medium supplemented with 2% sucrose and 1.2% phyto-agar for 5 days (Figure 1). The growth chamber conditions were 22 ± 1 °C, long-day (16 h light/8 h dark), and light intensity 100 μmol m−2 s−1.
- Confocal microscopy
- For DPBA-kaempferol, apply the emission spectrum (475-500 nm).
- For DPBA-quercetin, apply the emission spectrum (585-619 nm).
- Remember to take an additional bright field picture for control.
Data analysis
Arrange the data as following order: (1) Bright field; (2) Kaempferol; and (3) Quercetin. Please check some previous publications for details (Nguyen et al., 2013, 2015 and 2016; Vu et al., 2015).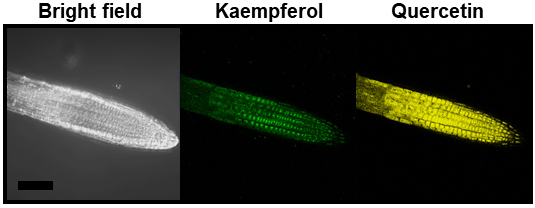
Figure 2. Accumulation of flavonols (kaempferol and quercetin) in the root tips of 5-day-old Arabidopsis seedlings (wild-type). Scale bar = 100 μm.
Recipes
- DPBA staining solution
Notes:Reagent Amount for 100 ml DPBA 0.25 g Triton X-100 20 μl Milli-Q water up to 100 ml - Some crystals of DPBA can be retained and seen after mixing.
- This staining solution can be stored at -20 °C for further uses.
Acknowledgments
I would like to appreciate Dr. Cuong Thach Nguyen (Nguyen Tat Thanh University, Vietnam) and Dr. Minh Tan Nguyen (UCLA School of Dentistry, US) for their critical reading this protocol. This protocol was derived from previous publications (Nguyen et al., 2013, 2015 and 2016). The authors declare that they have no conflict of interest.
References
- Lewis, D. R., Ramirez, M. V., Miller, N. D., Vallabhaneni, P., Ray, W. K., Helm, R. F., Winkel, B. S. and Muday, G. K. (2011). Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol 156(1): 144-164.
- Nguyen, H. N., Kim, J. H., Hyun, W. Y., Nguyen, N. T., Hong, S. W. and Lee, H. (2013). TTG1-mediated flavonols biosynthesis alleviates root growth inhibition in response to ABA. Plant Cell Rep 32(4): 503-514.
- Nguyen, N. H., Jeong, C. Y., Kang, G. H., Yoo, S. D., Hong, S. W. and Lee, H. (2015). MYBD employed by HY5 increases anthocyanin accumulation via repression of MYBL2 in Arabidopsis. Plant J 84(6): 1192-1205.
- Nguyen, N. H., Kim, J. H., Kwon, J., Jeong, C. Y., Lee, W., Lee, D., Hong, S. W. and Lee, H. (2016). Characterization of Arabidopsis thaliana FLAVONOL SYNTHASE 1 (FLS1) -overexpression plants in response to abiotic stress. Plant Physiol Biochem 103: 133-142.
- Sheahan, J. J. and Rechnitz, G. A. (1992). Flavonoid-specific staining of Arabidopsis thaliana. Biotechniques 13(6): 880-883.
- Vu, T. T., Jeong, C. Y., Nguyen, H. N., Lee, D., Lee, S. A., Kim, J. H., Hong, S. W. and Lee, H. (2015). Characterization of Brassica napus Flavonol Synthase Involved in Flavonol Biosynthesis in Brassica napus L. J Agric Food Chem 63(35): 7819-7829.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Nguyen, N. H. (2020). A Protocol for Flavonols, Kaempferol and Quercetin, Staining in Plant Root Tips. Bio-protocol 10(19): e3781. DOI: 10.21769/BioProtoc.3781.
Category
Plant Science > Plant biochemistry > Metabolite
Plant Science > Plant physiology > Phenotyping
Biochemistry > Other compound > Flavonoid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









