- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Intracellular Ca2+ Concentration in the Human Pathogens Trypanosomatids Leishmania mexicana and Trypanosoma cruzi by the Use of the Fluorescent Ca2+ Indicator Fura-2
(*contributed equally to this work) Published: Vol 10, Iss 18, Sep 20, 2020 DOI: 10.21769/BioProtoc.3766 Views: 3770
Reviewed by: Alexandros AlexandratosEvangelia XingiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An in vitro DNA Sensor-based Assay to Measure Receptor-specific Adhesion Forces of Eukaryotic Cells and Pathogens
Maurizio Wack [...] E. Ada Cavalcanti-Adam
Sep 5, 2020 5654 Views

Ex vivo Culture and Contractile Force Measurements of Non-human Primate Heart Slices
Christine M. Poch [...] Alessandra Moretti
Jul 5, 2023 1493 Views

An Ex Vivo Lung Histoculture Model for Studying Pulmonary Infection and Immune Response With SARS-CoV-2 as an Example of RNA Virus
Elena V. Maryukhnich [...] Elena J. Vasilieva
Dec 20, 2025 740 Views
Abstract
Ca2+ is an essential signaling messenger in all eukariotic cells, playing a pivotal role in many cellular functions as cell growth control (differentiation, fertilization and apoptosis), secretion, gene expression, enzyme regulation, among many others. This basic premise includes trypanosomatids as Trypanosoma cruzi and various species of Leishmania, the causative agents of Chagas disease and leishmaniasis respectively, where intracellular Ca2+ concentration ([Ca2+]i) has been demonstrated to be finely regulated. Nevertheless [Ca2+]i has been difficult to measure because of its very low cytoplasmic concentration (typically around 50-100 nM), when compared to the large concentration in the outside milieu (around 2 mM in blood). The development of intracellular fluorescent Ca2+-sensitive indicators has been of paramount importance to achieve this goal. The success was based on the synthesis of acetoximethylated derivative precursors, which allow the fluorescent molecules typically composed of many hydrophilic carboxyl groups responsible for its high affinity Ca2+-binding (and therefore very hydrophilic), to easily cross the plasma membrane. Once in the cell interior, unspecific esterases split the hydrophobic moiety from the fluorescent backbone structure, releasing the carboxyl groups, transforming it in turn to the acid form of the molecule, which remain trapped in the cytoplasm and regain its ability to fluoresce in a Ca2+-dependent manner. Among them, Fura-2 is by far the most used, because it is a ratiometric (two different wavelength excitation and one emission) Ca2+ indicator with a Ca2+ affinity compatible with the [Ca2+]i. This protocol essentially consists in loading exponential phase parasites with Fura-2 and recording changes in [Ca2+]i by mean of a double wavelength spectrofluorometer. This technique allows the acquisition of valuable information about [Ca2+]i changes in real time, as a consequence of diverse stimuli or changes in conditions, as addition of drugs or different natural modulators.
Keywords: Intracellular Ca2+ measurementsBackground
Aiming to develop new drugs against infections caused by different trypanosomatids, efforts have been invested in the elucidation of the physiological mechanism responsible for intracellular ionic homeostasis, in particular Ca2+ ions, which are known to play a pivotal role as a second messenger in Trypanosoma cruzi and Leishmania spp.The intracellular Ca2+ concentration ([Ca2+]i) is finely regulated (Benaim and García., 2011; Benaim et al, 2020) in these kinetoplastids. Leishmaniases are vector-borne parasitic neglected diseases caused by at least 20 species of the genus Leishmania, and are transmitted between mammalian hosts by female sandflies (Burzaet al., 2018). Chagas disease is another particularly neglected human infection, caused by Trypanosoma cruzi, transmitted by triatomine bugs when infectious parasites in the feces of the vector enter in the skin of the mammalian host at the bite site where scratching is provoked (Santos et al., 2020).
Prior to Fura-2, the most popular method for measuring [Ca2+]i in cells was to monitor the fluorescence of an indicator called Quin-2. Nevertheless this fluorophore has severe limitations due to its short excitation wavelength, non-ratiometric nature and low quantum yield (Grynkiewicz et al., 1985). Also, Quin-2 produces the quenching of large amounts of intracellular Ca2+ that could strongly interfere with the minute changes in the [Ca2+]i that are the focus of this protocol. For this reason, Quin-2 has been used instead as an intracellular Ca2+ chelator, when information under this extreme cellular condition is required. However, BAPTA is most preferred for this use because even though it has a similar molecular structure and a comparable affinity for Ca2+ as does Quin 2, BAPTA has a very weak fluorescence emission, thus having less interference with the [Ca2+]i measurement (Moreno et al, 1994). Fura-2 indeed overcomes all these limitations. The main advantage of using ratiometric dyes like Fura-2, when compared to single wavelength probes, is that the ratio signal is independent of the dye concentration, illumination intensity, and optical path length, allowing thereby to accurately determine the concentration of intracellular calcium (Barreto-Chang and Dolmetsch, 2009). In cell suspensions, accurate determinations of [Ca2+]i changes can be made by the use of Fura-2, especially in combination with various available pharmacological agents (Patel et al., 2012).
The simple and relatively fast protocol presented here has been widely used in the search and evaluation of several potential chemotherapeutic anti-parasitic drugs to elucidate their mechanism of action against T. cruzi and several Leishmania spp. For example, one can determine the source of Ca2+ (intracellular vs. extracellular) in a particular rise of the [Ca2+]i observed in a particular condition, for example a treatment with a drug, in order to verify if the compound acts at the plasma membrane level (channels, receptors), at intracellular compartments (mitochondria, endoplasmic reticulum, acidocalcisomes) or at both (Benaim et al., 2020). This is simply achieved by performing the same experiment in the presence or absence (using EGTA, a Ca2+ chelating agent) of extracellular Ca2+.
A typical example of the Fura-2 use in Trypanosoma cruzi is depicted in Figure 1. In A (blue trace) it can be seen that addition of sphingosine (Sph) to epimastigotes loaded with Fura 2 induces an increase in [Ca2+]i, which is specific for this particular sphingolipid, since addition of Sphingosine-1-Phosphate (Sph-1-P), Ceramide-1-Phosphate (Cer-1-P) or Ceramide (Cer) were without any discernible effect. On the other hand, when EGTA was added to study the effect of Sph in the absence of extracellular calcium (red trace), the sphingolipid, instead of inducing an increase in the [Ca2+]i, caused a small decrease.This could represent a release of the low cytoplasmic Ca2+ to the extracellular space passing by the channel, when itencountered the chelator EGTA. In Figure1B are depicted the effects of nifedipine, a classical human L Type VGCC antagonist on the parasite channel. The green trace shows that nifedipine fully blocks the action of Sph added (indicated by a green arrow). As it is observed (Black trace) the miltefosine, is also able to open this channel, mimicking its effect in L. mexicana (Benaim et al., 2013). Miltefosine is the only oral drug approved for the treatment of visceral leishmaniasis and is also effective against T. cruzi. But, differently to the effect of the natural sphingolipid, nifedipine was not able to totally block the action of this drug (Blue trace), suggesting that these compounds act via a different mechanism on the Ca2+ channel.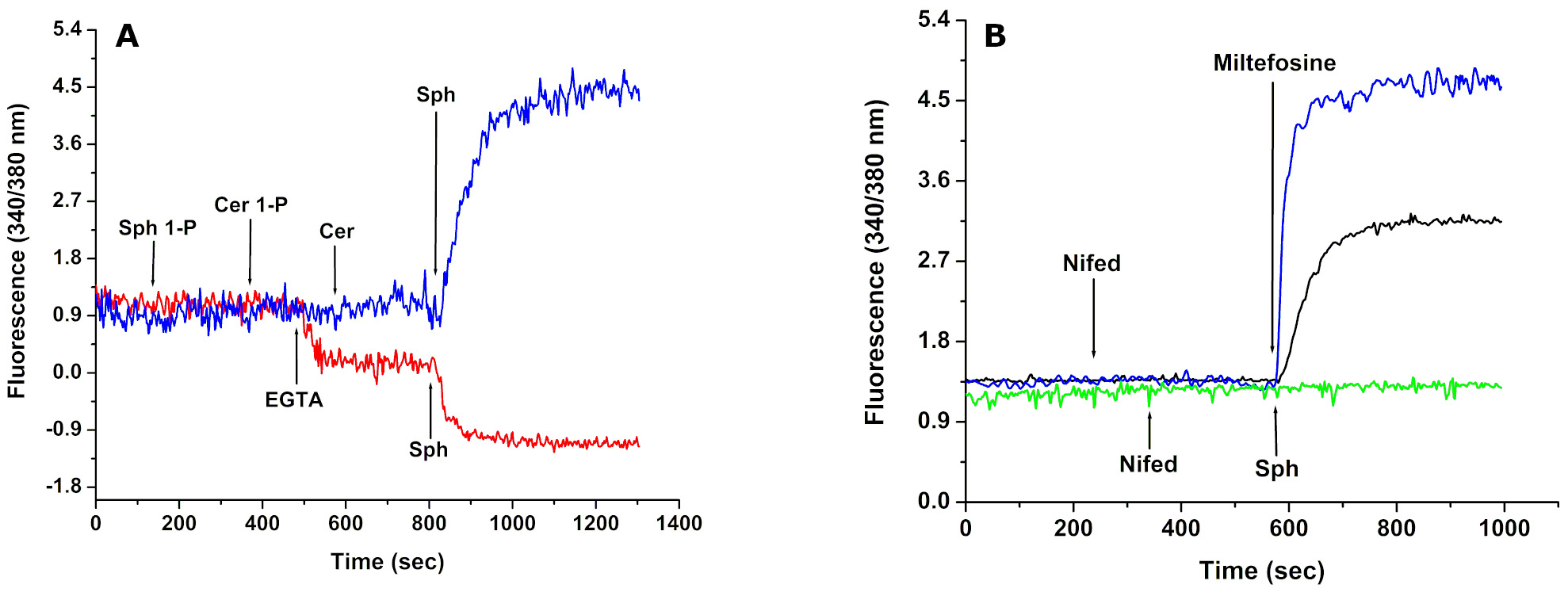
Figure 1. Effect of Sphingosine-1-P (Sph-1-P), Ceramide -1-P (Cer-1-P),Ceramide (Cer) and Sphingosine (Sph), Miltefosine and Nifedipine (a specific L-type VGCC channel blocker) on the intracellular Ca2+ concentration of Trypanosoma cruzi. A. Sph-1-P, Cer-1-P, Cer and Sph were added sequentially to a final concentration of 10 µM in the presence of 2 mM CaCl2 (blue trace) and in the absence of extracellular Ca2+ (red trace). B. Sphingosine (10 µM, arrow) was added after addition of nifedipine (4 µM, arrow) in the presence of extracellular Ca2+ (green trace). Miltefosine (4 µM) was added in the presence of extracellular CaCl2 (blue trace). Nifedipine (40 µM, arrow) was added before the addition of Miltefosine (4 µM) in the presence of extracellular Ca2+ (black trace). (see text for details). Taken from Rodriguez-Duran et al. (2019) FEBS J, 286, 3909-3925. Copyright 2019. FEBS PRESS.
Finally, we used as example the effect of a potent anti-tuberculosis drug known to affect T. cruzi, L. mexicana (Benaim et al., 2020) and L. donovani (Gil et al., 2020) which instead of acting through the plasma membrane Ca2+ channels as miltefosine does, it exerts its action on intracellular organelles, like the parasites’ mitochondrion and/or acidocalcisomes. Thus, it can be observed in Figure 2, that the effect of the drug is the same in the presence (A) or in the absence (B) of extracellular Ca2+, since the cation is released from the mentioned intracellular organelles.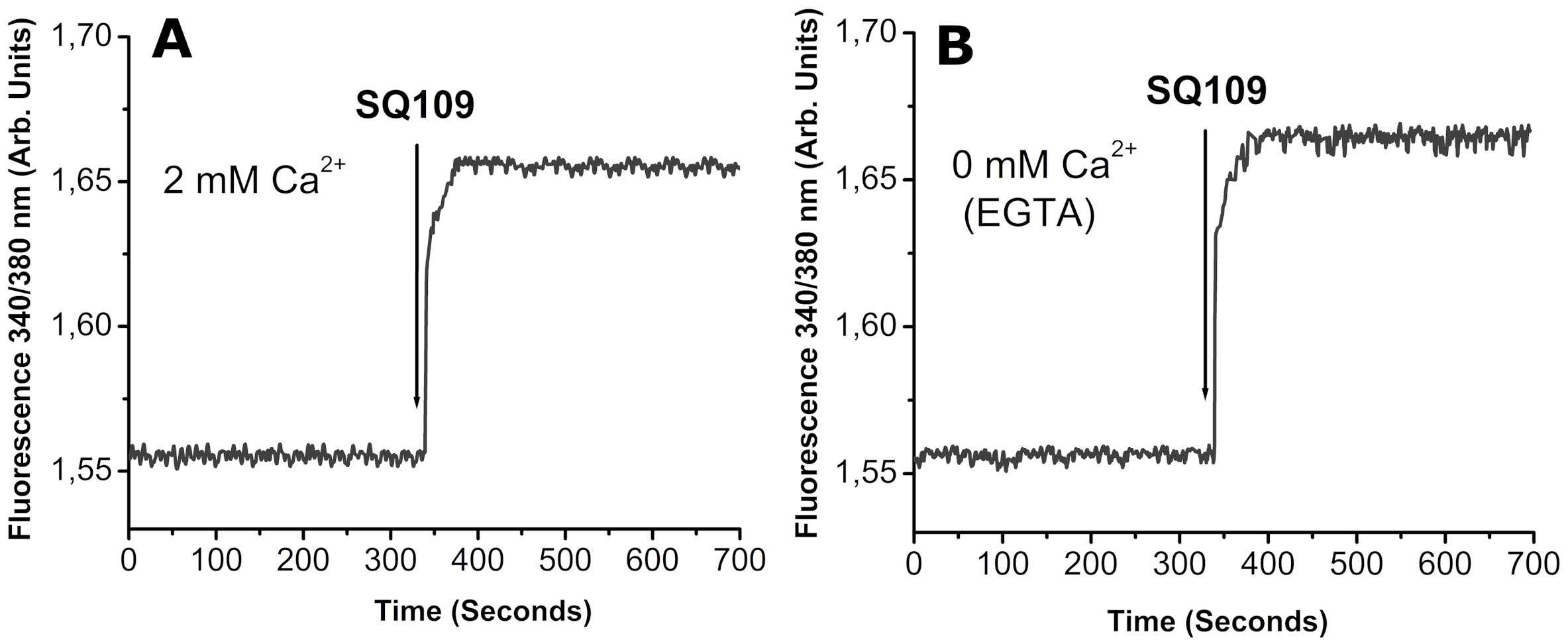
Figure 2. Effects of the antituberculosis drug SQ109 on Ca2+ fluxes in Leishmania mexicana. (A) Intracellular Ca2+ concentration in the presence of 2 mM external Ca2+ and (B) Same as panel A but in the absence of external Ca2+ (2 mM EGTA). Taken from García-García et al. (2016) Antimicrob Agents Chemother. 60: 6386-6389, Copyright 2016. American Chemical Society.
Materials and Reagents
- Pluronic acid F-127 (Thermo Fisher Scientific, InvitrogenTM, catalog number: P3000MP )
20% (w/v) stock in DMSO. An amphiphilic mild detergent that facilitates Fura-2 AM solubilization - Probenecid (Thermo Fisher Scientific, InvitrogenTM, catalog number: P36400 )
An inhibitor of the anionic transporters improving intracellular accumulation of the fluorophore - Fura-2 acetoxymethyl ester (Fura-2 AM) (Thermo Fisher Scientific, InvitrogenTM, catalog number: F1225 )
- Digitonin (Merck, Sigma-AldrichTM, catalog number: 300410 )
- EGTA (Ethylene-bis(oxyethylenenitrilo)tetraacetic acid) (Merck, Sigma-AldrichTM, catalog number: E3889 )
- Calcium chloride (CaCl2) (Merck, Sigma-AldrichTM, catalog number: C1016 )
- Potassium chloride (KCl) (Merck, Sigma-AldrichTM, catalog number: P9333 )
- Potassium phosphate monobasic (KH2PO4) (Merck, Sigma-AldrichTM, catalog number: P0662 )
- Sodium phosphate dibasic (Na2HPO4) (Merck, Sigma-AldrichTM, catalog number: S0876 )
- Magnesium sulfate (MgSO4) (Merck, Sigma-AldrichTM, catalog number: M7506 )
- Sodium chloride (NaCl) (Merck, Sigma-AldrichTM, catalog number: S7653 )
- HEPES (Merck, Sigma-AldrichTM, catalog number: H0887 )
- Glucose (Merck, Sigma-AldrichTM, catalog number: G8270 )
- Modified Tyrode’s loading buffer (MTLB) (pH 7.4) (see Recipes)
- MTLB without Ca2+(pH 7.4) (see Recipes)
Equipment
- Neubauer chamber
- Spectrofluorometer with Fast Filter Accessory (PerkinElmer, model: LS-55 ) or Spectrofluorometer HITACHI DW 7000
- Benchtop Centrifuge (Eppendorf, model: 5415D )
- Quartz SUPRASIL Macro/Semi-micro Cell (PerkinElmer, catalog number: B0631132 )
Software
- FL WinLab (PerkinElmer)
- Excel® (Microsoft)
- OriginPro 6 (OriginLab Corporation, https://www.originlab.com/)
Procedure
- Stock solutions/Buffers
- Digitonin (MW: 1229.3 g/mol) stock at 5 mM in distilled water.
- EGTA (MW: 380.35 g/mol) stock at 500 mM (pH 8) in distilled water.
- Fura-2 AM (MW: 1001.86 g/mol) stock at 1 mM in DMSO.
- Modified Tyrode’s loading buffer (MTLB) (see Recipes).
- MTLB without Ca2+ (see Recipes).
- Probenecid (MW: 285.36 g/mol) stock at 1 M in DMSO.
- Parasite loading with Fura-2 AM
- Count parasites using a Neubauer chamber and calculate the title.
- Collect 1 x 108 parasites at exponential phase by centrifugation at 600 x g for 2.5 min and discard the supernatant.
- Wash twice with 500 µl of MTLB at 29 °C and discard supernatant.
- Resuspend the parasites in 500 µl MTLB and add 1 µl Fura-2 AM (1:1,000 from stock to final concentration 1 µM), 1.2 µl probenecid (3:1,250) from stock to final concentration 2.4 mM), and 1.25 µlpluronic acid (1:400 from stock to final concentration 0.05%).
- Incubate the parasites at 29 °C at darkness with continuous stirring for 2 h.
- Wash Fura-2 loaded parasites with 500 µl of loading buffer, in either the presence or absence of Ca2+.
- Transfer 500 µl of the parasite suspension to a quartz cuvette and place it in a spectrofluorometer with gentle stirring at 29 °C.
- Adjust the spectrofluorometer at 340 and 380 nm for excitation (Ex) and at 510 nm for emission (Em).
- Start measurement using FL WinLab software.
- Controls
To end the experiment, add the followings reagents (the data they generated are needed for analysis of results):- When the monitor trace is stable (constant slope), meaning that Ca2+ is no changing further add digitonin at 30 µM to obtain the maximal ratio of fluorescence (Rmax).
- Wait until the monitor trace is stable again and add EGTA at 10 mM to obtain the minimal ratio of fluorescence (Rmin).
Data analysis
- Each experiment must be performed at least in triplicates.
- [(Ca2+)i] is evaluated as described by Grynkiewicz et al. (1985) by applying the following equation:
[Ca2+]i= Kd × (R – Rmin/Rmax – R) × Fmin/Fmax
Kd: Dissociation constant of Fura-2 (244 nM).
R: Ratio of the fluorescence emission at 510 nm obtained by alternating the excitation between 340 nm and at 380 nm by mean of the fast filter accessory at a given experimental condition.
Rmax: Ratio of the fluorescence emission at 510 nm obtained by alternating the excitation between 340 nm and at 380 nm under saturated Ca2+ concentrations (in the presence of digitonin to permeabilize the parasite plasma membrane).
Rmin: Ratio of the fluorescence emission at 510 nm obtained after alternating the excitation between 340 nm and at 380 nm in the absence of Ca2+ (in the presence of digitonin and presence of EGTA to chelate any remanent Ca2+).
Fmax: Fluorescence of Fura-2 at 380 nm under saturated Ca2+ concentrations
Fmin: Fluorescence of Fura-2 at 380 nm in the absence of Ca2+
Maximum and minimum values are obtained after the addition of 30 µM digitonin, which allows Ca2+ flow into the interior of the cell and thus, Fura-2 will display maximal fluorescence. Then, 10 mMEGTis added to chelate all Ca2+ to nominal 0 level (minimal fluorescence). - Post acquisition data must be converted to a suitable format for Excel®, and then those rows and columns should be copied on the OriginPro software and be plotted as a XY graph, where the X is the time values and Y 340/380 relation values or intracellular Ca2+ concentration (nM).
Notes
- It is strongly recommended the use of recently (maximum 1 month) isolated parasites from mice, instead of parasites after many culture passages. Fura-2 AM must be cleaved to Fura-2 and this cleavage is carried out by unspecified intracellular esterases. Parasites recently obtained from infected mice (or infected macrophages) have much higher levels of esterases, when compared to parasites after many passages, since these enzymes are lost by selection in frequently passed parasite culture.
- The incubation time for a successful parasite load can vary extremely, depending on the amount of esterases present in the parasite population at a given moment, which is very variable. In some cases a 2 h incubation is enough, but in others, loading time can take even 6 h. Even more, in some cases loadingwithFura 2 may not be achieved, because of total lack of esterases in that particular batch.
- Since Fura-2 AM is photosensitive, it is strongly recommended to cover aliquots with aluminum foil.
- It is well known that Fura-2 AM and other acetoximethyl esters from other fluorescent Ca2+ indicators (as Rhod 2-AM and Fluo 3-AM) can be accumulated and transformed to the fluorescent species inside organelles like endoplasmic reticulum, mitochondrion and nucleus. In this regard, combinations of incubation time and temperature for loading the fluorescence probes must be taken in consideration to avoid this phenomenon. On the other hand, this could nevertheless constitute a large advantage, because this can be rationally used to load these organelles with the fluorescent probes, by manipulating incubation time and temperature, and thus performing difficult tasks as study Ca2+ movements inside mitochondria and endoplasmic reticulum, otherwise very difficult to measure (for example, see Benaim et al., 2006).
Recipes
- 1.Modified Tyrode’s loading buffer (MTLB) (pH 7.4)
137 mM NaCl
4 mM KCl
1.5 mM KH2PO4
8.5 mM Na2HPO
11 mM glucose
1 mM CaCl2
0.8 mM MgSO4
20 mM HEPES-NaOH - MTLB without Ca2+ (pH 7.4)
137 mM NaCl
4 mM KCl
1.5 mM KH2PO4
8.5 mM Na2HPO4
11 mM glucose
0.8 mM MgSO4
100 µM EGTA
20 mM HEPES-NaOH
Acknowledgments
Supported by Fondo Nacional de Ciencia, Tecnología e Investigación, Venezuela (FONACIT) (grants 2017000274 and 2018000010) and the Consejo de Desarrollo Científico y Humanístico-Universidad Central de Venezuela (CDCH-UCV) grant PG-03-8728-2013/2 to G.B.This protocol was essentially extracted from Benaim et al. (2014) and Rodriguez-Duran et al. (2019)
Competing interests
There are no conflicts of interest or competing interest.
References
- Barreto-Chang, O. L. and Dolmetsch, R. E. (2009). Calcium imaging of cortical neurons using Fura-2 AM. J Vis Exp(23): e1067.
- Benaim, G., Casanova, P., Hernandez-Rodriguez, V., Mujica-Gonzalez, S., Parra-Gimenez, N., Plaza-Rojas, L., Concepcion, J. L., Liu, Y. L., Oldfield, E., Paniz-Mondolfi, A. and Suarez, A. I. (2014). Dronedarone, an amiodarone analog with improved anti-Leishmania mexicana efficacy. Antimicrob Agents Chemother 58(4): 2295-2303.
- Benaim, B. and García, C. R. (2011). Targeting calcium homeostasis as the therapy of Chagas' disease and leishmaniasis - a review. Trop Biomed 28(3): 471-481.
- Benaim, G., Garcia-Marchan, Y., Reyes, C., Uzcanga, G. and Figarella, K. (2013). Identification of a sphingosine-sensitive Ca2+ channel in the plasma membrane of Leishmania mexicana. Biochem Biophys Res Commun 430(3): 1091-1096.
- Benaim, G., Paniz-Mondolfi, A. E., Sordillo, E. M. and Martinez-Sotillo, N. (2020). Disruption of Intracellular Calcium Homeostasis as a Therapeutic Target Against Trypanosoma cruzi. Front Cell Infect Microbiol 10: 46.
- Benaim, G., Sanders, J. M., García-Marchan, Y., Colina, C., Lira, R., Caldera A. R., Payares, G., Sanoja, C., Burgos, J. M., Leon-Rossell, A., Concepcion, J. L., Schijman, A. G., Levin, M., Oldfield, E. and Urbina, J. A. (2006). Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem 49(3): 892-899.
- Burza, S., Croft, S. L. and Boelaert, M. (2018). Leishmaniasis. Lancet 392(10151): 951-970.
- García-García, V., Oldfield, E. and Benaim, G. (2016). Inhibition of Leishmania mexicana Growth by the Tuberculosis Drug SQ109. Antimicrob Agents Chemother 60(10): 6386-6389.
- Gil, Z., Martinez-Sotillo, N., Pinto-Martinez, A., Mejias, F., Martinez, J. C., Galindo, I., Oldfield, E. and Benaim, G. (2020). SQ109 inhibits proliferation of Leishmaniadonovani by disruption of intracellular Ca2+ homeostasis, collapsing the mitochondrial electrochemical potential (ΔΨm) and affecting acidocalcisomes. Parasitol Res 119(2): 649-657.
- Grynkiewicz, G., Poenie, M. and Tsien, R. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6): 3440-3450.
- Moreno, S. N., Silva, J., Vercesi, A. E. and Docampo, R. (1994). Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med 180(4): 1535-1540.
- Patel, A., Hirst, R. A., Harrison, C., Hirota, K. and Lambert, D. G. (2012). Measurement of [(Ca2+)]i in whole cell suspensions using Fura-2. Method Mol Biol 937: 37-47.
- Rodriguez-Duran, J., Pinto-Martinez, A., Castillo, C. and Benaim, G. (2019). Identification and electrophysiological properties of a sphingosine-dependent plasma membrane Ca2+ channel in Trypanosoma cruzi. FEBS J 286(19): 3909-3925.
- Santos, S. S., de Araujo, R. V., Giarolla, J., Seoud, O. E. and Ferreira, E. I. (2020). Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: a review. Int J Antimicrob Agents 55(4): 105906.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Rey-Cibati, A., Valladares-Delgado, M. and Benaim, G. (2020). Determination of Intracellular Ca2+ Concentration in the Human Pathogens Trypanosomatids Leishmania mexicana and Trypanosoma cruzi by the Use of the Fluorescent Ca2+ Indicator Fura-2. Bio-protocol 10(18): e3766. DOI: 10.21769/BioProtoc.3766.
Category
Microbiology > Microbe-host interactions > Ex vivo model
Cell Biology > Cell-based analysis > Ca2+ homeostasis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












