- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Expression and Purification of Arabidopsis Transmembrane Protein BCM1 in Saccharomyces cerevisiae
Published: Vol 10, Iss 18, Sep 20, 2020 DOI: 10.21769/BioProtoc.3758 Views: 4665
Reviewed by: ANSUL LOKDARSHIRunlai HangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Proteoliposomes for Studying Lipid-protein Interactions in Membranes in vitro
Helmut Kirchhoff
Oct 20, 2021 3354 Views

A Novel Method for Measuring Mitochondrial Respiratory Parameters in Wheat Paleae (Paleae Superior) Using the XF24 Analyzer
Daniel Schniertshauer and Jörg Bergemann
Aug 5, 2023 1352 Views

Seed Collection in Temperate Trees—Clean, Fast, and Effective Extraction of Populus Seeds for Laboratory Use and Long-term Storage
Naima Bhutta [...] Katharina Bräutigam
Feb 5, 2024 2037 Views
Abstract
Heterologous expression and purification of transmembrane proteins have remained a challenge for decades hampering detailed biochemical and structural characterization of key enzymes and their interacting regulators in multiple metabolic pathways. An in-depth study on the newly identified Arabidopsis thaliana integral membrane protein BALANCE OF CHLOROPHYLL METABOLISM 1 (BCM1) showed a stimulatory effect of the BCM1 on magnesium chelatase, the first enzyme of chlorophyll biosynthesis, through interaction with the GENOMES UNCOUPLED 4 (Wang et al., 2020). Here, we report a detailed and optimized method for heterologous expression and purification of His-tagged BCM1 in Saccharomyces cerevisiae. Following this method, we obtained native BCM1 used for in vitro enzymatic assay of magnesium chelatase (Wang et al., 2020). Currently, the crystallization studies of the BCM1 are underway. This protocol could be adapted to purify BCM1-like transmembrane proteins from eukaryotic organisms for enzymatic and structural studies.
Keywords: A transmembrane proteinBackground
Identification of post-translational regulators which directly modulate the enzymatic activities of chlorophyll synthesis enzymes can greatly improve our understanding of molecular mechanisms, by which plants maintain highly efficient chlorophyll biosynthesis during leaf greening (Brzezowski et al., 2015). However, the detailed biochemical analyses of chlorophyll synthesis enzymes and their interacting proteins have been restricted by the availability of recombinant proteins in vitro. We recently identified a post-translational regulator BALANCE OF CHLOROPHYLL METABOLISM 1 (BCM1), which simultaneously stimulates chlorophyll biosynthesis and delays chlorophyll breakdown, thereby conferring chlorophyll homeostasis during leaf development (Wang et al., 2020). To examine BCM1’s effect on the enzymatic activity of magnesium chelatase (MgCh), the first enzyme of chlorophyll biosynthesis, we expressed and purified His-tagged BCM1 in S. cerevisiae. It has been shown that BCM1 is able to stimulate MgCh activity in vitro (Wang et al., 2020). Because BCM1 has six transmembrane domains, BCM1 will be used an example of multiple-pass protein herein. Thus, we provide an optimized method for expression and purification of BCM1 in S. cerevisiae. Although prokaryotic purification system hosted by Escherichia coli has been widely used to express hydrophilic proteins from prokaryotic and eukaryotic organisms, overexpression of BCM1 in Escherichia coli cells leads to accumulation of BCM1 aggregate and inclusion bodies instead of properly folded proteins at the membranes. In comparison with cell-free protein expression systems and other eukaryotic expression systems, such as mammalian and insect expression systems, the yeast protein expression method described here enables a large-scale purification of integral membrane proteins with high yield and low cost.
Materials and Reagents
- Sterile pipette tips
- Eppendorf microcentrifuge tubes 1.5 ml (Eppendorf, catalog number: 00 30121694 )
- Falcon, conical centrifuge tubes 50 ml (Corning, catalog number: 14-432-22 )
- 0.45 μm filter (VWR, catalog number: 28145-479 )
- 14 ml Open-top thinwall untra-clear centrifuge tube (Beckman Coulter, catalog number: 344060 )
- Dispensable plastic spin column (Thermo Fisher Scientific, catalog number: 10220544 )
- SnakeSkin Dialysis Tubing 10 kDa cut-off (Thermo Fisher Scientific, catalog number: 68100 )
- Saccharomyces cerevisiae L40ccua strain
- pDR296-His-BCM1 plasmid
- Salmon sperm (Thermo Fisher Scientific, catalog number: 15632011 )
- Demineralized water
- Bacto yeast extract (Cal Roth, catalog number: 2904 )
- Bacto peptone (Cal Roth, catalog number: 8952 )
- Glucose monohydrate (Cal Roth, catalog number: 6780 )
- Adenine sulfate (Sigma-Aldrich, catalog number: A3159 )
- Agar-Y (MP Biomedicals, catalog number: 4019012 )
- Yeast nitrogen base (YNB) with ammonium sulfate (MP Biomedicals, catalog number: 4027412 )
- Synthetic dropout/-tryptophan (SD/-Trp) (MP Biomedicals, catalog number: 4511012 )
- Glycerol (Sigma-Aldrich, catalog number: G5516 )
- n-dodecyl-β-D-maltoside (β-DM) (Sigma-Aldrich, catalog number: D4641 )
- Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA·Na2) (Sigma-Aldrich, catalog number: ED2SS )
- Tris(hydroxymethyl)aminomethane (Tris) (Cal Roth, catalog number: A411 )
- Hydrochloric acid (HCl) (VWR, catalog number: BDH7204 )
- Sodium hydroxide (NaOH) (Fisher Scientific, catalog number: BP359-212 )
- Lithium acetate (LiAc) (Cal Roth, catalog number: 5447 )
- Polyethylene glycol 4000 (PEG4000) (Sigma-Aldrich, catalog number: 8.0749 0)
- Liquid nitrogen
- cOmplete, mini, EDTA-free protease inhibitor tablets (Roche Diagnostics, catalog number: 11873580001 )
- Glass beads (425-600 μm) (Sigma-Aldrich, catalog number: G8772 )
- Sodium chloride (NaCl) (Cal Roth, catalog number: 9265 )
- Potassium chloride (KCl) (Cal Roth, catalog number: 6781 )
- Sodium phosphate monobasic dihydrate (NaH2PO4·2H2O) (Cal Roth, catalog number: T879 )
- Potassium dihydrogen phosphate (KH2PO4) (Cal Roth, catalog number: 3904 )
- Ni-NTA agarose resin (Thermo Fisher Scientific, catalog number: 88223 )
- Imidazole (Sigma-Aldrich, catalog number: I2399 )
- YPD medium (930 ml) (see Recipes)
- 40% (w/v) glucose (100 ml) (see Recipes)
- 0.2% (w/v) Adenine sulfate (see Recipes)
- YPDA medium (1 L) (see Recipes)
- SD/-Trp medium (1 L) (see Recipes)
- 80% (v/v) glycerol (100 ml) (see Recipes)
- 10x Tris-EDTA (TE) buffer (200 ml) (see Recipes)
- 1 M LiAc (200 ml) (see Recipes)
- 50% (w/v) PEG4000 (100 ml) (see Recipes)
- 10x phosphate-buffered saline (PBS) buffer (1 L) (see Recipes)
- 10% (w/v) β-DM (see Recipes)
- 1x TE buffer (see Recipes)
- TE/LiAc buffer (see Recipes)
- Polyethylene glycol (PEG)/LiAc buffer (see Recipes)
- Solubilization buffer (10 ml) containing 1% (w/v) β-DM (see Recipes)
- Wash buffer containing 0.0256% (w/v) β-DM and 20 mM imidazole (20 ml) (see Recipes)
- Elute buffer containing 0.0256% (w/v) β-DM (see Recipes)
- Dialysis buffer (1 L) containing 0.0256% (w/v) β-DM (see Recipes)
Equipment
- Glassware
250 ml Erlenmeyer flask
200 ml Erlenmeyer flask
2 L Erlenmeyer flask
1 L Erlenmeyer flask
100 ml Erlenmeyer flask
1 L transparent glass media bottle
250 ml transparent glass media bottle
100 ml transparent glass media bottle - Rotor JA-25.50
- Rotor FA-48-45-11
- Milli-Q Reference Water Purification System (EMD Millipore, catalog number: Z00QSV0WW )
- Clean bench (Labconco, model: 3440009 )
- Water bath
- Temperature controlled shaker incubator
- Vortex mixer (Scientific Industries, model: Vortex-Genie 2 )
- Cold room
- Deep freezer (-20 °C)
- Ultra-low temperature freezer (-80 °C)
- Centrifuge (Eppendorf, model: 5430 R )
- High speed centrifuge (Beckman Coulter, model: Avanti J-26XP )
- Ultracentrifuge (Beckman Coulter, model: Optima L-80 XP )
Procedure
- Transformation of S. cerevisiae with pDR296-His-BCM1 plasmid
- The nucleotide sequences encoding mature BCM1 (amino acid 55-382) with depletion of N-terminal chloroplast transit peptide from Arabidopsis is cloned into the yeast expression vector pDR296. The construct contains an N-terminal histidine (His) tag to facilitate purification of BCM1 by means of immobilized nickel-affinity chromatography (Figure 1).
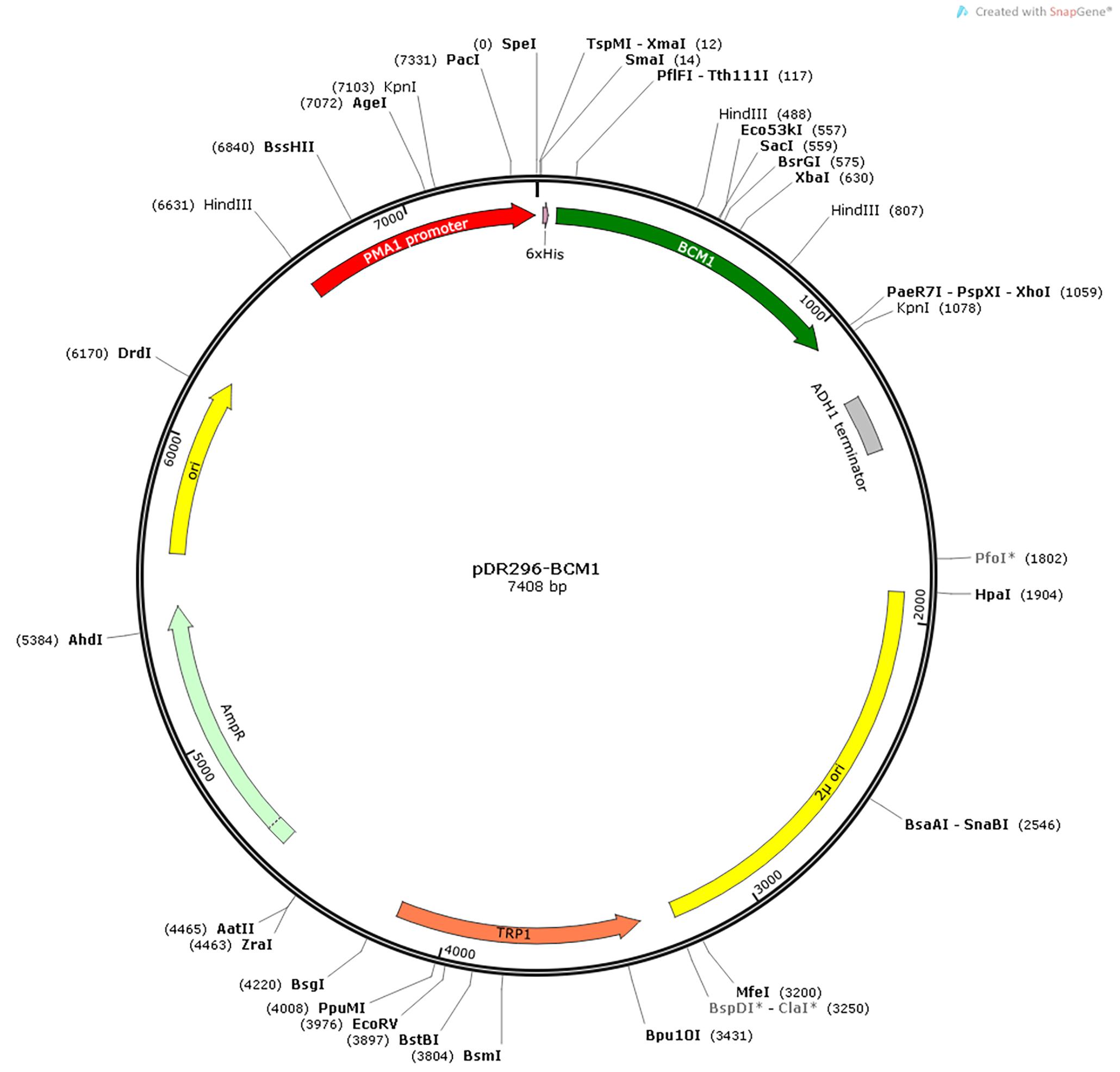
Figure 1. Plasmid map of pDR296-BCM1. Unique restriction sites and important features in the plasmids are indicated. PMA1 promoter, transcription promoter for the S. cerevisiae plasma membrane ATPase 1 (PMA1) gene. ADH1 terminator, transcription terminator for the S. cerevisiae alcohol dehydrogenase 1 (ADH1) gene. 2μ ori, yeast 2μ plasmid origin of replication. TRP1, tryptophan biosynthesis protein. AmpR, the gene encoding β-lactamase, which confer resistance to ampicillin. - Streak out a glycerol stock of S. cerevisiae L40ccua on a YPDA agar plate and incubate the plate at 30 °C for two days.
- Inoculate 5 ml of YPDA medium with 1 colony of S. cerevisiae L40ccua in a 14 ml Falcon tube and grow at 30 °C and 250 rpm overnight.
- Transfer the overnight culture to 100 ml YPDA medium in a 250 ml Erlenmeyer flask and continue to grow at 30 °C and 250 rpm for 2-4 h until the optical density at 600 nm (OD600) of the culture reaches between 0.4-0.6.
- Harvest cells by centrifugation at 1,000 x g for 5 min at room temperature using rotor JA-25.50.
- Resuspend cells in demineralized water by vortexing for 5-10 s and harvest then cells by centrifugation at 1,000 x g for 5 min at room temperature using rotor JA-25.50.
- Resuspend cells in 1.5 ml TE/LiAc buffer (the cell titer is ~3 x 109 cells/ml). These are now yeast competent cells.
- Add 0.1-0.2 μg pDR296-His-BCM1 plasmids, 10 μl of denatured salmon sperm DNA, and 100 μl of yeast competent cells to a 1.5 ml Eppendorf tube and mix them well by pipetting for 2-3 times.
- Add 600 μl of freshly prepared PEG/LiAc buffer to the tube and mix them well by vortex for 5 s.
- Incubate the mixture at 30 °C for 30 min with shaking at 200 rpm.
- Add 70 μl of DMSO to the culture and mix well by gentle inversion.
- Heat the tube in a 42 °C water bath for 15 min, do not shake.
- Place the tube on ice for 1-2 min.
- Pellet cells by centrifugation at 12,000 x g for 5 s at room temperature using rotor FA-48-45-11.
- Resuspend cells in demineralized water and harvest then cells by centrifugation at 12,000 x g for 5 s at room temperature using rotor FA-48-45-11.
- Resuspend cells in 200 μl of 1x TE buffer and spread 100 μl of the transformation mixture on a SD/-Trp agar plate.
- Incubate the agar plate at 30 °C for 2-4 days until emergence of colonies.
- Pick a single colony from the transformation plate using a sterile pipette tip and inoculate 5 ml of YPDA medium in a 14 ml Falcon tube at 30 °C and 250 rpm overnight.
- Add 250 μl of 80% (v/v) glycerol to 750 μl of the overnight culture in a 1.5 ml Eppendorf tube and mix well by vortexing for 5 s.
- Flash freeze the tube in liquid nitrogen and store the glycerol stock at -80 °C for future use.
- The nucleotide sequences encoding mature BCM1 (amino acid 55-382) with depletion of N-terminal chloroplast transit peptide from Arabidopsis is cloned into the yeast expression vector pDR296. The construct contains an N-terminal histidine (His) tag to facilitate purification of BCM1 by means of immobilized nickel-affinity chromatography (Figure 1).
- Large scale expression of His-BCM1 in S. cerevisiae
- Streak out a glycerol stock of transformed S. cerevisiae L40ccua on a SD/-Trp agar plate and incubate the plate at 30 °C for two days.
- Inoculate 50 ml of SD/-Trp medium with 2-3 colonies of S. cerevisiae L40ccua from Step B1 in a 200 ml Erlenmeyer flask and grow at 30 °C and 250 rpm overnight.
- Transfer the overnight culture to 1 L of SD/-Trp medium in a 2 L Erlenmeyer flask and grow at 30 °C and 250 rpm for 3-4 h until the OD600 of the culture reaches between 0.4-0.6.
- Harvest cells by centrifugation at 1,000 x g for 5 min at 4 °C using rotor FA-48-45-11.
- Flash freeze the pellet in liquid nitrogen and store at -80 °C for future use.
- Analyze the expression of His-BCM1 in S. cerevisiae cells by immunoblotting (Figure 2).
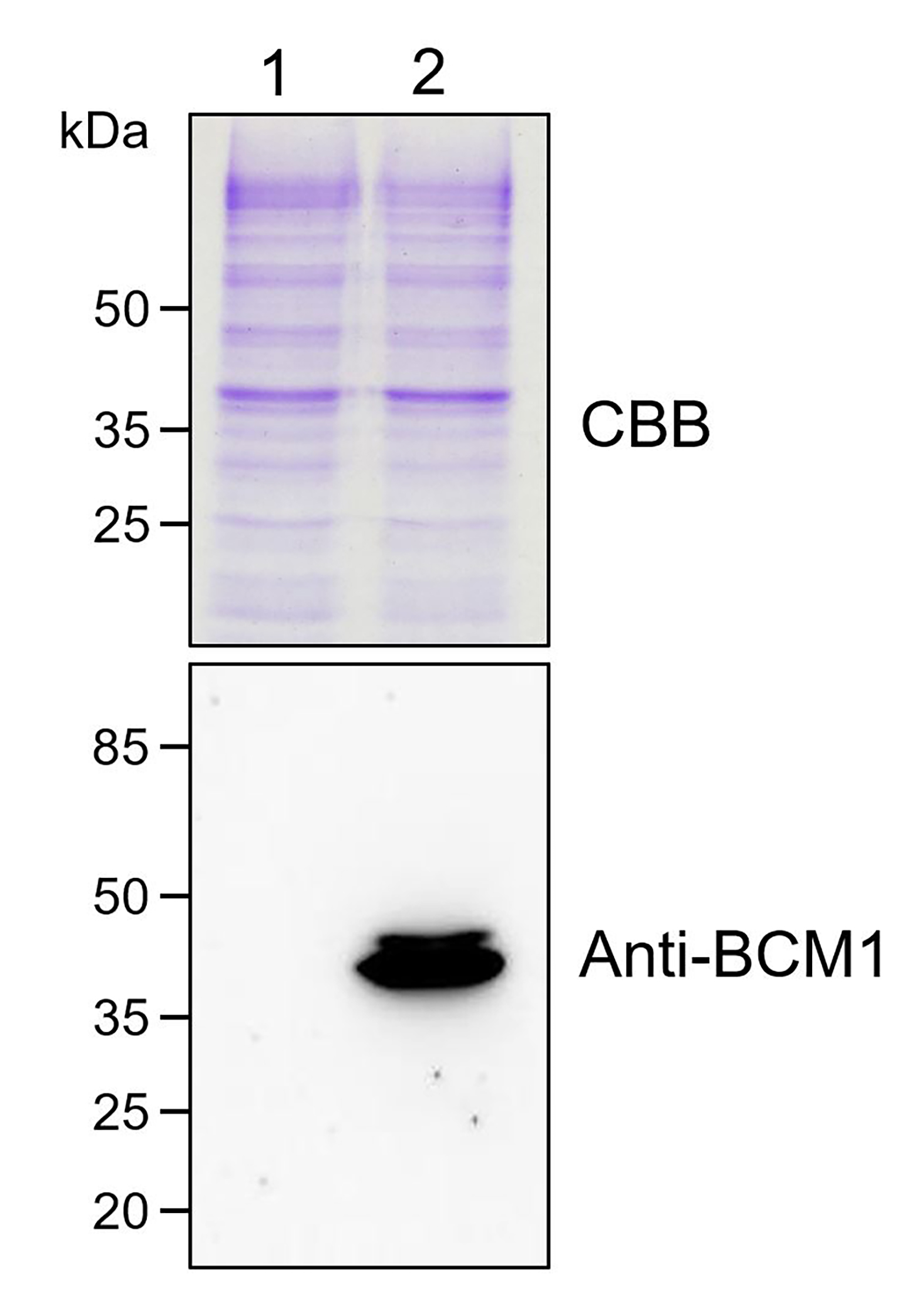
Figure 2. Immunoblotting analysis of His-BCM1 expression in S. cerevisiae. Lane 1: total lysate of untransformed S. cerevisiae cells. Lane 2: total lysate of transformed S. cerevisiae cells. Total yeast proteins were stained with Coomassie Brilliant Blue (CBB, upper panel) and probed with BCM1 antibody (lower panel), respectively. The immunoblotting analysis was conducted as described previously (Wang et al., 2020).
- Purification of His-BCM1 from total yeast membrane extracts
- Thaw the S. cerevisiae cell pellet obtained in Step B5 on ice.
- Resuspend the cells in chilled 20 ml of 1x phosphate-buffered saline (PBS) buffer supplemented with 2 tablets of protease inhibitor.
- Add 3 g chilled glass beads and vortex at maximum speed 5-7 times for 1 min, each time keeping the cells on ice for 1 min between vortexing.
- Remove unbroken yeast cells and glass beads by centrifugation at 1,000 x g for 5 min at 4 °C using rotor JA-25.50.
- Transfer supernatant to a clean 14-ml ultracentrifuge tube using a plastic pipette and pellet total yeast membranes by ultracentrifugation at 40,000 x g for 30 min at 4 °C using rotor SW 40-Ti.
- Resuspend the yeast membrane pellet in 10 ml of chilled solubilization buffer by gentle pipetting for 5-10 times.
- Incubate the suspension on ice at 100 rpm for 30 min.
- Pellet insoluble debris by centrifugation at 40,000 x g for 30 min at 4 °C using rotor SW 40-Ti and transfer the supernatant to a clean 50 ml tube.
- Add 1 ml of the Ni-NTA agarose resin (pre-equilibrated with solubilization buffer) in the supernatant and mix on a roller mixer at 4 °C for 1 h.
- Transfer the suspension to a 10 ml disposable plastic spin column and let the suspension run through by gravity.
- Wash the Ni-NTA agarose resin with 10 ml of wash buffer and let the wash buffer run through by gravity.
- Incubate Ni-NTA agarose resin with 2 ml of elution buffer on ice for 1-2 min and collect then flow through.
- Remove aggregates in the elute by centrifugation at 20,000 x g for 10 min at 4 °C using rotor FA-48-45-11.
- Dialyse the elute against 1 L of dialysis buffer at 4 °C for 1 h. Change the dialysis buffer and dialyse at 4 °C overnight.
- Transfer the elute to clean 1.5 ml Eppendorf tubes.
- Add 50 μl of glycerol to 1 ml of the elute and mix them well by gentle pipetting for 3-5 times.
- Divide the protein solution into 100 μl aliquots on ice.
- Flash freeze the aliquots in liquid nitrogen and store at -80 °C for future use.
- Analyze the purified His-BCM1 protein using 12% SDS-PAGE (Figure 3).
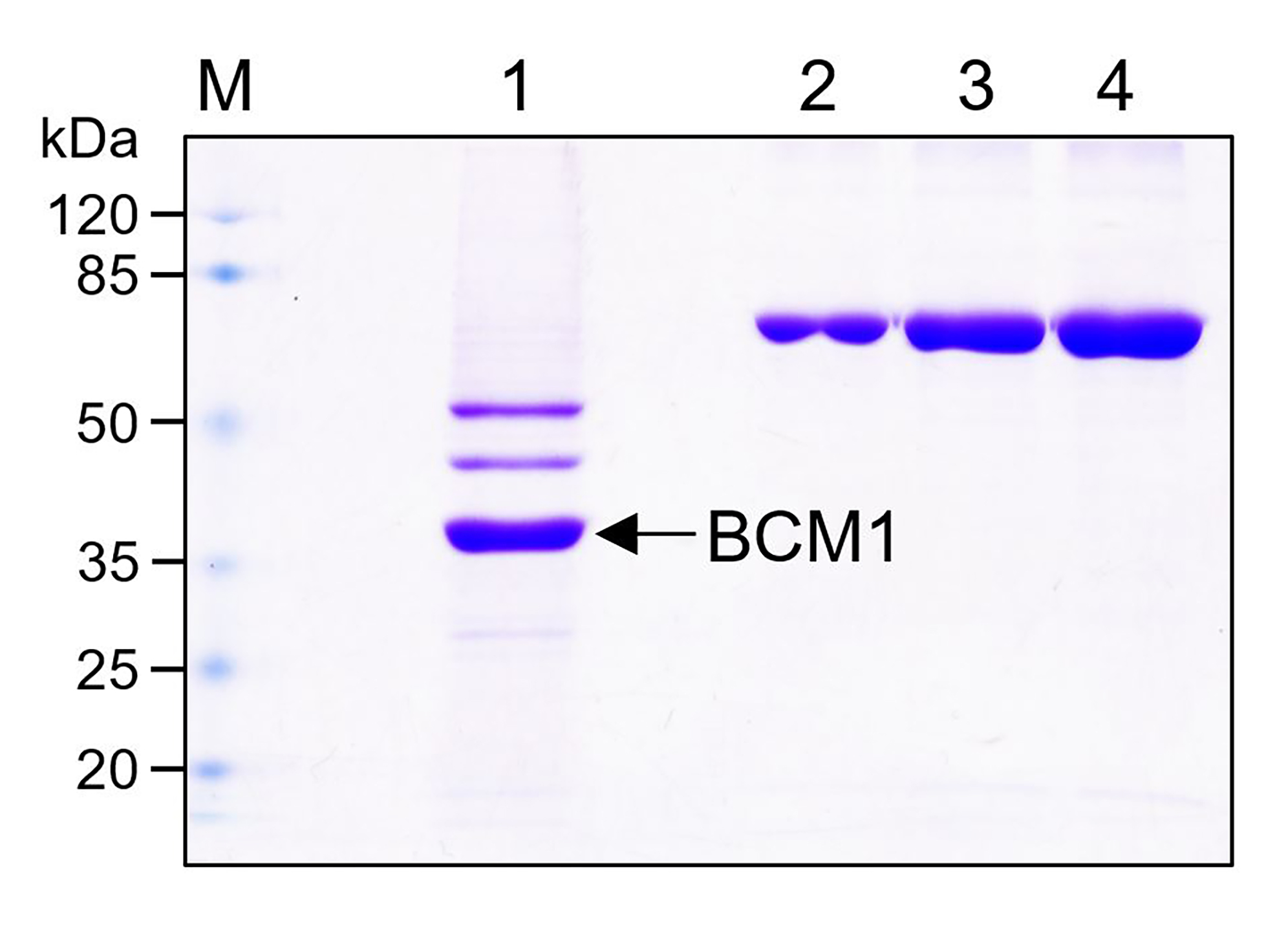
Figure 3. SDS-PAGE analysis of purified His-BCM1. Lane M: protein marker; Lane 1: Purified His-BCM1 (approximately 36.5 kDa) indicated by the arrow; Lanes 2-4: 0.5, 1, and 2 μg bovine serum albumin standard.
Notes
- It is necessary to boil salmon sperm DNA at 100 °C for 5 min and chill immediately on ice before use.
- To induce the expression of pDR296-His-BCM1 in S. cerevisiae, we use the SD/-Trp medium instead of YPDA medium.
Recipes
- Media and stock solution
- YPD medium (930 ml)
- Dissolve 10 g Bacto yeast extract and 20 g Bacto peptone in 930 ml of demineralized water in a 1 L transparent glass media bottle
- Add 20 g Agar-Y only for making solid agar plates
- Autoclave at 121 °C for 20 min on a liquid cycle
- Store for up to 6 months at room temperature
- Dissolve 10 g Bacto yeast extract and 20 g Bacto peptone in 930 ml of demineralized water in a 1 L transparent glass media bottle
- 40% (w/v) glucose (100 ml)
- Dissolve 40 g glycerol in 40 ml of demineralized water in a 50 °C water bath in a 100 ml Erlenmeyer flask
- Fill up to 100 ml with demineralized water
- Sterilize by filtering through a 0.45 μm filter
- Store for up to 1 month at 4 °C
- 0.2% (w/v) Adenine sulfate
- Dissolve 0.1 g Adenine sulfate in 50 ml of demineralized water in a 100 ml Erlenmeyer flask
- Sterilize by filtering through a 0.45 μm filter
- This solution is freshly prepared
- Dissolve 0.1 g Adenine sulfate in 50 ml of demineralized water in a 100 ml Erlenmeyer flask
- YPDA medium (1 L)
- Add 50 ml of 40% glucose and 20 ml of 0.2% (w/v) Adenine sulfate to 930 ml of YPD medium under aseptic conditions in a 1 L sterilized Erlenmeyer flask
- This medium is freshly prepared
- Add 50 ml of 40% glucose and 20 ml of 0.2% (w/v) Adenine sulfate to 930 ml of YPD medium under aseptic conditions in a 1 L sterilized Erlenmeyer flask
- SD/-Trp medium (1 L)
- Dissolve 20 mg SD/-Trp and 6.7 g YNB in 950 ml of demineralized water in a 1 L transparent glass media bottle
- Adjust to pH 5.8 with NaOH
- Add 20 g Agar-Y only for making solid agar plates
- Autoclave at 121 °C for 20 min on a liquid cycle
- Add 50 ml of 40% (w/v) glucose 950 ml of the medium under aseptic conditions
- Store for up to 6 months at room temperature
- Dissolve 20 mg SD/-Trp and 6.7 g YNB in 950 ml of demineralized water in a 1 L transparent glass media bottle
- 80% (v/v) glycerol (100 ml)
- Add 80 ml of glycerol to 20 ml of demineralized water in a 100 ml transparent glass media bottle
- Autoclave at 121 °C for 20 min on a liquid cycle
- Store for up to 6 months at room temperature
- Add 80 ml of glycerol to 20 ml of demineralized water in a 100 ml transparent glass media bottle
- 10x TE buffer (200 ml)
- Dissolve 2.42 g Tris and 0.744 g EDTA·Na2 in 160 ml of demineralized water in a 250 ml transparent glass media bottle
- Adjust to pH 8.0 with hydrochloric acid
- Fill up to 200 ml with demineralized water
- Sterilize by filtering through a 0.45 μm filter
- Store for up to 6 months at room temperature
- Dissolve 2.42 g Tris and 0.744 g EDTA·Na2 in 160 ml of demineralized water in a 250 ml transparent glass media bottle
- 1 M LiAc (200 ml)
- Dissolve 13.2 g LiAc in 160 ml of demineralized water in a 250 ml transparent glass media bottle
- Adjust to pH 7.5 with acetic acid
- Fill up to 200 ml with demineralized water
- Sterilize by filtering through a 0.45 μm filter
- Store for up to 6 months at room temperature
- Dissolve 13.2 g LiAc in 160 ml of demineralized water in a 250 ml transparent glass media bottle
- 50% (w/v) PEG4000 (100 ml)
- Dissolve 50 g PEG4000 in 50 ml of demineralized water in a 50 °C water bath in a 100 ml transparent glass media bottle
- Fill up to 100 ml with demineralized water
- Sterilize by filtering through a 0.45 μm filter
- Store for up to 3 months at room temperature
- Dissolve 50 g PEG4000 in 50 ml of demineralized water in a 50 °C water bath in a 100 ml transparent glass media bottle
- 10x PBS buffer (1 L)
- Dissolve 80 g NaCl, 2 g KCl, 15.6 g NaH2PO4·2H2O and 2.4 g KH2PO4 in 800 ml of demineralized water in a 1 L transparent glass media bottle
- Adjust to pH 7.4
- Fill up to 1 L with demineralized water
- Autoclave at 121 °C for 20 min on a liquid cycle
- Store for up to 6 months at room temperature
- Dissolve 80 g NaCl, 2 g KCl, 15.6 g NaH2PO4·2H2O and 2.4 g KH2PO4 in 800 ml of demineralized water in a 1 L transparent glass media bottle
- 10% (w/v) β-DM
- Dissolve 0.1 g β-DM in 1 ml of demineralized water in a 1.5 ml Eppendorf tube
- Store for up to 6 months at -20 °C
- Dissolve 0.1 g β-DM in 1 ml of demineralized water in a 1.5 ml Eppendorf tube
- YPD medium (930 ml)
- Buffer
- 1x TE buffer (100 ml)
- Add 10 ml of 10x TE buffer to 90 ml of demineralized water under aseptic conditions in a 100 ml transparent glass media bottle
- This buffer is freshly prepared
- Add 10 ml of 10x TE buffer to 90 ml of demineralized water under aseptic conditions in a 100 ml transparent glass media bottle
- TE/LiAc buffer (100 ml)
- Add 10 ml of 10x TE buffer and 10 ml of 1 M LiAc to 90 ml of demineralized water in a 100 ml transparent glass media bottle under aspartic conditions
- This buffer is freshly prepared
- Add 10 ml of 10x TE buffer and 10 ml of 1 M LiAc to 90 ml of demineralized water in a 100 ml transparent glass media bottle under aspartic conditions
- PEG/LiAc buffer (10 ml)
- Add 1 ml of 10x TE buffer and 1 ml of 1 M LiAc to 8 ml of 50% (w/v) PEG4000 in a 14 ml Falcon tube under aspartic conditions
- This buffer is freshly prepared
- Add 1 ml of 10x TE buffer and 1 ml of 1 M LiAc to 8 ml of 50% (w/v) PEG4000 in a 14 ml Falcon tube under aspartic conditions
- Solubilization buffer (10 ml) containing 1% (w/v) β-DM
- Add 5 ml of 10% (w/v) β-DM to 45 ml of 1x PBS buffer in a 100 ml transparent glass media bottle
- This buffer is freshly prepared
- Add 5 ml of 10% (w/v) β-DM to 45 ml of 1x PBS buffer in a 100 ml transparent glass media bottle
- Wash buffer containing 0.0256% (w/v) β-DM and 20 mM imidazole (20 ml)
- Dissolve 27.2 mg imidazole to 20 ml of 1x PBS buffer, supplemented with 51.2 μl of 10% (w/v) β-DM in a 50 ml transparent glass media bottle
- This buffer is freshly prepared
- Dissolve 27.2 mg imidazole to 20 ml of 1x PBS buffer, supplemented with 51.2 μl of 10% (w/v) β-DM in a 50 ml transparent glass media bottle
- Elution buffer containing 0.0256% (w/v) β-DM and 300 mM imidazole (20 ml)
- Dissolve 0.408 g imidazole to 20 ml of 1x PBS buffer, supplemented with 51.2 μl of 10% (w/v) β-DM in a 50 ml transparent glass media bottle
- This buffer is freshly prepared
- Dissolve 0.408 g imidazole to 20 ml of 1x PBS buffer, supplemented with 51.2 μl of 10% (w/v) β-DM in a 50 ml transparent glass media bottle
- Dialysis buffer (1 L) containing 0.0256% (w/v) β-DM
- Dissolve 0.256 g β-DM in 1 L of 1x PBS buffer in a 1 L transparent glass media bottle
- This buffer is freshly prepared
- Dissolve 0.256 g β-DM in 1 L of 1x PBS buffer in a 1 L transparent glass media bottle
- 1x TE buffer (100 ml)
Acknowledgments
This work was funded by the Alexander von Humboldt Foundation to P.W., and the Deutsche Forschungsgemeinschaft to P. W. (WA 4599/2-1), and to B.G. (FOR2092, GR 936/18-1 and SFB TR175, subproject C04). This protocol was adapted from a previously described method (Wang et al., 2020).
Competing interests
We declare no conflicting or competing interests.
References
- Brzezowski, P., Richter, A. S. and Grimm, B. (2015). Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta 1847(9): 968-985.
- Wang, P., Richter, A. S., Kleeberg, J. R. W., Geimer, S. and Grimm, B. (2020). Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat Commun 11(1): 1254.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, P. and Grimm, B. (2020). Expression and Purification of Arabidopsis Transmembrane Protein BCM1 in Saccharomyces cerevisiae. Bio-protocol 10(18): e3758. DOI: 10.21769/BioProtoc.3758.
Category
Plant Science > Plant physiology > Metabolism
Biochemistry > Protein > Expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









