- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fluorescence Measurement and Calibration of Intracellular pH in Starfish Oocytes
Published: Vol 10, Iss 19, Oct 5, 2020 DOI: 10.21769/BioProtoc.3778 Views: 4519
Reviewed by: Imre GáspárAbhijit KaleAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Rapid and solvent-free, 2-hydroxyethyl methacrylate (HEMA)-acrylamide (AAm) copolymer-based optical clearing of tissue for fluorescent imaging
Yanran Wang [...] Kefeng Wu
Nov 20, 2025 1509 Views

Characterizing Tissue Oxygen Tension During Neurogenesis in Human Cerebral Organoids
Yuan-Hsuan Liu and Hsiao-Mei Wu
Nov 20, 2025 1704 Views

Monitoring of Sperm-Independent Calcium Oscillations in Immature Oocytes of Mice
Sae Horiike [...] Hidehiko Ogawa
Feb 5, 2026 65 Views
Abstract
Oocyte maturation is a process wherein an oocyte arrested at prophase of meiosis I resumes meiosis to become a fertilizable egg. In starfish ovaries, a hormone released from follicle cells activates the oocytes, resulting in an increase in their intracellular pH (pHi), which is required for spindle assembly. Herein, we describe a protocol for pHi measurement in living oocytes microinjected with the pH-sensitive dye BCECF. For in vivo BCECF calibration, we treated oocytes with artificial seawater containing CH3COONH4 to clamp pHi, injected pH-standard solutions, and converted the BCECF fluorescence intensity ratios to pHi values. Of note, if the actual pHi is higher or lower than the known pH of injected standard solutions, the BCECF fluorescence intensity ratio will decrease or increase, respectively. On the other hand, the pH of the injected solution displaying no change in fluorescence intensity should be considered the actual pHi. These methods for pHi calibration and clamping are simple and reproducible.
Keywords: Intracellular pHBackground
Intracellular pH (pHi) measurement is an extremely useful method for the study of cell biology since pHi plays important roles in a variety of cell processes, such as gametes’ activation (Johnson and Epel, 1976; Shen and Steinhardt, 1978; Tilney et al., 1978), cell division (Schuldiner and Rozengurt, 1982; Moolenaar et al., 1983; Anand and Prasad, 1989; Karagiannis and Young, 2001), and cancer cell survival (Grillo-Hill et al., 2015).
An oocyte at prophase, during meiosis I, is quiescent until hormonal stimulation resume meiosis. Studies on starfish oocytes have reported that the hormone 1-methyladenine (1-MA) binds to an unidentified receptor on the plasma membrane of oocytes (Tadenuma et al., 1992), dissociating the heterotrimeric GTP-binding protein (G) α-subunit (Gα) from the βγ subunit (Gβγ), which activates phosphatidylinositol-3 kinase (PI3K) (Chiba et al., 1993; Sadler and Ruderman, 1998). Thereafter, serum- and glucocorticoid-regulated kinase (SGK) is phosphorylated and activated by the target of rapamycin complex2 (TORC2) and 3-phosphoinositide-dependent protein kinase 1 (PDK1) (Hiraoka et al., 2019; Hosoda et al., 2019). Starfish SGK is required for Na+/H+ exchanger (NHE) dependent pHi increase from ~6.7 to ~6.9 in the ovarian oocytes (Harada et al., 2010; Moriwaki et al., 2013; Hosoda et al., 2019). Simultaneously, SGK phosphorylates Cdc25 and Myt1, inducing the de-phosphorylation and activation of cyclin B–Cdk1, causing germinal vesicle breakdown (GVBD) (Hiraoka et al., 2019). Importantly, both pHi increase and GVBD are required for spindle assembly of ovarian oocytes at metaphase I (Harada et al., 2003; Hosoda et al., 2019), followed by MI arrest at pHi 6.9 until spawning (Moriwaki et al., 2013).
Fluorescence indicators, including 2',7'-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF) have been used to measure pHi (Rink et al., 1982; Bassil et al., 2013; Behbahan et al., 2014; Mortera et al., 2015). BCECF is a pH-sensitive and ratiometric dye requiring dual-excitation. To calibrate pHi in vivo, BCECF-loaded cells are treated with buffer containing the K+/H+ exchanger nigericin and K+ at high concentration since pHi is expected to become equal to the extracellular pH, adjusted to known values in the presence of nigericin, if extracellular K+ is equal to intracellular K+ (Thomas et al., 1979; Rink et al., 1982). However, it is difficult to use this method when the intracellular K+ concentration is unknown. In another study, in vitro BCECF calibration without cells was performed to estimate pHi; however, the pKa of intracellular BCECF is sometimes greater than that of the in vitro solution (Boyarsky et al., 1996). Permeabilization of the cell membrane with digitonin or Triton-X-100 is another method used for pHi calibration (Rink et al., 1982); however, the fluorescence ratio is often unstable owing to cell lysis (Harada et al., 2003; Moriwaki et al., 2013).
Weak bases and acids can directly affect pHi since all membranes are permeable to uncharged molecules (Roos and Boron, 1981). For example, NH4Cl in seawater forms NH4+ and NH3, and the uncharged NH3 penetrates the cell membrane and binds to intracellular H+, thus increasing the pHi. In contrast, CH3COONa dissolved in seawater forms CH3COO- and CH3COOH; thereafter, CH3COOH penetrates the cell membrane, releasing H+ and decreasing pHi (Hamaguchi et al., 1997). Similarly, CH3COONH4 dissolved in seawater forms NH4+, NH3, CH3COO-, and CH3COOH. NH3 and CH3COOH easily penetrate the cell membrane, and bind to or release H+, respectively. Of note, in seawater at a higher pH, the NH3 concentration is greater than that of CH3COOH, resulting in a higher NH3 concentration and an increase in pHi by forming NH4+. In contrast, at a lower pH, the seawater-derived intracellular CH3COOH concentration is higher than that of NH3, thus decreasing the pHi (Figure 1). Moreover, the increase in NHE-dependent pHi is inhibited in sodium-free artificial seawater. In fact, using sodium-free artificial seawater (ASW) containing CH3COONH4 (modified ASW) at various pH values, we were previously able to clamp the pHi of starfish oocytes at desired pH values (Moriwaki et al., 2013; Hosoda et al., 2019).
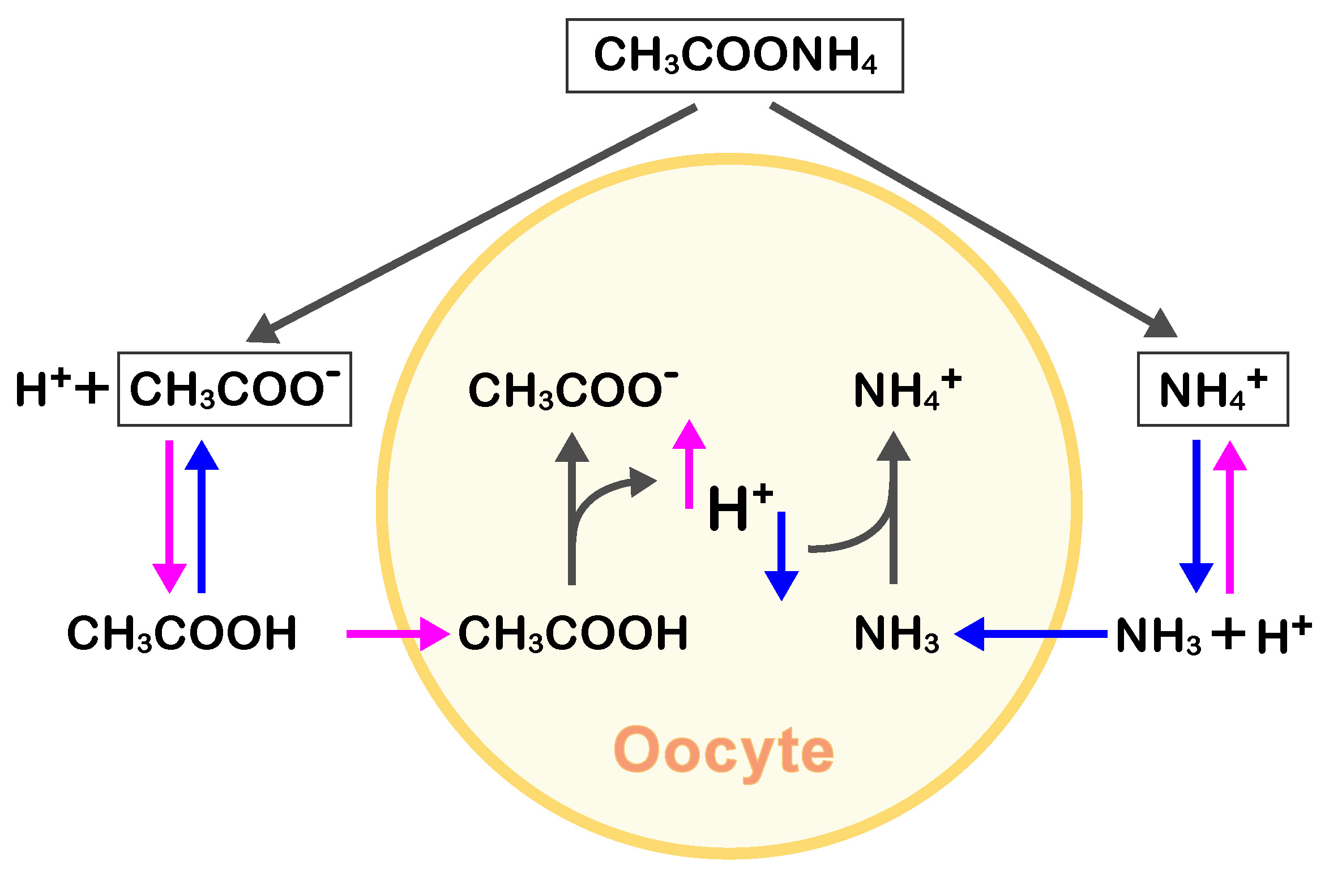
Figure 1. Diagram showing the CH3COONH4-based equilibrium in seawater and its effect on intracellular pH. In seawater with a higher pH, the NH3 concentration is greater than that of CH3COOH (blue arrows) due to equilibrium shift, resulting in a higher intracellular NH3 concentration in oocytes, permeable to the uncharged form of NH3. Then, intracellular NH3 in oocytes increases pHi via NH4+ formation. Conversely, at a lower seawater pH, the intracellular uncharged CH3COOH concentration is higher than that of NH3, thus decreasing the pHi (magenta arrows).
Furthermore, we could estimate the actual pHi of oocytes in modified ASW via the injection of standard solutions. When the actual (or real) pHi is higher than the known pH of the injected standard solution, the BCECF fluorescence intensity ratio decreases since the pHi is also decreased upon injection of standard solutions at lower pH values. On the other hand, when the actual pHi is lower than the known pH of the injected standard solutions, the abovementioned ratio increases. Thus, the actual pHi should be between these two pH values. Indeed, based on this premise, we were able to select the injection solutions at the actual pHi, resulting in no change in the intensity ratio upon their administration (Moriwaki et al., 2013; Hosoda et al., 2019).
Here, to calibrate the pHi of oocytes stimulated with 1-MA in normal SW, we treated immature oocytes with modified ASW, clamping pHi at higher and lower values, and thus yielding high and low BCECF fluorescence intensity ratios. Thereafter, we injected these oocytes with the standard solutions to estimate the actual pHi values. Using these references, we conducted a two-point calibration, forming a straight line crossing the two points. Although a calibration using ≥ 3 points is achievable, we confirmed that the two-points’ calibration graph was linear–as per the calibration data obtained using more than three points (Moriwaki et al., 2013; Hosoda et al., 2019). Thus, we could convert the fluorescence ratios of maturing oocytes in normal seawater to pHi, using this standard linear calibration graph/function-based method (Moriwaki et al., 2013; Hosoda et al., 2019).
Materials and Reagents
- Glass capillary for a glass micropipette, Microcap, 50 μl (Drummond Scientific Company, catalog number: 1-000-0500)
A micropipette was made using the puller PC-100 system, as per the manufacturer’s instructions (Narishige, https://products.narishige-group.com/group1/PC-100/pipette/english.html). The properties (length + outer/inner diameter) of the ideal capillaries are shown in Figure 2A. To control precisely the flow volume out of the glass micropipette, a constriction was made using a handmade loop of platinum wire. Briefly, the micropipette was passed through the loop of the platinum wire. Then, the micropipette (3-5 mm from the end) was heated until a constriction of 3.5-5.0 µm in diameter was formed via the application of an electric current to the loop (see also Hiramoto, 1974). - Cover glasses (Matsunami, 18 mm x 18 mm, thickness 0.12-0.17 mm)
- Silicone oil (Shin-Etsu Chemical Co.Ltd. catalog number: KF-96-100CS ) (Figure 2C)
- A dextran (10-kD) BCECF conjugate (Fisher Scientific, InvitrogenTM, catalog number: D1878 ) (Figure 2B)
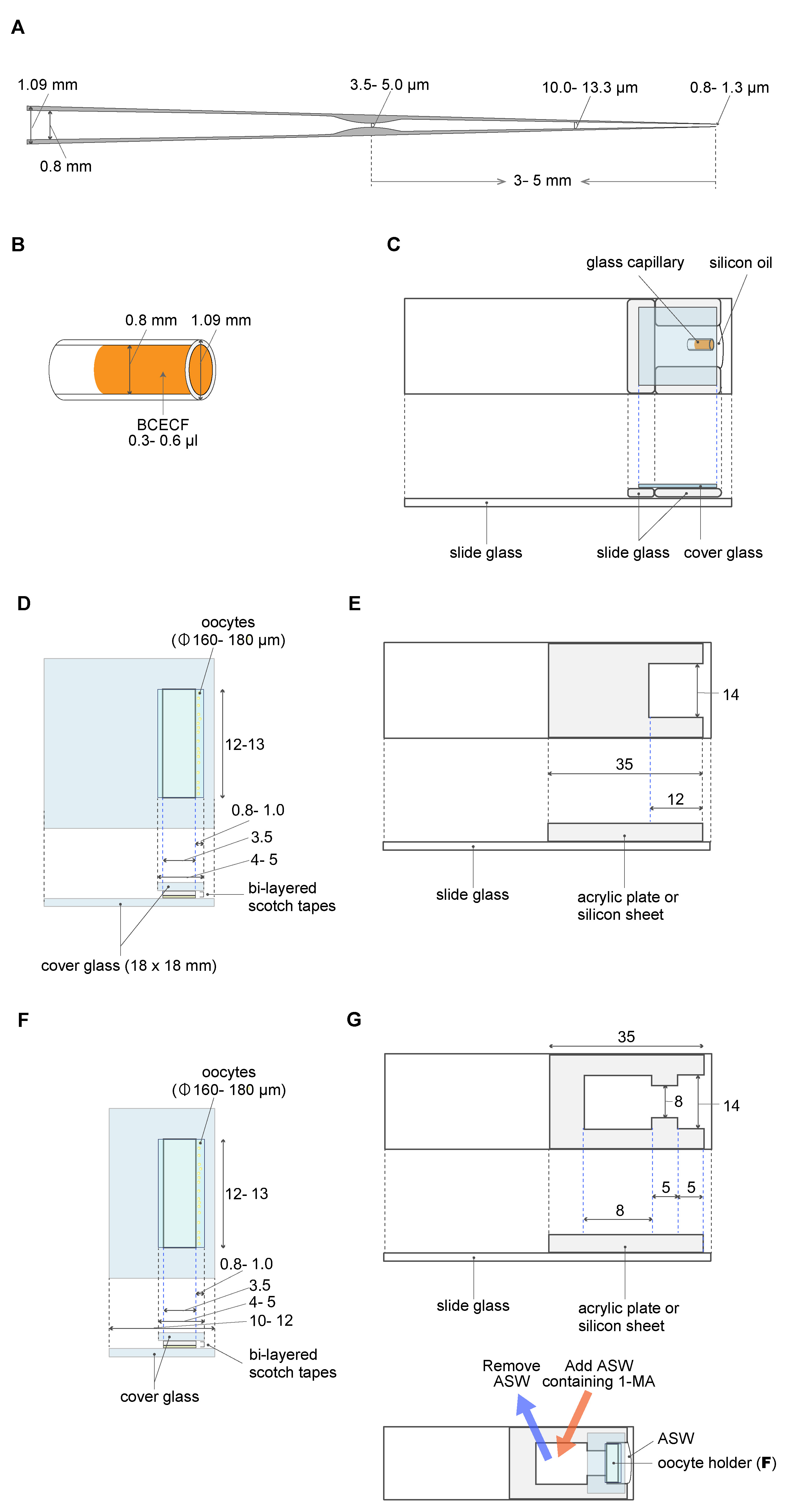
Figure 2. Materials and equipment used for oocyte manipulation. A. The constriction made in a glass micropipette acts as a brake. B. BCECF solution in a glass capillary. C. BCECF solution in a glass capillary is set in the capillary holding chamber filled with silicone oil. The glass micropipette (A) filled with silicone oil is inserted into the BCECF solution; 20 pL BCECF is aspirated into the micropipette. D. The oocyte holder. E. The manipulation chamber. F. The oocyte holder for 1-MA treatment. G. The manipulation chamber for 1-MA treatment. The chamber has an opening that allows the removal of an ASW volume (25%) and its replacement by the same volume of ASW containing 1-MA. D-G. All measurements are represented in mm unless otherwise indicated. - 1-methyladenine (KANTO CHEMICAL CO. INC., catalog number: 20131-1A )
- Potassium aspartate (Tokyo Chemical Industry Co., Ltd., catalog number: A0922 )
- HEPES, 2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (DOJINDO, catalog number: 340-01371 )
- Pipes, Piperazine-1,4-bis (2-ethanesulfonic acid) (DOJINDO, catalog number: 347-02224 )
- KOH (Wako Pure Chemical Industries, Ltd., catalog number: 168-21815)
- NP-40 (Nonidet P-40. Nacalai Tesque, catalog number: 252-23 )
- NaCl (FUJIFILM Wako Pure Chemical Corporation, catalog number: 191-01665 )
- KCl (KANTO CHEMICAL CO. INC., catalog number: 32326-00 )
- MgCl2•7H2O (Wako Pure Chemical Industries, Ltd., catalog number: 135-00165 )
- MgSO4•7H2O (Wako Pure Chemical Industries, Ltd., catalog number: 131-00405 )
- H3BO3 (Showa Chemical Co. Ltd., catalog number: 0214-6250 )
- CH3COONH4 (Wako Pure Chemical Industries, Ltd., catalog number: 019-02835 )
- CaCl2•2H2O (Wako Pure Chemical Industries, Ltd., catalog number: 031-00435 )
- Calcium-free ASW (see Recipes)
- ASW (see Recipes)
- Modified ASW pH 6.8 (see Recipes)
- Standard pH solutions at pH 6.40-6.75 or 6.80-7.40 (see Recipes)
- BCECF solution (see Recipes
Equipment
- CMOS camera (Hamamatsu Photonics K.K., ORCA-Flash2.8, model: C11440-10C )
- ECLIPSE Ti-U fluorescent microscope (Nikon Instech)
- 4× 0.20 NA CFI super Fluor lens (Nikon Instech)
- Navi h pH meter (HORIBA, pH METER, F-52)
- Screw-controlled microinjector (Narishige, model: IM-9B )
Software
- HCImage U11158-02, 03 (Hamamatsu Photonics K.K., https://hcimage.com/)
Procedure
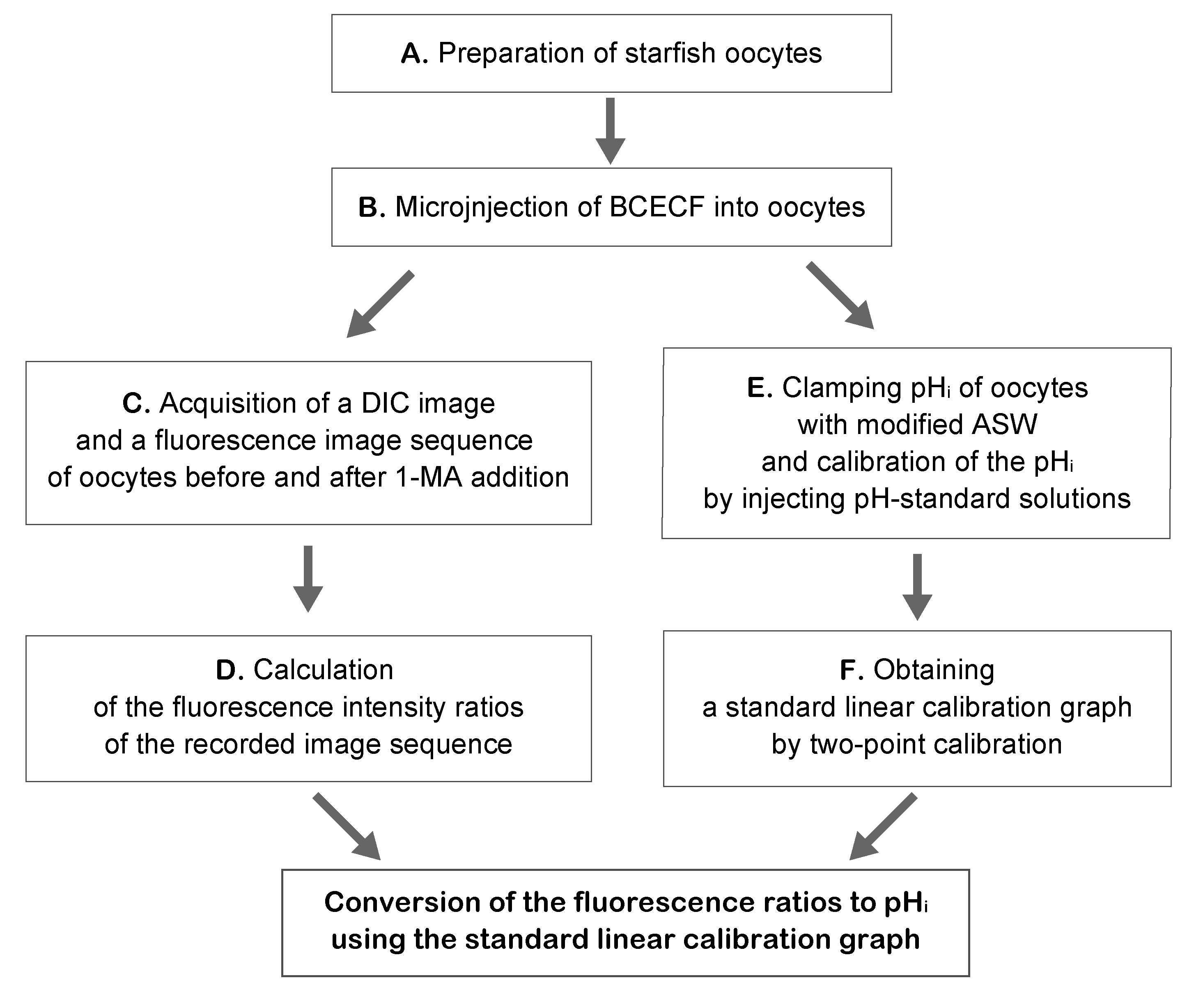
Figure 3. Flowchart representing the procedure overview (Procedures A to F)
- Oocyte preparation (Video 1)
Video 1. Oocyte Preparation. This video was made at Ochanomizu Univ. according to guidelines from the Ochanomizu Univ. on Animal Care and approved by the Animal Research Ethics Board of Ochanomizu University.
Maintain starfish (Asterina pectinifera) in laboratory aquariums supplied with circulating seawater at 14 °C (Murabe et al., 2020).- Isolate oocytes or ovaries from female animals and treat them with calcium-free artificial seawater to eliminate follicle cells.
- Maintain follicle-free oocytes in artificial seawater (ASW) until use.
- Microinjection of BCECF into oocytes (Video 2)
Video 2. Microinjection technique- Generate a glass micropipette having a constriction of a few micrometers in diameter to control the flow speed out of the micropipette (Hiramoto, 1974; see also Materials and Reagents 1 and Figure 2A).
- Fill the constricted micropipette with silicon oil and connect it to the screw-controlled microinjector ( IM-9B Narishige).
- Microscopically aspirate 20 pl BCECF solution into the micropipette (Figures 2A-2C).
- Microinject the BCECF solution into a starfish oocyte sandwiched between the two coverslips of an oocyte holder (Chiba et al., 1992) (Figures 2D and 2E).
- Incubate oocytes injected with BCECF in ASW for 1 h at 23 °C before measuring the BCECF fluorescence intensity or the pHi.
Note: Incubation for 1 h is required for diffusion of injected BCECF.
- Image acquisition (sequence-imaging) of the oocytes injected with BCECF before and after 1-MA stimulation (Video 3)
Video 3. pHi measurement using BCFCF- To evaluate the camera “noise”, acquire a dark image (no light is delivered to the CMOS camera controlled by the HCImage acquisition system, which is connected to a fluorescence microscope equipped with differential interference contrast (DIC) optics including a 4× 0.20 NA CFI super Fluor lens).
- Set oocytes pre-injected with BCECF (as shown in Procedure B) in a manipulation chamber for 1-MA treatment at 23 °C (Figures 2F and 2G).
- Acquire a DIC image of 8-15 oocytes (Figure 4A).
- Acquire a fluorescence image of oocytes to configure the region of interest (ROI) (Figure 4B): surround the cytoplasm excluding undiffused BCECF and the GV area in the oocyte image to define the ROI, using the HCImage Analysis software. Most of the injected BCECF is diffusely distributed throughout the cytoplasm 1 h after injection; however, a small, brighter fraction, is usually retained at the site of injection near an oil drop. Importantly, this brighter region should be excluded from the ROIs (Figures 4C and 4D).
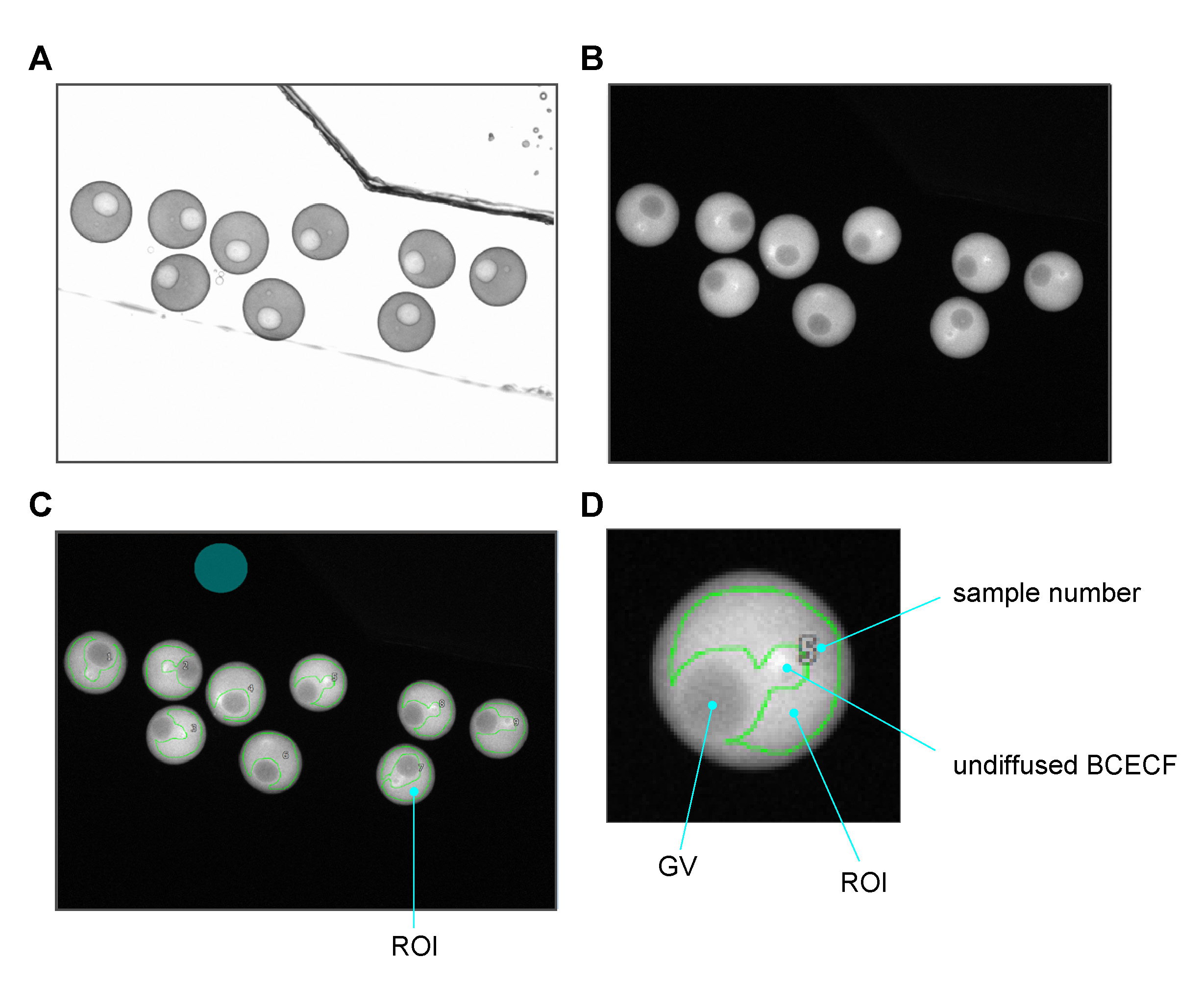
Figure 4. Oocytes’ images acquisition. A. A representative oocytes’ DIC image. B. A fluorescence image of oocytes injected with BCECF. C. The ROI is defined using a green line. The background area is also colored in green. D. An enlarged oocyte image showing the ROI. The GV area is excluded from the ROI since GVBD causes a rapid change in fluorescence intensity. - Select an area without oocytes to get the background fluorescence (Figure 4C).
- Before treatment with 1-MA, acquire fluorescence microscopic images of BCECF-injected oocytes, excited every 10 s at 436 nm and 495 nm for more than 5 min. This excitation alternation is obtained from a Xenon lamp using a filter wheel under the computational control of the HCImage acquisition system. Then, the emitted light passes through a dichroic beam splitter at 505 nm and through a 510-560 nm emission filter, and finally through the CMOS camera and is recorded by the HCImage acquisition system.
- Pause the CMOS camera, and aspirate 25% of the volume of ASW and subsequently add 25% of the volume of ASW containing 2 µM 1-MA (final concentration: 0.5 µM).
- Immediately after addition of 1-MA, turn on (or resume) the CMOS camera to obtain fluorescence images of the oocyte for 2-30 min. Refer to the original study for more detailed information regarding the procedure and prototypical images (Hosoda et al., 2019; Figure S5F, left panel) (see also Video 3).
- Calculation of the average fluorescence intensity ratios, using the recorded images of BCECF-injected oocytes and the HCImage analysis system
- Open an Image Display window showing the fluorescence microscopic image sequence of the oocyte. If oocytes move during recording, new ROIs should be defined according to the procedure in Step C4.
- Calculate the fluorescence intensity ratios between 436 nm and 495 nm excitation from 8-15 oocytes, using the HCImage Analysis software.
- Average the ROI ratios of 8-15 oocytes at each time point.
- After the experiments, convert the average ratios to pHi, using the procedure outlined in Procedure F.
- pHi clamping with modified ASW (sodium-free ASW containing CH3COONH4) and calibration after injection of standard solutions with known pH values
Note: For oocytes treated with modified ASW at pH 6.8, the pHi is expected to be clamped at ~7.0, because the pHi values become ~0.2 higher than the pH values of the modified ASW (Moriwaki et al., 2013; Hosoda et al., 2019).To calibrate accurately the clamped pHi, inject different sets of oocytes with at least three types of pH-standard solutions. When the actual (or real) pHi is higher than the known pH of the injected standard solution, the BCECF fluorescence intensity ratio will decrease after injection since the pHi also decreases upon injection of the standard solution at a lower pH. Conversely, when the actual pHi is lower than the known pH of the injected standard solution, the BCECF fluorescence intensity ratio will increase. Thereafter, the actual pHi should be between the pH values of the two injected standard solutions. When the actual pHi is equal to that of the injected solution, the intensity ratio does not change. Using this method, pHi differences as low as 0.05 can be detected. In this section, taking as an example modified ASW at pH 6.8, the pHi of unstimulated oocytes was clamped, and different sets of clamped oocytes were injected with different pH-standard solutions (pH 7.00, 7.05, 7.10) to estimate the values of clamped pH; readers can expect to see three different outcomes as shown in Figures 5A, 5B and 5C, that allowed the calibration of pHi.- Remove BCECF-injected oocytes from the oocyte holder (Figure 2D) after Procedure B, and recover them in 20 μl ASW, using an adjustable-volume micropipette. Then, transfer them immediately to 1 ml sodium-free ASW containing CH3COONH4 at pH 6.8 (modified ASW pH 6.8). Thereafter, gently agitate the oocytes for 15 s.
- Transfer the oocytes in 20 μl modified ASW at pH 6.8 to freshly modified ASW (1 ml) at pH 6.8, and gently agitate the oocytes for 15 s. Repeat this procedure 5 times to completely eliminate Na+ (with modified ASW at pH 6.8).
- Sandwich immature oocytes with two cover glasses using the oocyte holder (Figure 2D).
- Define the ROI of an oocyte and select an area without oocytes, as background fluorescence–Steps C4 and C5.
Note: The procedures in Steps E1 to E4 should be completed within 20 min to obtain reproducible data. - Record a fluorescence image sequence of an oocyte for ≥ 100 s.
- To inject the pH-standard solution, pause the CMOS camera.
- Inject oocytes with 2% of the volume per oocyte of a pH-standard solution at pH 7.00; of note modified ASW at pH 6.8 may clamp pHi at ~7.0 (Moriwaki et al., 2013; Hosoda et al., 2019).
- Rapidly turn on the CMOS camera system to record the changes in the fluorescence intensity ratio for at least 20 s.
- Measure the average fluorescence intensities inside the ROI and calculate the ratio of the averaged intensities between the 436 nm and 495 nm excitation wavelengths, using the HCImage analysis software. A typical result is shown (Figure 5A). In this particular example, the fluorescence intensity ratio decreased upon injection of the pH-standard solution. Thus, the clamped pHi is estimated to be higher than pH 7.00. Therefore, in the next step, a standard solution with a pH higher than 7.00 should be used, in a way to cause an increase in the fluorescence intensity ratio, and ultimately to determine the real pHi.
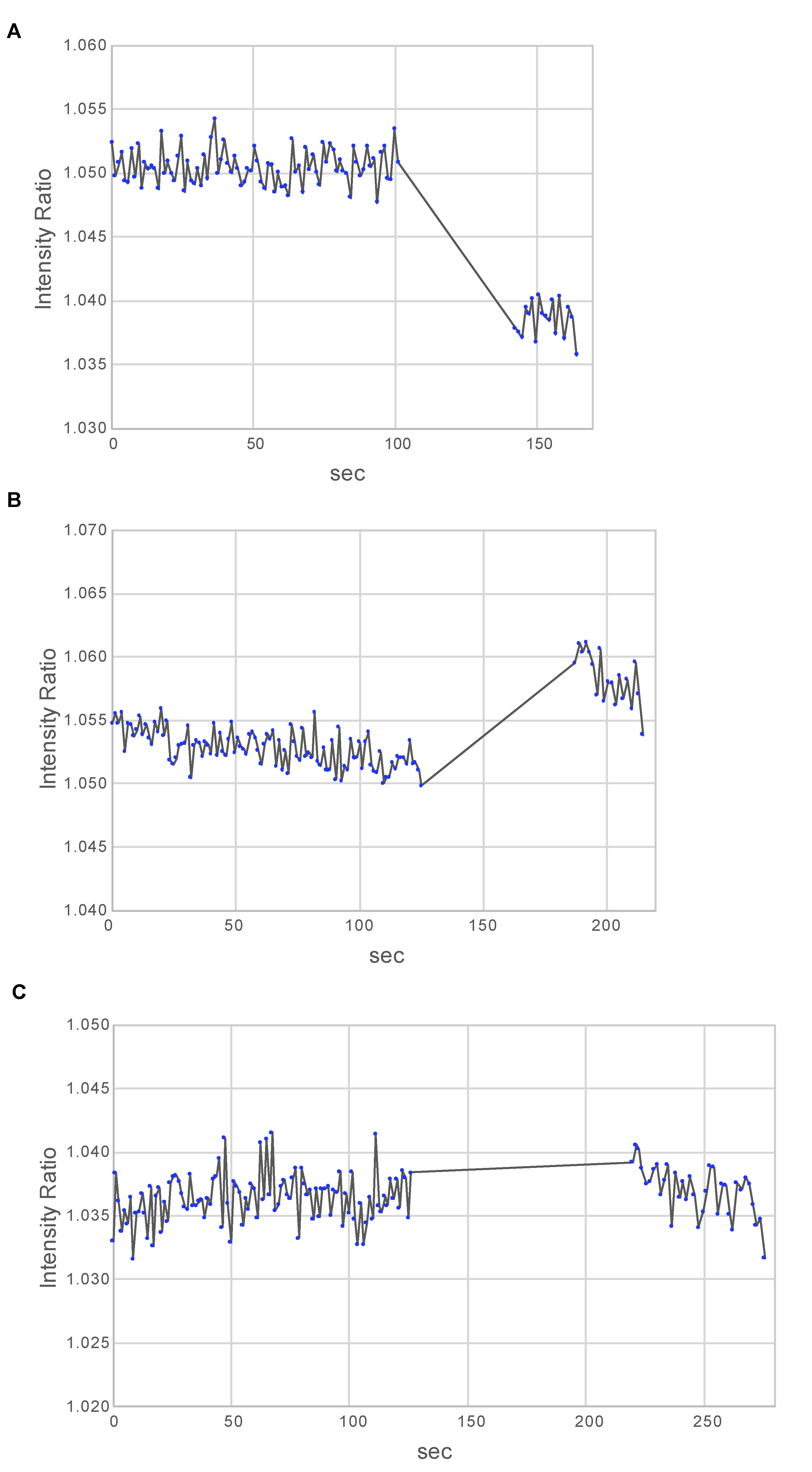
Figure 5. Estimation of the pHi in oocytes treated with modified ASW at pH 6.8. A. The standard solution at pH 7.00 was injected between 100 s and 140 s. Clamped pHi was estimated to be higher than pH 7.00 in this oocyte. B. The standard solution at pH 7.10 was injected between 125 s and 185. Clamped pHi was estimated to be lower than pH 7.10. C. The pH-standard solution at pH 7.05 was injected between 125 s and 220 s. No change in the BCECF fluorescence intensity ratio was observed. Therefore, the clamped pHi is estimated to be pH 7.05. - After the procedure described in Steps E1-E6, inject the oocytes with 2% volume per oocyte of a standard solution at pH 7.10; because pH 7.00 decreased the fluorescence intensity ratio (Figure 5A), pH 7.10 is expected to increase this ratio.
- Turn on the CMOS camera system and calculate the fluorescence ratio for the standard solution at pH 7.10, as mentioned in Steps E7-E9.
- A typical result is shown (Figure 5B). As expected, the fluorescence intensity ratio increased upon injection of the pH-standard solution. Thus, the clamped pHi is estimated to be lower than pH 7.10 and higher than pH 7.00. Therefore, to find out a standard solution with an adequate pH value causing no change of the fluorescence intensity ratio, standard solutions 7.00 ≤ pH ≤ 7.10 should be considered.
- After the procedure described in Steps E1-E6, inject the oocytes with 2% of the volume per oocyte of a standard solution at pH 7.05, and then turn on the CMOS camera system and calculate the respective fluorescence ratio, as mentioned in Steps E7-E9.
- A typical result is shown (Figure 5C). As expected, the fluorescence intensity ratio did not change upon injection of the pH-standard solution, indicating that the actual pHi should be equal to that of the injected solution (= pH 7.05).
- Calibration of pHi in normal seawater
Note: The procedure in F should be conducted on the same day when the procedure in C is performed.- To conduct a two-point calibration, treat immature oocytes (n = 8-15) with modified ASW to clamp the pHi at higher and lower values, and obtain the respective BCECF high- and low-intensity ratios. Usually, modified SW at pH 6.4 (pHi ~6.6) and that at pH 7.3 (pHi ~7.5) can be used. The actual pHi of oocytes in modified ASW is estimated as described in Procedure E.
- Calculate the average values for each point.
- Plot fluorescence ratios against pHi 6.6 and pHi 7.5 to obtain a standard linear calibration graph/function. More detailed information regarding this procedure is already provided in the original study (Hosoda et al., 2019; Figure S5D and E). Convert the ratio data from procedure E to pHi values, using the standard linear calibration graph/function. Again, refer to the procedure in the original study for more detailed information (Hosoda et al., 2019; Figure S5F)
Recipes
- Calcium-free ASW
476 mM NaCl
10 mM KCl
36 mM MgCl2
18 mM MgSO4
20 mM H3BO3, adjusted to pH 8.2 - ASW
462 mM NaCl
10 mM KCl
36 mM MgCl2
18mM MgSO4
10 mM CaCl2
20 mM H3BO3, adjusted to pH 8.2 - Modified ASW pH 6.8
480 mM choline chloride
55 mM MgCl2
5 mM KCl
10 mM Pipes
10 mM HEPES
20 mM CH3COONH4
9.2 mM CaCl2
pH 6.8 is adjusted by KOH - Standard pH solutions at pH 6.40-6.75 or 6.80-7.40
0.5 M Pipes for pH 6.40-6.75; pH is adjusted by KOH
0.5 M HEPES for pH 6.80-7.40; pH is adjusted by KOH - BCECF solution
2 mM dextran (10-kD) conjugate BCECF
100 mM potassium aspartate
10 mM HEPES
0.05% NP-40, pH 7.2
pH 7.2 is adjusted by KOHNote: The pH of the ASW, the modified ASW, and the standard solutions should be adjusted at 23 °C using a Navi h pH meter (HORIBA), which has a resolution of 0.001 pH units. All experiments associated with pHi measurement should be performed at 23 °C.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant no. 17K07405), the Takeda Science Foundation, and the Cooperative Program provided by the Atmosphere and Ocean Research Institute, University of Tokyo. This protocol was adapted from Moriwaki et al., 2013 and Hosoda et al., 2019 (for pHi calibration).
Competing interests
The authors declare no competing financial interests.
Ethics
Experiments were conducted at Ochanomizu Univ. according to guidelines from the Ochanomizu Univ. on Animal Care and approved by the Animal Research Ethics Board of Ochanomizu University.
References
- Anand, S. and Prasad, R. (1989). Rise in intracellular pH is concurrent with 'start' progression of Saccharomyces cerevisiae. J Gen Microbiol 135(8): 2173-2179.
- Bassil, E., Krebs, M., Halperin, S., Schumacher, K. and Blumwald, E. (2013). Fluorescent Dye Based Measurement of Vacuolar pH and K+. Bio-protocol 3(13): e810.
- Behbahan, I. S. S., McBrian, M. A. and Kurdistani, S. K. (2014). A Protocol for Measurement of Intracellular pH. Bio-protocol 4(2): e1027.
- Boyarsky, G., Hanssen, C. and Clyne, L. A. (1996). Superiority of in vitro over in vivo calibrations of BCECF in vascular smooth muscle cells. FASEB J 10(10): 1205-1212.
- Chiba, K., Tadenuma, H., Matsumoto, M., Takahashi, K., Katada, T. and Hoshi, M. (1992). The primary structure of the alpha subunit of a starfish guanosine-nucleotide-binding regulatory protein involved in 1-methyladenine-induced oocyte maturation. Eur J Biochem 207(3): 833-838.
- Chiba, K., Kontani, K., Tadenuma, H., Katada, T. and Hoshi, M. (1993). Induction of starfish oocyte maturation by the beta gamma subunit of starfish G protein and possible existence of the subsequent effector in cytoplasm. Mol Biol Cell 4(10): 1027-1034.
- Grillo-Hill, B. K., Choi, C., Jimenez-Vidal, M. and Barber, D. L. (2015). Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. Elife 4: e03270.
- Hamaguchi, M. S., Watanabe, K. and Hamaguchi, Y. (1997). Regulation of intracellular pH in sea urchin eggs by medium containing both weak acid and base. Cell Struct Funct 22(4): 387-398.
- Harada, K., Oita, E. and Chiba, K. (2003). Metaphase I arrest of starfish oocytes induced via the MAP kinase pathway is released by an increase of intracellular pH. Development 130(19): 4581-4586.
- Harada, K., Fukuda, E., Hirohashi, N. and Chiba, K. (2010). Regulation of intracellular pH by p90Rsk-dependent activation of an Na+/H+ exchanger in starfish oocytes. J Biol Chem 285(31): 24044-24054.
- Hiramoto, Y. (1974). A method of microinjection. Exp Cell Res 87(2): 403-406.
- Hiraoka, D., Hosoda, E., Chiba, K. and Kishimoto, T. (2019). SGK phosphorylates Cdc25 and Myt1 to trigger cyclin B-Cdk1 activation at the meiotic G2/M transition. J Cell Biol 218(11): 3597-3611.
- Hosoda, E., Hiraoka, D., Hirohashi, N., Omi, S., Kishimoto, T. and Chiba, K. (2019). SGK regulates pH increase and cyclin B-Cdk1 activation to resume meiosis in starfish ovarian oocytes. J Cell Biol 218(11): 3612-3629.
- Johnson, J. D. and Epel, D. (1976). Intracellular pH and activation of sea urchin eggs after fertilisation. Nature 262(5570): 661-664.
- Karagiannis, J. and Young, P. G. (2001). Intracellular pH homeostasis during cell-cycle progression and growth state transition in Schizosaccharomyces pombe. J Cell Sci 114(Pt 16): 2929-2941.
- Moolenaar, W. H., Tsien, R. Y., van der Saag, P. T. and de Laat, S. W. (1983). Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature 304(5927): 645-648.
- Moriwaki, K., Nakagawa, T., Nakaya, F., Hirohashi, N. and Chiba, K. (2013). Arrest at metaphase of meiosis I in starfish oocytes in the ovary is maintained by high CO2 and low O2 concentrations in extracellular fluid. Zoolog Sci 30(11): 975-984.
- Mortera, P., Zuljan, F., Magni, C. and Alarcón, S. H. (2015). Loading of cells with fluorescent probe to study intracellular acid-base homeostasis in lactic acid bacteria. Bio-protocol 5(2): e1380.
- Murabe, N., Okumura, E., Chiba, K., Hosoda, E., Ikegami, S., and Kishimoto, T. (2020). The Starfish Asterina pectinifera: Collection and Maintenance of Adults and Rearing and Metamorphosis of Larvae. Methods in Mol Biol (in press).
- Rink, T. J., Tsien, R. Y. and Pozzan, T. (1982). Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol 95(1): 189-196.
- Roos, A. and Boron, W. F. (1981). Intracellular pH. Physiol Rev 61(2): 296-434.
- Sadler, K. C. and Ruderman, J. V. (1998). Components of the signaling pathway linking the 1-methyladenine receptor to MPF activation and maturation in starfish oocytes. Dev Biol 197(1): 25-38.
- Schuldiner, S. and Rozengurt, E. (1982). Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A 79(24): 7778-7782.
- Shen, S. S. and Steinhardt, R. A. (1978). Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature 272(5650): 253-254.
- Tadenuma, H., Takahashi, K., Chiba, K., Hoshi, M. and Katada, T. (1992). Properties of 1-methyladenine receptors in starfish oocyte membranes: involvement of pertussis toxin-sensitive GTP-binding protein in the receptor-mediated signal transduction. Biochem Biophys Res Commun 186(1): 114-121.
- Thomas, J. A., Buchsbaum, R. N., Zimniak, A. and Racker, E. (1979). Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18(11): 2210-2218.
- Tilney, L. G., Kiehart, D. P., Sardet, C. and Tilney, M. (1978). Polymerization of actin. IV. Role of Ca++ and H+ in the assembly of actin and in membrane fusion in the acrosomal reaction of echinoderm sperm. J Cell Biol 77(2): 536-550.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hosoda, E. and Chiba, K. (2020). Fluorescence Measurement and Calibration of Intracellular pH in Starfish Oocytes. Bio-protocol 10(19): e3778. DOI: 10.21769/BioProtoc.3778.
- Hosoda, E., Hiraoka, D., Hirohashi, N., Omi, S., Kishimoto, T. and Chiba, K. (2019). SGK regulates pH increase and cyclin B-Cdk1 activation to resume meiosis in starfish ovarian oocytes. J Cell Biol 218(11): 3612-3629.
Category
Developmental Biology > Cell growth and fate > Oocyte
Cell Biology > Cell imaging > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











