- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Surgical Induction of Endometriosis in Female Mice
(*contributed equally to this work) Published: Vol 10, Iss 18, Sep 20, 2020 DOI: 10.21769/BioProtoc.3763 Views: 4429
Reviewed by: Zinan ZhouFrancisco NavarreteMario Valentino

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2540 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
Endometriosis is a common gynecological disease characterized by the presence of endometrial tissue outside the uterine cavity. It is frequently associated with pain, infertility and a reduced quality of life, and it lacks adequate treatment. Several rodent models of endometriosis have been developed through heterologous and homologous transplantation of endometrial tissue into the abdominal compartment. Here we describe a surgical procedure to generate a syngeneic model of endometriosis in immunocompetent mice with intact uterine and ovarian tissues. In this model, four uterine fragments from a donor mouse at diestrus are sutured to the abdominal wall of a recipient mouse. One month after surgeries, endometrial implants develop into cysts with glandular epithelium and stroma, mimicking the endometriotic lesions observed in women with endometriosis. Therefore, this mouse model provides a valuable tool to study the pathophysiology of endometriosis and the efficacy of potential treatments.
Keywords: EndometriosisBackground
Endometriosis is a chronic gynecological condition defined by the growth of endometrial tissue outside the uterus, mainly in pelvic and abdominal surfaces (Zondervan et al., 2018). It affects 10% of women in reproductive age and it is associated with pain, infertility and reduced quality of life (Fourquet et al., 2011; Márki et al., 2017; Zondervan et al., 2018). Available pharmacological and surgical therapies for endometriosis have undesired effects and fail providing long-term alleviation of the symptoms (Falcone and Flyckt, 2018). Hence, new therapeutic strategies are needed, and their development relies on the establishment of animal models that recapitulate the pathophysiological and behavioral features of clinical endometriosis.
Endometriosis occurs spontaneously in human and nonhuman primates (D’Hooghe et al., 1992; Hadfield et al., 1997). In baboons, endometriosis has also been experimentally induced by injection of menstrual effluent into the pelvis (D’Hooghe et al., 1995), resulting in a model that closely mimics endometriosis in women. However, the use of nonhuman primates is limited by economical and ethical issues (D’Hooghe et al., 2009). In this scenario, rodents are cost-effective and widely available, representing a very useful tool for endometriosis research (Grümmer, 2006). Different heterologous and homologous rodent models have been developed to study the etiology of endometriosis and the efficacy of compounds on its development and symptoms. On the one hand, heterologous models involve xenotransplantation of human endometrial tissue–ectopic, eutopic or cultured stromal and epithelial cells–into immunodeficient mice (Greaves et al., 2017). These models allow the evaluation of potential therapies on endometriotic lesions that maintain human histological characteristics (Hull et al., 2008), however, the lack of a normal immunological response represents an important limitation for the study of this chronic inflammatory condition. On the other hand, homologous models have been developed through surgical transplantation or injection of endometrial tissue from the same animal or a syngeneic donor into the peritoneal cavity of immunocompetent animals (Vernon and Wilson, 1985; Somigliana et al., 1999; Fattori et al., 2020). Syngeneic transplantation of endometrial tissue avoids complications related to the resection of one uterine tissue from the same animal and allows studying the contribution of host and donor cells on endometriosis (Zhao et al., 2016). Among syngeneic models, the one presented here involves suturing of uterine fragments and has the advantage of high reproducibility of the lesions, allowing easy identification and size comparison when testing disease-modifying drugs.
Here we describe the implementation of a syngeneic surgical model of endometriosis in immunocompetent mice with intact uterine tissue and ovarian function. This model reproduces the ectopic endometrial growths observed in clinical endometriosis as well as some of its symptoms, including persistent pain hypersensitivity in the lower abdomen, anxiety-related behavior and potential cognitive deficits (Escudero-Lara et al., 2020). Furthermore, we have used this model to show the efficacy of Δ9-tetrahydrocannabinol limiting the development of endometriosis and its pain-related manifestations (Escudero-Lara et al., 2020). Since the present approach allows the use of immunocompetent mice with an unaltered estrous cycle, we consider this resource a model with high construct and face validity, valuable for identification and assessment of novel therapeutic strategies for endometriosis.
Materials and Reagents
- 24 x 60 mm coverslips (Deltalab, Euroturbo®, catalog number: D102460 )
- 200 µl pipette tips (Daslab, catalog number: 162006 )
- Microscope slides (Avantor, VWRTM, catalog number: 631-1553 )
- Rolled cotton 100% (Acofarma, Acofar®, catalog number: 4957051 )
- Iodine-povidone (Meda Pharma SAU, Betadine®, catalog number: 716720.4 )
- No. 15 Sterile Disposable Scalpels (Swann Morton, catalog number: 0 505 )
- Petri dishes (BD, FalconTM, catalog number: 353001 )
- 0.7 METRIC, 45 cm (6-0 18’’) silk sutures (Alcon, catalog number: 184801 )
- 26 G needles (BD, MicrolanceTM, catalog number: 300300 )
- 1 ml syringes (BD, PlastipakTM, catalog number: 303172 )
- Reaction tubes, 1.5 ml (Sarstedt, catalog number: 72.690.001 )
- Isoflurane-absorbing charcoal filter (Bickford, OMNICON f/air, catalog number: 80120 )
- 8-week-old female C57Bl/6J mice (Charles Rivers Laboratories, JAXTM, strain code: 632)
Note: Housed in cages of four to five mice with water and food available ad libitum. Housing conditions are maintained at 21 ± 1 °C and 55 ± 10% relative humidity in a 12 h light/dark cycle (light on from 8 AM to 8 PM). - Saline solution 0.9% sodium chloride (ERN, Vitulia, catalog number: 999791.5 )
- Distilled water (dH2O)
- Medical Oxygen (Abelló Linde, Conoxia®, catalog number: 652547 .0)
- Isoflurane (Virbac, Vetflurane®, catalog number: 575837-4 )
- Ophthalmic ointment (Nicox, Xilin, catalog number: 171324 )
- Optimal cutting temperature compound (O.C.T) (Sakura Finetek, Tissue-Tek® O.C.T.TM, catalog number: 4583 )
- Cryomolds (Sakura Finetek Tissue-Tek® Cryomold®, catalog number: 4565 )
- Papanicolaou's solution 1a Harris' hematoxylin solution (Merck Millipore, catalog number: 104302 )
- Eosin G or Y 0.5% alcoholic (Diapath, catalog number: C0353 )
- Xylene Cyanol FF (Sigma-Aldrich, catalog number: X4126 )
- Mounting medium for microscopy (Deltalab, VITROCLUD®, catalog number: A20250 )
- Gelatin (Sigma-Aldrich, catalog number: G9391 )
- KCr(SO4)2•12H2O (Merck Millipore, EMSURE®, catalog number: 101036 )
- NaH2PO4•2H2O (Sigma-Aldrich, catalog number: 71505 )
- Na2HPO4•12H2O (Sigma-Aldrich, catalog number: 71649 )
- NaCl (Sigma-Aldrich, catalog number: S7653 )
- PFA (Merck Millipore, catalog number: 104005 )
- NaOH (Merck Millipore, catalog number: 109137 )
- Sodium azide (Sigma-Aldrich, catalog number: S2002 )
- Sucrose (Merck Millipore, catalog number: 107687 )
- 100% ethanol (Merck Millipore, EMSURE®, catalog number: 1009831 )
- Gentamicin (Laboratorios Normon, Genta-Gobens®, catalog number: 999037.7 )
- Meloxicam (Boehringer Ingelheim, Metacam®, catalog number: 210028 )
- Methylene blue (Scharlau, EsssentQ®, catalog number: AZ02030025 )
- Gelatinized microscope slides (see Recipes)
- 0.3 mg/ml gentamicin (see Recipes)
- 0.2 mg/ml meloxicam (see Recipes)
- 0.01 M phosphate buffered saline (PBS) (see Recipes)
- 4% paraformaldehyde (PFA) (see Recipes)
- Cryoprotectant solution: 30% sucrose with 0.1% sodium azide (see Recipes)
- 96% and 70% ethanol (see Recipes)
- 0.01% methylene blue in saline solution (see Recipes)
Equipment
- P20 pipette (Gilson, model: PIPETMAN® P20, catalog number: F123600 )
- Plastic staining jars and baskets (Brand, Brand®, catalog number: 471800 )
- Light microscope (Leica Microsystems, model: DM6000 B ) with camera (Leica Microsystems, model: DFC300 FX )
- Anesthesia circuit including isoflurane vaporizer (Midmark, Isoflurane VIP 3000®-Well-Fill, catalog number: 91305430 ), tubing, regulating valves, nose cones and induction chamber (Midmark, catalog number: 93805108 ) (Figure 1)
- Cold light source (Leica Microsystems, model: CLS 50 X )
- Electric hair-clipper (BBraun, Aesculap®, model: ISIS , catalog number: GT421 )
- Heating pads (Daga, catalog number: N2P 220-230 )
- Small serrated-semi curved tip forceps (Allgaier Instrumente, A-line endoscopy, catalog number: 08-515-005 )
- Haemostatic forceps (Allgaier Instrumente, A-line endoscopy, catalog number: 13-028-120 )
- Rounded tip scissors (Medicon, catalog number: 02.50.62 )
- Dissection scissors (Medicon, catalog number: 02.70.14 )
- Microdissection scissors (Dimeda, catalog number: 09.102.11 )
- Cryostat (Leica Biosystems, model: CM3050 )
- Macro Zoom Microscope (Olympus, model: MVX10 ) with camera (Olympus, model: DP71 )
- -80 °C freezer (Thermo Fisher, -86 C ULT Freezer, model: 917 )
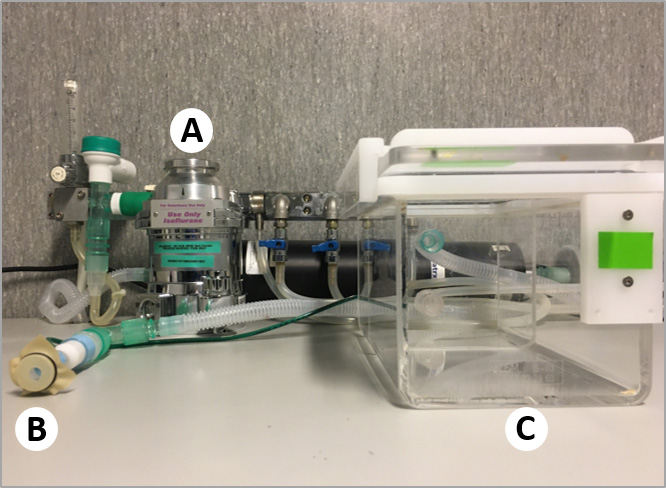
Figure 1. Anesthesia circuit. A. Isoflurane vaporizer. B. Nose cone. C. Induction chamber.
Software
- Leica Application Suite software v4.0 (Leica Microsystems, www.leica-microsystems.com)
- Cell^D Life Science documentation software (Matrix Optics, www.matrixoptics.com)
Procedure
- Selection of donors in diestrus: estrous cycle determination
- Place the mouse on the cage grid, and gently pull the tail backwards to induce the mouse gripping the grid and pulling forward. With the other hand, grasp the mouse by the scruff of the neck and pin the tail between the palm and the fourth finger of the hand holding the scruff.
- Place the pipette tip at the opening of the vaginal canal and flush the vagina 5 times with 20 μl of saline solution in and out.
- Place the resulting fluid onto a gelatinized slide.
- Let the drop air-dry.
- Stain with 0.01% methylene blue for 2 min.
- Wash twice in distilled water for 2 min.
- Let air-dry.
- Take images of stained cells using the 40x objective of a light microscope to determine the stage of the estrous cycle (Figure 2):
- In proestrus, most cells are nucleated.
- In estrus, mostly cornified epithelial cells are present.
- In metestrus, cornified epithelial cells and leukocytes are found.
- In diestrus, leukocytes are predominant, and some nucleated and cornified epithelial cells are present.
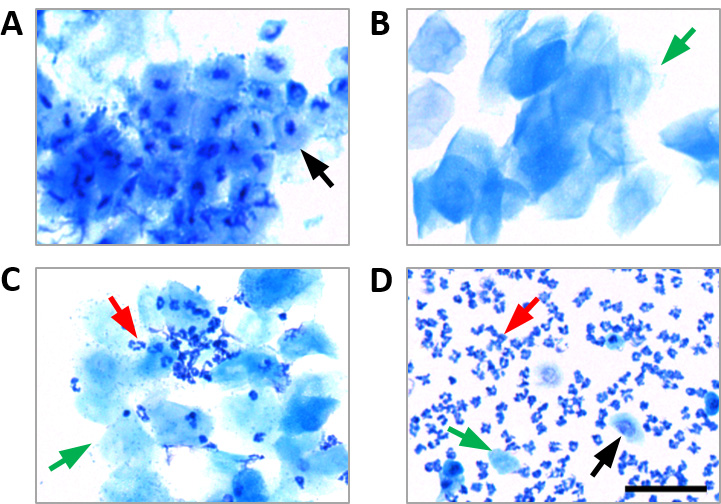
Figure 2. Vaginal cytology from mice in each stage of the estrous cycle. A. Proestrus. B. Estrus. C. Metestrus. D. Diestrus. Nucleated epithelial cells, cornified epithelial cells, and leukocytes are indicated by black, green and red arrows, respectively. Scale bar = 50 μm. - Obtention of endometrial and control tissues: excision of uterine fragments and abdominal fat
- Euthanize the donor mouse in diestrus by cervical dislocation.
- Make a midline incision on the abdomen using a scalpel and excise the uterus (Figures 3A and 3B).
- Place the uterus in a Petri dish containing cold saline solution (4 ºC, on ice) (Figure 3C).
- Place abdominal fat of a size similar to the uterus in another Petri dish containing cold saline solution (Figure 3D).
- Strip one excised uterine horn and open it longitudinally with microdissection scissors, exposing the endometrium (Figures 3E and 3F).
Note: Keep the other uterine horn unopened and in cold saline solution until used to induce endometriosis on a second recipient mouse. - Use a new scalpel to cut the uterine horn or the fat in four squares with each side measuring 2 x 2 mm (Figures 3G and 3H).
- Keep the uterine fragments in cold saline solution until needed.
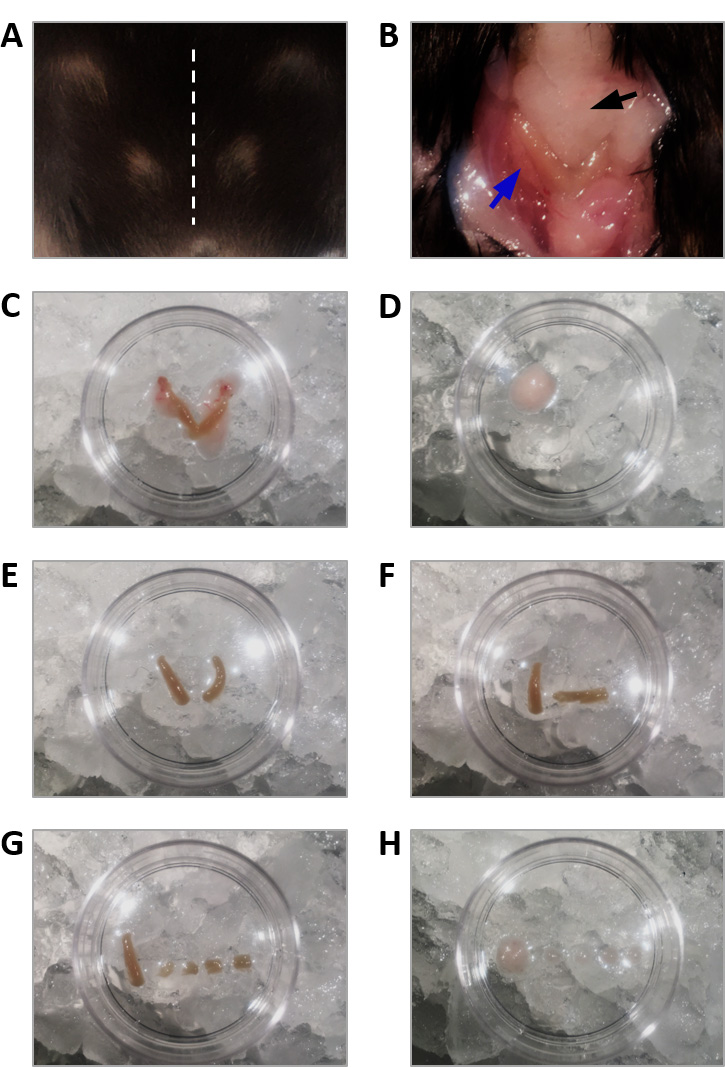
Figure 3. Excision of uterine fragments and abdominal fat. A. A midline incision (dashed line) is made on the abdomen of the euthanized donor mouse. B. The uterus (blue arrow) and the abdominal fat (black arrow) are exposed through the incision. C. The uterus is excised and placed in a Petri dish containing cold saline solution. D. Abdominal fat tissue is also excised and placed in another Petri dish containing cold saline solution. E. The uterine horns are stripped. F. One uterine horn is opened longitudinally, exposing the endometrium. G. The opened uterine horn is cut in four fragments of 2 x 2 mm that are used for the endometriosis surgery. H. Abdominal fat is also cut in similar-sized fragments that are used for the control surgery. - Surgical induction of endometriosis: peritoneal implantation of ectopic endometrium
- Anesthetize the recipient mouse with 4% V/V isoflurane in medical oxygen (2 L/min) in the induction chamber.
- Once anesthetized, place the mouse on top of a heating pad and maintain anesthesia with 2.5% V/V isoflurane in medical oxygen (2 L/min) using a nose cone.
- Apply ophthalmic ointment to avoid drying of the eyes.
- Shave the abdomen of the mouse using an electric hair-clipper.
- Clean the skin with a piece of cotton soaked in iodine-povidone solution (Figure 4A).
- Make a 1 cm midline incision on the skin using a scalpel, ending approximately 0.5 cm cranial to the vaginal ending.
- Insert closed rounded tip scissors between the skin and the abdominal wall and open them to detach the skin from the muscle.
- Make a 1 cm incision on the abdominal wall using the small scissors and use blunt forceps to expose its inner surface (Figure 4B).
- Suture the uterine fragments into the abdominal wall (two on each side of the incision) with loose square knots of 6-0 black silk. Uterine fragments must be sutured with the endometrial surface facing the peritoneal cavity (Figures 4C and 4D).
- Close the muscular abdominal wall with three loose square knots of 6-0 black silk (Figure 4E).
- Close the skin with three square knots of 6-0 black silk.
- Clean the skin with a piece of cotton soaked in povidone-iodine solution (Figure 4F).
- Leave the animal lying over one side in a clean cage partially placed over a heating pad until it recovers.
- Whenever the use of analgesics and antibiotics does not interfere with the purpose of the experiment, give meloxicam (2 mg/kg, subcutaneously, injection volume 10 ml/kg) and gentamicin (1 mg/kg, intraperitoneally, injection volume 3 ml/kg) right after surgery and 24 h later.
- To induce endometriosis to a second recipient mouse, repeat the procedure from Step B4 using the remaining uterine horn excised from the same donor mouse.
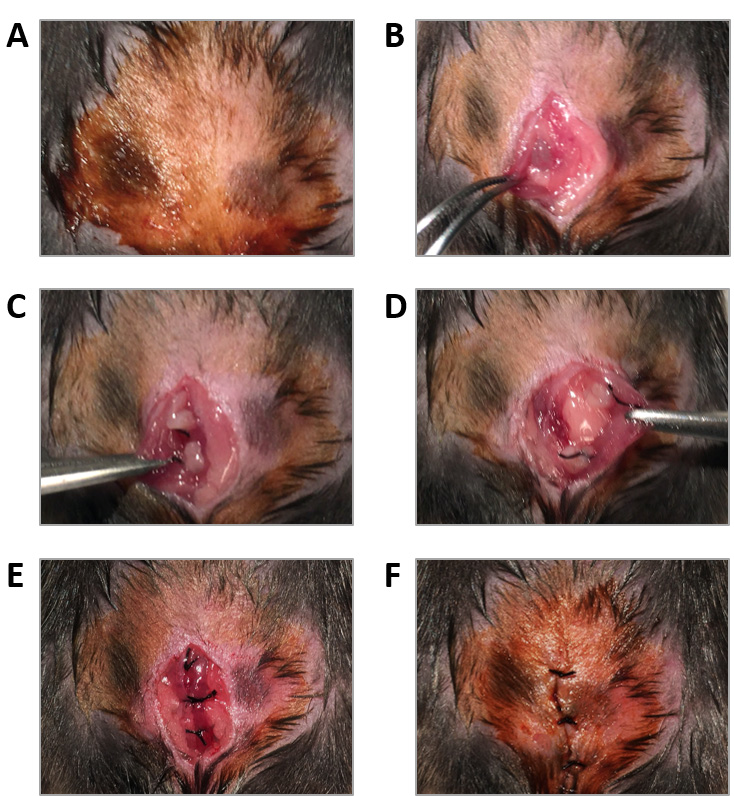
Figure 4. Surgical induction of endometriosis. A. The abdomen of an anesthetized recipient mouse is shaved and cleaned with iodine-povidone solution. B. The inner surface of the abdominal wall is exposed through a 1 cm incision. C and D. Two fragments of uterine tissue or fat from a donor mouse are sutured on each side of the incision. E. The muscular abdominal wall is closed with three loose square knots. F. The skin is closed with three square knots and cleaned with iodine-povidone solution. - Control mice: peritoneal implantation of abdominal fat
To obtain control mice repeat the procedure from Section C, substituting the Step C9 by suturing the fat fragments into the abdominal wall (two on each side of the incision) with loose square knots of 6-0 black silk. - Confirmation of endometriosis: histology of endometriotic lesions
Note: Establish the time of necropsy according to the purpose of the experiment. Control mice should not develop endometriotic lesions.- Perform a vaginal lavage as described in Section A if the stage of the estrous cycle at the time of sample collection needs to be considered.
- Euthanize the mouse by cervical dislocation.
- Use dissection scissors to make a dorsal incision in the back of the mouse at the level of the pelvis and peel the skin (Figure 5A).
- Remove the spine from S1 to L1 by making a first cut at the level of the most caudal rib and a second cut 1 cm caudal to the first one. Then, cut both sides along the spinal column (Figure 5A).
Note: Spinal cord and dorsal root ganglia can be isolated from the extracted fragment of spinal column and used for biochemical and immunohistochemical analyses. - Carefully pull out the intestines to allow the examination of the abdominal wall (Figure 5B).
- Excise the cystic endometriotic lesions and remove the surrounding fat tissue using dissection scissors and forceps. Special care should be taken to avoid lancing the fluid-filled cysts (Figure 5C).
- Place the endometriotic lesions in reaction tubes with paraformaldehyde 4% and allow tissue fixation for 4 h at 4 °C.
- Wash 3 x 5 min with 0.01 M PBS at room temperature.
- Keep samples in sucrose 30% with 0.1% sodium azide for 6 days to cryoprotect the tissue.
- Take the samples out of the sucrose solution, remove sucrose excess with filter paper, embed in cryomolds filled with O.C.T. and store at -80 °C.
- Section samples at 20 μm with a cryostat and mount onto gelatinized slides. Store at -20 °C.
- Stain with hematoxylin and eosin:
- Leave the slides in staining baskets and allow them to thaw.
- Sink in 100% ethanol for 5 min.
- Sink in 96% ethanol for 5 min.
- Sink in 70% ethanol for 5 min.
- Wash with distilled water.
- Immerse in Papanicolaou's solution 1a Harris' hematoxylin solution for 5 min.
- Wash in distilled water for 2 min.
- Immerse in Eosin G or Y 0.5% alcoholic for 30 s.
- Sink in 70% ethanol for 5 min.
- Sink in 96% ethanol for 5 min.
- Sink in 100% ethanol for 5 min.
- Clear with Xylene Cyanol FF for 5 min.
- Mount coverslip with mounting medium for microscopy.
- Allow to dry horizontally for 3 days.
- Capture images of stained sections using a macro zoom microscope for assessment of diameter and histological features (Figure 5D).
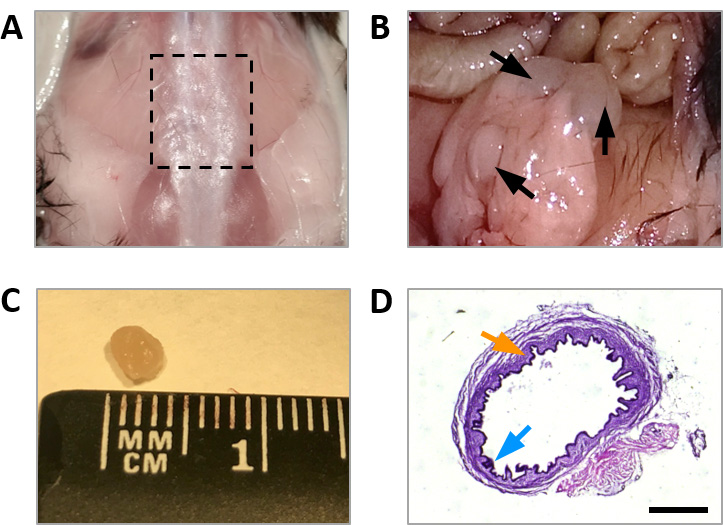
Figure 5. Endometriotic lesions recovered 32 days after surgical induction of endometriosis. A. Removal of the spine from S1 to L1 (dashed lines). B. Uterine fragments implanted into the abdominal wall develop into cysts. C. Endometriotic cyst of approximately 2.5 mm diameter. D. Hematoxylin and eosin stained section of an endometriotic lesion showing the presence of stroma (orange arrow) and glandular epithelium (blue arrow). Scale bar = 1 mm.
Notes
- Use different scalpels and silk sutures for uterine fragments or fat, and for muscle and skin.
- Mice should be monitored daily for at least 3 days after surgeries. If a mouse removes the skin sutures, briefly anesthetize with isoflurane (4% V/V for induction, 2.5% V/V for maintenance), wash the incision site with saline solution and close the skin with 6-0 black silk square knots. Afterwards, disinfect with iodine-povidone solution.
- Mice should be supervised following an adaptation of the Morton and Griffiths guidelines on the recognition of pain, distress and discomfort (Morton and Griffiths, 1985). This adapted protocol considers four variables, and a score is assigned for each variable:
- Weight loss:
0 Normal. There is no weight loss or the animal grows normally.
1 Weight loss less than 10%.
2 Weight loss between 10 and 20%. Alteration in the appearance or amount of stool.
3 Weight loss greater than 20%. The animal does not consume water or food. - Coat appearance:
0 Normal.
1 Hair in poor condition.
2 Hair in poor condition and ocular or nasal secretions.
3 Piloerection. - Movement/posture/behavior of the animal:
0 Normal.
1 Small changes.
2 Moderate changes.
3 Inactivity, aggressiveness, self-mutilation or vocalizations. - Aspect of the incision site:
0 Normal.
1 Slight redness.
2 Local edema.
3 Infection, darkening (signs of necrosis). - 0 to 3. The mouse does not require any corrective action.
- 4 to 7. The mouse requires the application of corrective actions.
- Equal to 8. The mouse should be euthanized.
Corrective measures include cleaning the wound with disinfectant and applying topical antibiotic, placing the cage on top of a heating pad and supplying nutrient-enriched hydrating gel or food pellets soaked in water to facilitate feeding.
Recipes
- Gelatinized microscope slides
- Prepare 0.5% gelatin-coating solution
0.5 g gelatin
0.5 g KCr(SO4)2•12H2O
dH2O up to 400 ml
stir at 50 °C until dissolved - Gelatinize slides
Place the slides in staining baskets
Soak the baskets 3 x 30 s in 0.5% gelatin-coating solution, waiting 30 s between immersions
Blot excess solution onto filter paper and cover the baskets containing the slides
with aluminum foil
Dry in an incubator at 37 °C for 24 h
- Prepare 0.5% gelatin-coating solution
- 0.3 mg/ml gentamicin
31.25 µl gentamicin
5 ml saline solution 0.9% sodium chloride - 0.2 mg/ml meloxicam
0.2 ml meloxicam
4.8 ml saline solution 0.9% sodium chloride - 0.01 M phosphate buffered saline (PBS)
- Prepare Solution A: 0.2 M sodium phosphate monobasic
3.12 g NaH2PO4•2H2O
dH2O up to 100 ml - Prepare Solution B: 0.2 M sodium phosphate dibasic
28.65 g Na2HPO4•12H2O
dH2O up to 400 ml - Prepare 0.1 M phosphate buffer (PB), pH 7.4
100 ml Solution A
400 ml Solution B
500 ml dH2O - Prepare 0.1 M PBS
9 g NaCl
1,000 ml 0.1 M PB - Prepare 0.01 M PBS
100 ml 0.1 M PBS
1,000 ml dH2O
- Prepare Solution A: 0.2 M sodium phosphate monobasic
- 4% paraformaldehyde (PFA)
10 g PFA
1 ml 1 M NaOH
0.1 M PB (see Recipe 3) up to 250 ml - Cryoprotectant solution: 30% sucrose with 0.1% sodium azide
- Prepare 0.01 M PBS (see Recipe 3)
- Prepare 20% sodium azide solution
2 g sodium azide
dH2O up to 10 ml - Prepare cryoprotectant solution
30 g sucrose
0.5 ml 20% sodium azide
0.01 M PBS up to 100 ml
- 96% and 70% ethanol
- 96% ethanol
480 ml 100% ethanol
20 ml dH2O - 70% ethanol
350 ml 100% ethanol
150 ml dH2O
- 96% ethanol
- 0.01% methylene blue in saline solution
2 mg methylene blue
20 ml saline solution 0.9% sodium chloride
Acknowledgments
“Instituto de Salud Carlos III”, “Redes temáticas de investigación cooperativa en salud–Red de trastornos adictivos” (#RD16/0017/0020), “Ministerio de Ciencia, Innovación y Universidades”, MCIU (#SAF2017-84060-R-AEI/FEDER-UE), “Generalitat de Catalunya- Agència de Gestió d'Ajuts Universitaris i de Recerca-AGAUR" (#2017-SGR-669 and #ICREA Acadèmia2015) to R.M. are acknowledged.
This protocol was adapted from an established published procedure to induce endometriosis to rats (Somigliana et al., 1999), and has already been used by our group (Escudero-Lara et al., 2020).
Competing interests
The authors declare no conflicts of interest.
Ethic
Animal experimentation: All animal procedures were conducted in accordance with standard ethical guidelines (European Communities Directive 2010/63/EU and NIH Guide for Care and Use of Laboratory Animals, 8th Edition) and approved by autonomic (Generalitat de Catalunya, Departament de Territori i Sostenibilitat) and local (Comitè Ètic d'Experimentació Animal, CEEA-PRBB) ethical committees.
References
- D'Hooghe, T. M., Bambra, C. S., Isahakia, M. and Koninckx, P. R. (1992). Evolution of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) over a 12-month period. Fertil Steril 58(2): 409-412.
- D'Hooghe, T. M., Bambra, C. S., Raeymaekers, B. M., De Jonge, I., Lauweryns, J. M. and Koninckx, P. R. (1995). Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am J Obstet Gynecol 173(1): 125-134.
- D'Hooghe, T. M., Kyama, C. M., Chai, D., Fassbender, A., Vodolazkaia, A., Bokor, A. and Mwenda, J. M. (2009). Nonhuman primate models for translational research in endometriosis. Reprod Sci 16(2): 152-161.
- Escudero-Lara, A., Argerich, J., Cabanero, D. and Maldonado, R. (2020). Disease-modifying effects of natural Delta9-tetrahydrocannabinol in endometriosis-associated pain. Elife 9: e50356.
- Falcone, T. and Flyckt, R. (2018). Clinical Management of Endometriosis. Obstet Gynecol 131(3): 557-571.
- Fattori, V., Franklin, N. S., Gonzalez-Cano, R., Peterse, D., Ghalali, A., Madrian, E., Verri, W. A., Jr., Andrews, N., Woolf, C. J. and Rogers, M. S. (2020). Nonsurgical mouse model of endometriosis-associated pain that responds to clinically active drugs. Pain 161(6): 1321-1331.
- Fourquet, J., Baez, L., Figueroa, M., Iriarte, R. I. and Flores, I. (2011). Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril 96(1): 107-112.
- Greaves, E., Critchley, H. O. D., Horne, A. W. and Saunders, P. T. K. (2017). Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet Gynecol Scand 96(6): 644-658.
- Grümmer, R. (2006). Animal models in endometriosis research. Hum Reprod Update 12(5): 641-649.
- Hadfield, R. M., Yudkin, P. L., Coe, C. L., Scheffler, J., Uno, H., Barlow, D. H., Kemnitz, J. W. and Kennedy, S. H. (1997). Risk factors for endometriosis in the rhesus monkey (Macaca mulatta): a case-control study. Hum Reprod Update 3(2): 109-115.
- Hull, M. L., Escareno, C. R., Godsland, J. M., Doig, J. R., Johnson, C. M., Phillips, S. C., Smith, S. K., Tavare, S., Print, C. G. and Charnock-Jones, D. S. (2008). Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol 173(3): 700-715.
- Márki, G., Bokor, A., Rigo, J. and Rigo, A. (2017). Physical pain and emotion regulation as the main predictive factors of health-related quality of life in women living with endometriosis. Hum Reprod 32(7): 1432-1438.
- Morton, D. B. and Griffiths, P. H. (1985). Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 116(16): 431-436.
- Somigliana, E., Vigano, P., Rossi, G., Carinelli, S., Vignali, M. and Panina-Bordignon, P. (1999). Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum Reprod 14(12): 2944-2950.
- Vernon, M. W. and Wilson, E. A. (1985). Studies on the surgical induction of endometriosis in the rat. Fertil Steril 44(5): 684-694.
- Zhao, Y., Chen, Y., Kuang, Y., Bagchi, M. K., Taylor, R. N., Katzenellenbogen, J. A. and Katzenellenbogen, B. S. (2016). Multiple Beneficial Roles of Repressor of Estrogen Receptor Activity (REA) in Suppressing the Progression of Endometriosis. Endocrinology 157(2): 900-912.
- Zondervan, K. T., Becker, C. M., Koga, K., Missmer, S. A., Taylor, R. N. and Vigano, P. (2018). Endometriosis. Nat Rev Dis Primers 4(1): 9.
Article Information
Copyright
Escudero-Lara et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Escudero-Lara, A., Cabañero, D. and Maldonado, R. (2020). Surgical Induction of Endometriosis in Female Mice. Bio-protocol 10(18): e3763. DOI: 10.21769/BioProtoc.3763.
- Escudero-Lara, A., Argerich, J., Cabanero, D. and Maldonado, R. (2020). Disease-modifying effects of natural Delta9-tetrahydrocannabinol in endometriosis-associated pain. Elife 9: e50356.
Category
Immunology > Animal model > Mouse
Cell Biology > Tissue analysis > Physiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








