- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunohistochemistry of Kidney a-SMA, Collagen 1, and Collagen 3, in A Novel Mouse Model of Reno-cardiac Syndrome
Published: Vol 10, Iss 18, Sep 20, 2020 DOI: 10.21769/BioProtoc.3751 Views: 4400
Reviewed by: Xiaoyi ZhengFereshteh AzediAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views

Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood
Ilyssa E. Ramos [...] James M. Cherry
Nov 20, 2025 2336 Views
Abstract
Cardiorenal syndrome defines a synergistic pathology of the heart and kidneys where failure of one organ causes failure in the other. The incidence of cardiovascular mortality caused by this syndrome, is 20 fold higher in the end stage renal disease (ESRD) population compared to the population as a whole thus necessitating the need for improved therapeutic strategies to combat reno-cardiac pathologies.
Murine in vivo models play a major role in such research permitting precise genetic modification thus reducing miscellany, however presently there is no steadfast model of reno-cardiac syndrome in the most common genetically modified mouse strain, the C57BL/6 mouse. In this study we have modified an established model of chronic renal disease using adenine diet and extended the associated pathology achieving chronic renal failure and consequent reno-cardiac syndrome in the C57BL/6 mouse.
Eight week-old male C57BL/6 mice were acclimatized for 7 days before administration of a 0.15% adenine diet or control diet for 20 weeks after which the experiment was terminated and blood, urine and organs were collected and analyzed biochemically and by immunohistochemistry.
Administration of 0.15% adenine diet caused progressive renal failure resulting in a reno-cardiac syndrome confirmed by a significantly increased heart to body weight ratio (P < 0.0001). Blood biochemistry showed that adenine fed mice had significantly increased serum creatinine, urea (P < 0.0001), and a significantly reduced glomerular filtration rate (P < 0.05), while immunohistochemistry of the kidneys for α-SMA, collagen 1 and collagen 3 showed severe fibrosis.
We present a novel regimen of adenine diet which induces both chronic kidney disease and reno-cardiac syndrome in the C57BL/6 mouse strain. The non-surgical nature of this model makes it highly reproducible compared to other models currently available.
Background
Chronic kidney disease (CKD) results in vascular and cardiac dysfunction, termed cardio renal syndrome, defined more specifically as reno-cardiac syndrome (RCS) by Ronco et al. (2008). RCS is a predominant feature of end stage renal disease (ESRD) resulting in a 20 fold higher incidence of cardiovascular mortality (compared to the normal population as a whole) and cardiovascular events account for approximately 50% of mortality in ESRD patients (de Jager et al., 2009; Steenkamp et al., 2013). As the ESRD population is increasing worldwide research into novel therapies to combat RCS is vital.
Animal models have significantly aided research into many disease pathologies and the development of technology to genetically modify strains has enabled such models to become even more potent through better targeting of pathological genes.
The sub-total nephrectomy model has been up till now the most successful model of RCS and involves the surgical removal or negation of (5/6ths) of the kidney mass (Morrison, 1962). The resulting renal ischaemia/reduced nephron mass initiates hypertension and subsequent left ventricular hypertrophy (LVH) leading to progressive left ventricular dilatation (Kumar et al., 2014). In parallel, fibroblast activation leads to cardiac hypertrophy and fibrosis (Hewitson, 2012; Bursac, 2014). The disadvantages of this model is that it requires surgical expertise, expensive surgical facilities and that it is prone to inter and intra operator variability. The preferred rodent used in this model is the rat because in mice, this model is much less effective giving highly variable results (Hewitson et al., 2015). Further to this the C57BL/6 mouse strain, the preferred strain for genetic modification (Seong et al., 2004) has been reported to be resistant to the development of CKD by subtotal nephrectomy (Kren and Hostetter, 1999).
An alternative model of CKD is adenine induced. This non-surgical and therefore highly reproducible method was first described by Yokozawa et al. (1982), who noted that the metabolism of adenine differed from that of other purines and that it was nephrotoxic. However this chemical method although very successful in rats, has not flourished in mice because they do not like the adenine taste resulting in rapid weight loss leading to mortality after 4 weeks. Jia et al. (2013), improved the palatability of the diet by the addition of casein, extending the course of the administration to 8 weeks however this is not long enough to achieve RCS.
We found that we could conceal the adenine taste (without the need for addition of casein), by decreasing the concentration of the diet to 0.15% and by beginning its administration when the mice reached 9 weeks of age, extended the course of the regimen to 20 weeks in order to achieve CKD and subsequently RCS as seen by blood biochemistry and heart/bodyweight ratio data (Table 1), immunoblotting and immunohistochemistry.
Therefore we succeeded in developing a highly reproducible model which does not require surgical expertise or facilities (Kieswich et al., 2018).
Although we did not measure blood pressure (BP) we predict that the main cause of the RCS was haemodynamic due to kidney dysfunction and therefore, if BP was measured consciously, e.g., by a telemetric method, this could also prove to be a successful non-surgical model of hypertension.
Table 1. Results showing evidence of RCS. Control group (n = 6) received standard chow for 20 weeks. Adenine treated group (n = 10) received standard chow with the addition of 0.15% adenine for 20 weeks. Compared with animals receiving normal chow, a 20-week diet of 0.15% adenine caused significant increases in plasma urea (P < 0.0001) and creatinine (P < 0.0001), a significantly reduced glomerular filtration rate (GFR) (P < 0.05), and a significantly greater heart weight-to body weight ratio (P < 0.0001).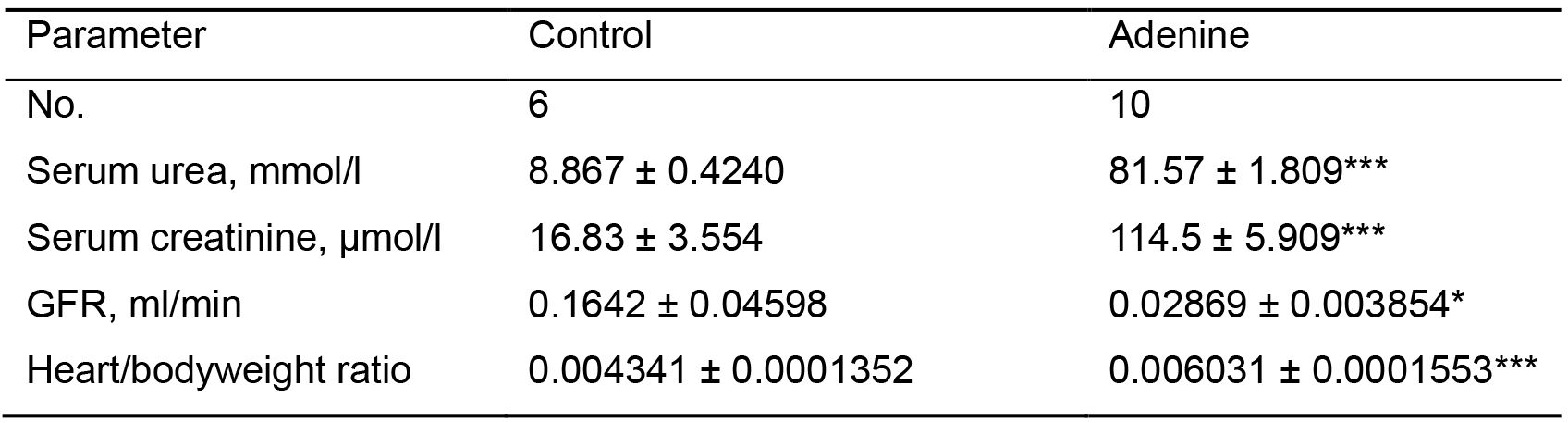
***P < 0.0001,*P < 0.05
Materials and Reagents
- 25 gauge needles (Fisher Scientific, catalog number: 10442204 )
- 1 ml syringes (Fisher Scientific, catalog number: 15849152 )
- Eppendorf tubes (Fisher Scientific, catalog number: 13094697 )
- Embedding cassettes (Fisher Scientific, Simport Acetal Histology Cassettes, catalog number: 10420823 )
- Glass slides (Fisher Scientific, catalog number: 12343138 )
- Pipettes and tips
ErgoOne Single-Channel Pipettes 20-200 µl (STARLAB (UK) Ltd, catalog number: S7100-2200 )
ErgoOne Single-Channel Pipettes 100-1,000 µl (STARLAB (UK) Ltd, catalog number: S7110-1000 )
Yellow Tips (Sterile), Racked (STARLAB (UK) Ltd, catalog number: S1111-0816-C )
1,000 µl Blue Graduated Tip (Sterile), Racked (STARLAB (UK) Ltd, catalog number: S1111-6811-C ) - Hydrophobic barrier pen (Vector Laboratories, ImmEdge Hydrophobic Barrier Pen, catalog number: H-4001 )
- Humidified chamber e.g., a plastic box with airtight lid (prevents tissue from drying out). Place wet tissue paper into the bottom of the box. To keep slides off the wet paper insert a perforated base (can be made by cutting pasteur pipettes into equal size pieces and taping them into a grid formation. Ensure it is level)
- Tissue paper
- Vessel with slide rack to hold approximately 400-500 ml
- Brush (for cleaning microtome blade between samples)
- Glass coverslips (Fisher Scientific, catalog number: 12343138 )
- 8-week old, male C57BL/6 mice (n = 6 per group) (Charles River, Margate, UK)
- 20 Kg Adenine diet (LBS-Biotech, Hookwood, UK, SDS diets, catalog number: 824534 ), RM1 + 0.15% Adenine, storage temperature 4 °C
Note: Buy 20 Kg 824534 RM1 diet (normal adenine concentration) from SDS diets at same time to use as control). For a detailed composition of the diet see attached file ‘Adenine diet composition’. - Ketamine 100 mg/ml solution (Narketan, MWI Animal Health, Somerset, UK, catalog number: 0 3120257 ), storage temperature–Room Temperature (RTP)
- Xylazine hydrochloride 2% w/v solution (Rompun, MWI Animal Health, Somerset, UK, catalog number: 0 2150569 ), storage temperature–RTP
- Buprenorphine 0.3 mg/ml solution (Vertegesic, MWI Animal Health, Somerset, UK, catalog number: 0 1300258 ), storage temperature–RTP
- Heparin Sodium 5,000 u/ml (MWI Animal Health, Somerset, UK, catalog number: 30394030 ), storage temperature–RTP
- Ethanol (Fisher Scientific, catalog number: 10233962 ), storage temperature–RTP
- Phosphate Buffered Saline (Fisher Scientific, PBS, catalog number: 10173433 ), storage temperature 4 °C
- Formalin (Sigma-Aldrich, catalog number: HT5014 ), storage temperature–RTP
- Xylene (Fisher Scientific, catalog number: 10467270 ), storage temperature–RTP
- Parrafin (Fisher Scientific, Thermo Scientific Richard-Allan Scientific Histoplast Paraffin, catalog number: 12683026 ), storage temperature–RTP
- Hydrogen peroxide (Fisher Scientific, H2O2, catalog number: 10687022 ), storage temperature 4 °C
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 ), storage temperature–RTP
- Bovine Serum Albumin (Fisher Scientific, BSA, catalog number: 11423164 ), storage temperature 4 °C
- Primary antibody, anti α-Smooth muscle actin antibody (Abcam, catalog number: ab5694 ), storage temperature -20 °C
- Primary antibody, anti Collagen I antibody (Abcam, catalog number: ab21286 ), storage temperature 4 °C
- Primary antibody, anti Collagen III antibody (Abcam, catalog number: ab7778 ), storage temperature 4 °C
- Secondary antibody, Goat Anti-Rabbit IgG H&L (HRP) (Abcam, catalog number: ab205718 ), storage temperature 4 °C
- Normal Goat serum (Abcam, catalog number: ab7481 ), storage temperature 4 °C
- DAB (Fisher Scientific, catalog number: 10006913 ), DAB storage temperature -20 °C. DAB Substrate kit storage temperature 4 °C
- Glacial acetic acid (Sigma-Aldrich, catalog number: A6283 ), storage temperature–RTP
- TRIS hydrochloride (Tris-HCl) (Sigma-Aldrich, catalog number: RES3098T-B7 ), storage temperature RTP
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 ), storage temperature–RTP
- Ammonium hydroxide (NH4OH) 30% solution (Sigma-Aldrich, catalog number: 221228-1L-A ), storage temperature RTP
- Sodium citrate (Sigma-Aldrich, Tri-sodium citrate (dihydrate), catalog number: W302600 ), storage temperature RTP
- Tween 20 (Sigma-Aldrich, catalog number: 93773 ), storage temperature RTP
- Hematoxylin (Vector Laboratories Ltd, VECTOR®HEMATOXYLINNUCLEAR COUNTERSTAIN, catalog number: H-3401 ), storage temperature RTP
- Mounting medium (Vector Laboratories, VectaMount Permanent Mounting Medium, catalog number: H-5000 ), storage temperature RTP
- Hydrochloric acid (Sigma-Aldrich, catalog number: 258148 ), storage temperature RTP
- Bluing solution (see Recipes)
- Acid rinse solution (see Recipes)
- Sodium citrate buffer (see Recipes)
- TBS buffer (see Recipes)
Equipment
- Dissection Forceps (Harvard Apparatus, Jewelers Forceps, No. 7, catalog number: 72-8685 )
- Scissors (Harvard Apparatus, Eye Scissors, Straight, Sharp/Sharp, catalog number: 2-8438 )
- Ear punch for identifying mice (Harvard Apparatus, Model 4.7.2 Forceps Type Punch, 2.0 mm hole, catalog number: 34-0137 )
- Fume hood (Fisher Scientific, Thermo Scientific Hyperclean TruAir Ductless Fume Hood, catalog number: 15613136 )
- Orbital shaker (Fisher Scientific, Stuart Orbital Shaker, catalog number: 10759145 )
- Vacuum Oven (Fisher Scientific, Technico, catalog number: 13025703 )
- Microtome and blade, Fisher Scientific, HM 325 Rotary Microtome, catalog number: 12052999 )
- Waterbath (Fisher Scientific, Clifton Unstirred Digital Water Bath, catalog number: 15700619 )
- Domestic stainless steel pressure cooker (e.g., Argos, Tower Compact 4 Litre Pressure Cooker, catalog number: 711/5008 )
- Hot plate (Fisher Scientific, Stuart Hotplate with Stirrer, catalog number: 11966558 )
- Centrifuge (Fisher Scientific, Thermo Scientific Heraeus Megafuge 8, catalog number: 15211026 )
- Microscope (3D Histech Ltd, Budapest, Hungary, Panoramic Scanning 250 microscope)
- Refrigerator (Fisher Scientific, Loughborough, UK, Liebherr, 12088281)
- Staining set consisting of multiple containers (Tissue Tek Slide Staining Dish, Sakura Finetek Europe, Alphen aan den Rijn, The Netherlands, S76316)
Software
- Panoramic Viewer software (3D Histech Ltd, Budapest, Hungary)
- ImageJ software (https://imagej.nih.gov)
- Excel (https://products.office.com/en-gb/excel)
- GraphPad Prism (https://www.graphpad.com/scientific-software/prism/)
Procedure
- Induction of reno-cardiac syndrome in mice and collection of tissue samples
- Allow at least 8 week old C57BL/6 strain mice to acclimatize for 7 days in animal facility with free access to food and water, in a room with 12 h light/dark cycle.
- On day 8 weigh mice and change cages/diets giving half mice normal chow and the other half chow containing 0.15% adenine.
- Continue with diets administration to mice for 20 weeks weighing them every week.
- Prepare anaesthetic Ketamine:Xylazine as 2:1 ratio. Dilute 1:10 with PBS. Weigh mouse and administer at 1.5 ml/kg, intraperitoneally.
After 3 min when mouse is anaesthetized (check pinch reflex i.e., pinch foot, there should be no response). - Administer buprenorphine (0.03 ml/Kg), intraperitoneally. Wait 3 min.
- Remove blood by cardiac puncture, performed by inserting heparinized 25 gauge needle attached to 1 ml syringe into left ventricle (see video link, Stewart and Schroeder, 2020), heparinize needle by briefly drawing up and expelling heparin from a tube, heparin may be reused for multiple needles.
- Approximately 0.5 ml of blood should be collected. Perform laparotomy, followed by thoracotomy. Remove heart and weigh. Blood should be centrifuged at 17,000 x g/3 min for separation of plasma and stored in refrigerator.
- Remove left and right kidneys. Wash in cold PBS briefly, then cut longitudinally and place into formalin for fixation.
- After 24 h make up a solution of 70% ethanol and transfer kidneys from formalin into 70% ethanol.
- After 24 h rinse tissue in PBS.
- Send blood to a service laboratory (IDEXX, Bioresearch, Ludwigsberg, Germany), for biochemistry measurements (plasma creatinine and urea) to determine degree of renal failure.
- Paraffinisation of tissue
- Dehydrate tissue
- Incubate in 50% ethanol for 10 min, room temperature (RTP).
- Then 70% ethanol for 10 min (RTP).
- Then 80% ethanol for 10 min (RTP).
- Then 95% ethanol for 10 min (RTP).
- Then 100% ethanol for 10 min (RTP). Repeat this step 2 more times.
- Then 2:1 ratio ethanol:xylene for 10 min (RTP).
- Then 1:1 ratio ethanol:xylene for 10 min (RTP).
- Then 1:2 ratio ethanol:xylene for 10 min (RTP).
- Then 100% xylene for 10 min (RTP). Repeat this step 2 more times.
- Transfer samples to a vacuum oven set at 56 °C.
- Then 2:1 ratio xylene:paraffin for 30 min, 56 °C.
- Then 1:1 ratio xylene:paraffin for 30 min, 56 °C.
- Then 1:2 ratio xylene:paraffin for 30 min, 56 °C.
- Then 100% paraffin for 2 h, 56 °C.
- Then 100% paraffin overnight, 56 °C.
- Embed longitudinally cut kidney in fresh paraffin orientated so internal side faces upwards in wax in embedding cassette.
- Sectioning
- Place embedding cassettes with samples on ice for approximately 2 h or preferably in refrigerator overnight to cool down and facilitate cutting.
- Cut 5 μm sections of kidney using microtome.
- Place into water bath set at 42 °C.
- Collect individual sections onto slides.
- Place into slide rack and leave to dry in oven at 37 °C overnight.
- Deparaffinize and rehydrate slides
- Take slides in rack and incubate in xylene for 3 min, room temperature (RTP). Repeat this step 2 more times.
- Then xylene:100% ethanol 1:1 ratio for 3 min (RTP).
- Then 100% ethanol for 3 min (RTP). Repeat this step.
- Then 95% ethanol for 3 min (RTP).
- Then 70% ethanol for 3 min (RTP).
- Then 50% ethanol for 3 min (RTP).
- Finally rinse off excess ethanol by rinsing under cold tap water. Leave slides rinsing under tap water until ready for antigen retrieval protocol (to prevent drying out).
- Antigen retrieval
- Pour enough of the sodium citrate pH 6.0 buffer in to the slide container to cover the slides and place into the pressure cooker.
- With the pressure cooker on the hotplate turn on to full power. Leave the pressure cooker lid resting on top (unfastened).
- Once the cooker is boiling, carefully transfer the slides from the tap water into it using forceps. Fasten the lid according to the manufacturer’s instructions.
- Allow the cooker to reach full pressure, and then time 3 min.
- After 3 min have elapsed, switch off the hot plate, carefully remove the cooker off the hotplate and place the pressure cooker in a sink.
- Activate the pressure release valve and run cold tap water over the cooker. Once de-pressurized, remove the lid and run cold tap water into the cooker for 10 min.
Slides are now ready for staining.
- Staining
- Add Triton X-100 to TBS buffer at 0.025% final concentration.
- Wash slides 2 x 5 mins in TBS Triton X-100 buffer on gently rotating orbital shaker.
- Prepare blocking buffer (TBS + 1% BSA + 10% normal serum).
- Block slides in blocking buffer for 2 h on shaker, RTP.
- Remove slides and wipe with tissue paper (around sections).
- Use hydrophobic barrier pen to draw around sections.
- Prepare primary antibody:
anti SMA antibody ( ab5694 diluted 1:250 in TBS buffer/1% BSA)
or
anti collagen I ( ab21286 diluted 1:200 in TBS buffer/1% BSA)
or
anti collagen III ( ab7778 diluted 1:400 in TBS buffer/1% BSA). - Prepare humidified chamber (plastic box with tray lined with wet tissue paper, under a raised platform formed of taped together Pasteur pipettes, see Figure 1 below). Place slides flat on top with sections facing up. Apply antibody onto sections on slides. Close lid.

Figure 1. Humidified chamber. A. Pasteur pipettes taped together. B. Wet tissue paper in box. C. Slides layed on top. D. Lid closed tightly.
- Incubate at 4 °C overnight.
- Wash slides 5 min in TBS/0.025% Triton X-100, gently shaking, RTP.
- Repeat wash.
- Prepare 0.3% H2O2 in TBS
- Apply onto sections, incubate 15 min, gently shaking, RTP.
- Detection step
- Prepare secondary antibody, Goat Anti-Rabbit IgG H&L (HRP) ( ab205718 ) 1:1,000 dilution in TBS/1% BSA.
- Apply onto slides and incubate for 1 h, gentle shaking, RTP.
- Add DAB chromagen onto slide, 10 min with gentle shaking, RTP.
- Rinse slides under running tap water for 5 min.
- Counter stain with hematoxylin. Add Vector Hematoxylin onto slide, 5 min with gentle shaking, RTP.
- Rinse slides under running tap water until water is colourless.
- Dip slides into acid rinse 10 times.
- Dip slides into tap water 10 times.
- Add Bluing solution onto slide, 1 min with gentle shaking, RTP.
- Dip slides into tap water 10 times.
- Dehydrate tissue, clear and mount
- Incubate slides in 95% ethanol for 10 min, room temperature (RTP).
- Then 100% ethanol for 10 min (RTP). Repeat this step 2 more times.
- Then 100% xylene for 10 min (RTP). Repeat this step 2 more times.
- Mount by draining slides
- Apply a drop of mounting medium onto section.
- Cover with coverslip ensuring there are no air bubbles.
- Dehydrate tissue
- Imaging acquisition
Images were recorded using a Panoramic Scanning 250 microscope (3D Histech Ltd, Budapest, Hungary) at 20x magnification (Figure 2).
Data analysis
Statistical analysis was performed using GraphPad Prism 5 Software (GraphPad Software Inc, CA, US). Values are presented as mean ± SEM of n observations. Differences between adenine-treated mice and controls were calculated using the unpaired t-test. P < 0.05 was considered to be statistically significant.
- To calculate increase in α-SMA staining between samples open file with a control sample slide in ImageJ. i.e., click ‘File’ then select ‘Open’. Invert the image by clicking ‘Edit’ then select ‘Invert’. Take a background measurement by drawing a rectangle approximately 0.5 cm2 on the dark area surrounding the image. Click ‘Analyze’ then select ‘Measure’. A value will appear in a data file called ‘Results’ which opens automatically. Next drag the same rectangle over the kidney and take 6 random measurements of kidney (by clicking ‘Measure’ each time). Repeat with 2 more control slides and then with 3 Adenine treated kidney slides (ensure that the rectangle is exactly the same size for all slides).
- Save all the picture and results files. Finally transfer data to an Excel file by clicking ‘Copy’ in the ‘Results’ files. Subtract background value from all measurements. Calculate average for each slide (Table 2).
Table 2. Data in Excel spreadsheet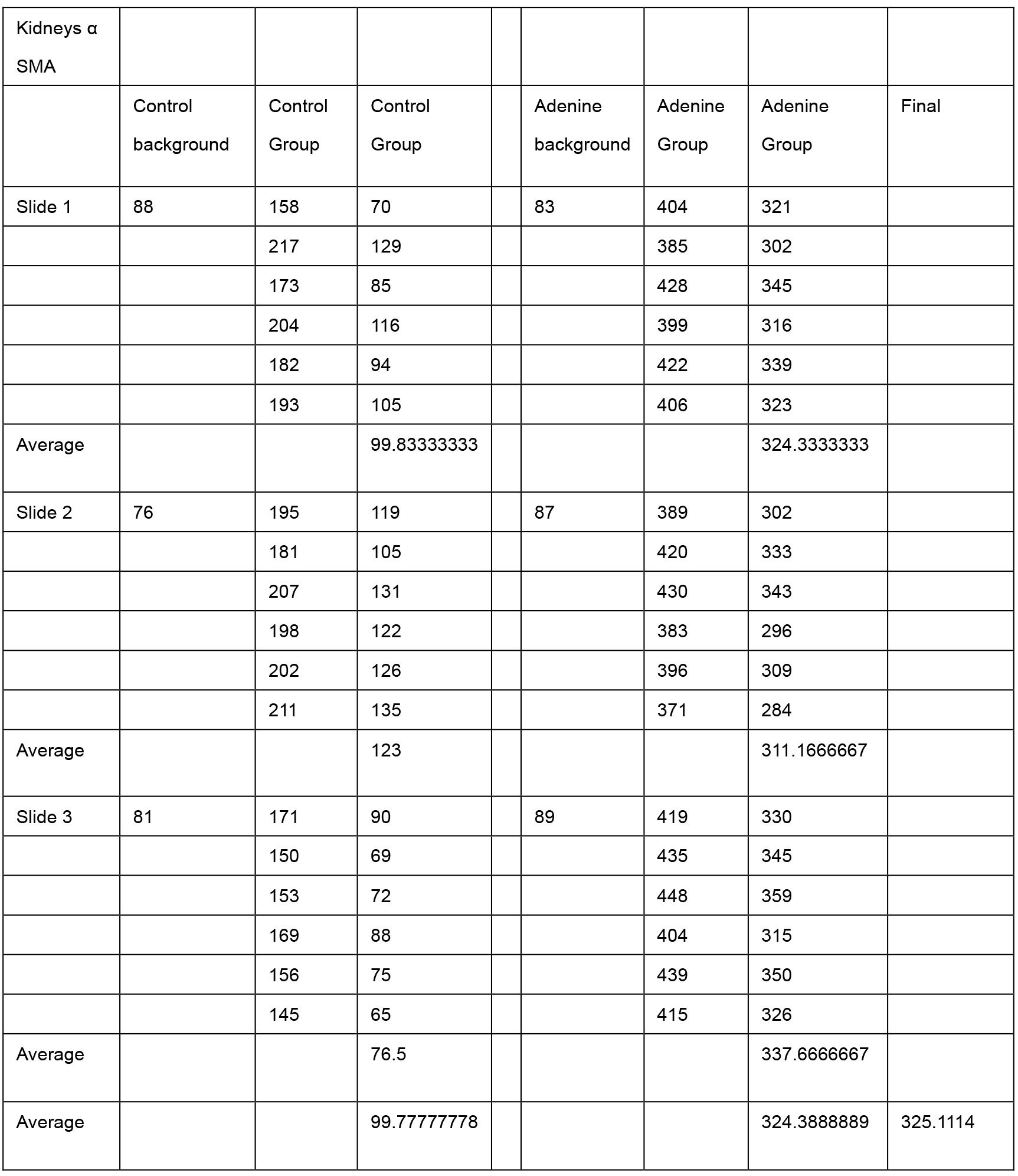
- Input results into GraphPad Prism to convert into a column graph (Table 3).
Table 3. Data in GraphPad Prism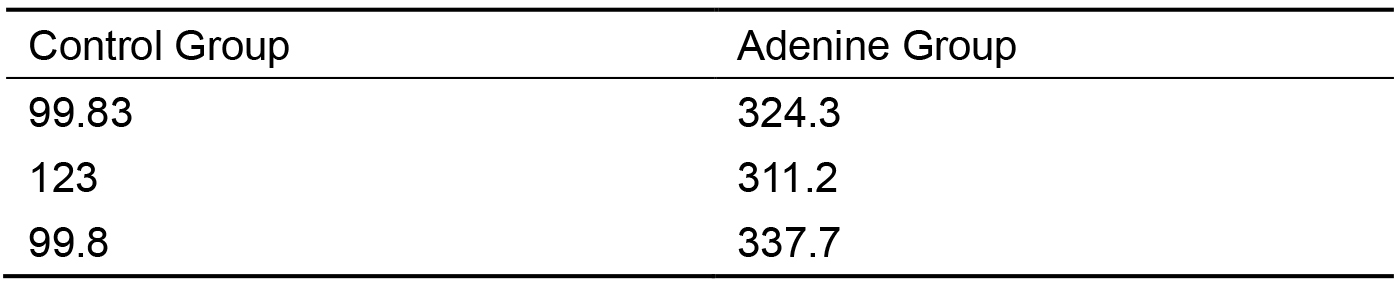
- Do an (mean ± SEM) unpaired t-test to calculate statistical significance between the 2 columns (Table 4). Repeat with collagen I and then collagen III.
Table 4. Statistical analysis of data in GraphPad Prism
- Click on ‘Graphs’ icon to obtain graph. Edit graphs (Figure 2).
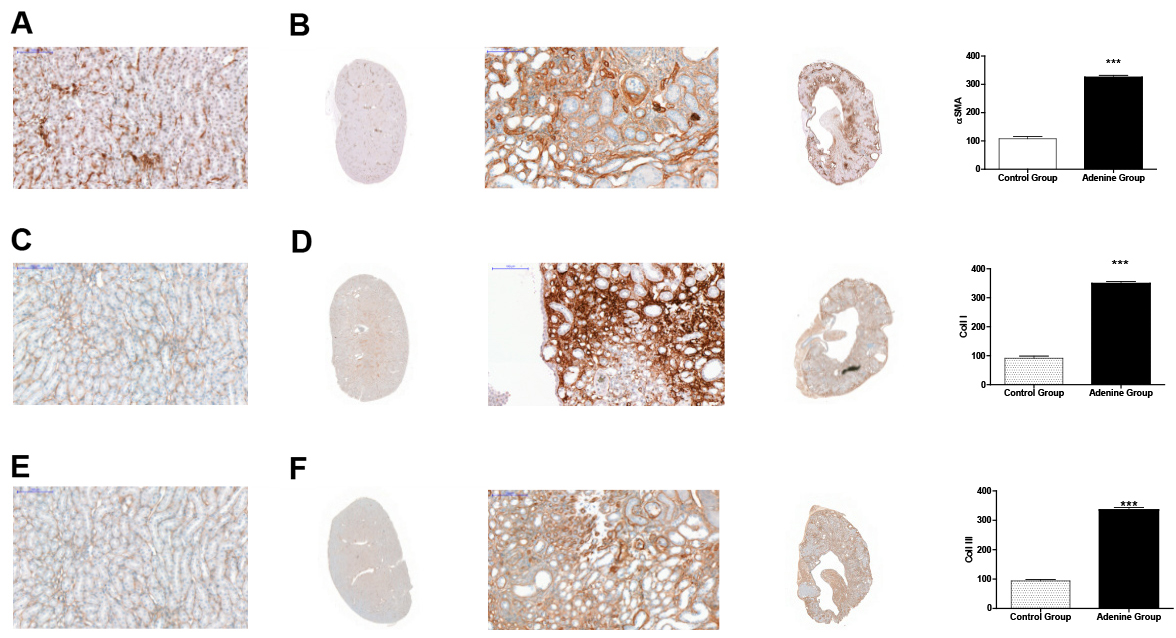
Figure 2. Evidence for renal hypertrophy through immunostaining for α-SMA, collagen I, and collagen III. Control group (n = 6) received standard chow for
20 weeks. Adenine treated group (n = 10) received standard chow with the addition of 0.15% adenine for 20 weeks. Immunostaining of kidneys showed a
significant increase in α-SMA staining of adenine group (B) compared to control group (A) (P < 0.0001). A significant increase in collagen I staining of
adenine group (D) compared to control group (C) (P < 0.0001). A significant increase in collagen III staining of adenine group (F) compared to control group
(E) (P < 0.0001), mean ±SEM unpaired t-test. Representational images (3 mice per group).
- Do an (mean ± SEM) unpaired t-test to calculate statistical significance between the 2 columns (Table 4). Repeat with collagen I and then collagen III.
Notes
- Animal experiments must be performed in accordance with national legal guidelines for animal research and with local ethical committee approval.
- Do not administer diet to animals below 9 weeks old.
- Do not fix tissue in formalin for more than 24 h as over fixation may inhibit antigen: antibody binding.
- Confirm induction of CKD from blood biochemistry results before proceeding with Procedure B. Paraffinisation of tissue.
- Steps using xylene should be performed inside a fume hood.
Recipes
- Bluing solution
1.5 ml NH4OH (30% stock) + 98.5 ml of 70% ethanol. Store at RTP for up to 3 months - Acid rinse solution
2 ml glacial acetic acid + 98 ml of deionized or distilled H2O
Store at RTP for up to 3 months - Sodium citrate buffer
10 mM Sodium citrate, 0.05% Tween 20, pH 6.0. Dissolve 2.94 g Tri-sodium citrate (dihydrate) in 1 L distilled water and adjust pH to 6.0 with 1 N HCl- Add 0.5 ml Tween 20 and mix well
- Store at RTP for up to 3 months or at 4 °C for longer
- TBS buffer
50 mM Tris-Cl, pH 7.5, 150 mM NaCl- Dissolve 6.05 g Tris and 8.76 g NaCl in 800 ml of H2O
- Adjust pH to 7.5 with 1 M HCl and make volume up to 1 L with H2O
- TBS is stable at 4 °C for 3 months
Acknowledgments
We acknowledge the following funding bodies which made this study possible:
Julius Kieswich–Barts and the Royal London Hospital Diabetic Kidney Disease Centre.
Jianmin Chen–China Scholarship Council (grant number 201206240146).
Samira Alliouachene–Barts and the Royal London Hospital Diabetic Kidney Disease Centre.
Paul Caton–Barts and the Royal London Hospital Diabetic Kidney Disease Centre.
Kieran McCafferty–Barts and the Royal London Hospital Diabetic Kidney Disease Centre.
C. Thiemermann–William Harvey Research Foundation.
Muhammad Yaqoob–Barts and the Royal London Hospital Diabetic Kidney Disease Centre.
The funding body played no role in the design of the study nor in the collection, analysis, or interpretation of data, nor in the writing of the manuscript. A brief version of this protocol appeared in BMC Nephrology (Kieswich et al., 2018).
Competing interests
The authors declare no competing interests.
Ethics
Animal experiments were conducted in accordance with UK Home Office Animals (Scientific Procedures) Act 1986, with local ethical committee approval. Under Home Office Project Licence no: 70/8350 which was granted after review by the Animal Welfare and Ethical Review Body (AWERB) of Queen Mary University, London.
References
- Bursac, N. (2014). Cardiac fibroblasts in pressure overload hypertrophy: the enemy within? J Clin Invest 124(7): 2850-2853.
- de Jager, D. J., Grootendorst, D. C., Jager, K. J., van Dijk, P. C., Tomas, L. M., Ansell, D., Collart, F., Finne, P., Heaf, J. G., De Meester, J., Wetzels, J. F., Rosendaal, F. R. and Dekker, F. W. (2009). Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302(16): 1782-1789.
- Hewitson, T. D. (2012). Fibrosis in the kidney: is a problem shared a problem halved? Fibrogenesis Tissue Repair 5(Suppl 1): S14.
- Hewitson, T. D., Holt, S. G. and Smith, E. R. (2015). Animal models to study links between cardiovascular disease and renal failure and their relevance to human pathology. Front Immunol 6: 465.
- Jia, T., Olauson, H., Lindberg, K., Amin, R., Edvardsson, K., Lindholm, B., Andersson, G., Wernerson, A., Sabbagh, Y., Schiavi, S. and Larsson, T. E. (2013). A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol 14: 116.
- Kieswich, J. E., Chen, J., Alliouachene, S., Caton, P. W., McCafferty, K., Thiemermann, C. and Yaqoob, M. M. (2018). A novel model of reno-cardiac syndrome in the C57BL/ 6 mouse strain. BMC Nephrol 19(1): 346.
- Kren, S. and Hostetter, T. H. (1999). The course of the remnant kidney model in mice. Kidney Int 56(1): 333-337.
- Kumar, S., Bogle, R. and Banerjee, D. (2014). Why do young people with chronic kidney disease die early? World J Nephrol 3(4): 143-155.
- Morrison, A. B. (1962). Experimentally induced chronic renal insufficiency in the rat. Lab Invest 11: 321-332.
- Ronco, C., Haapio, M., House, A. A., Anavekar, N. and Bellomo, R. (2008). Cardiorenal syndrome. J Am Coll Cardiol 52(19): 1527-1539.
- Seong, E., Saunders, T. L., Stewart, C. L. and Burmeister, M. (2004). To knockout in 129 or in C57BL/6: that is the question. Trends Genet 20(2): 59-62.
- Steenkamp, R., Shaw, C. and Feest, T. (2013). UK Renal Registry 15th annual report: Chapter 5 survival and causes of death of UK adult patients on renal replacement therapy in 2011: national and centre-specific analyses. Nephron Clin Pract 123 Suppl 1: 93-123.
- Stewart K. and Schroeder V A. (2020). JoVE Science Education Database. Lab Animal Research. Blood Withdrawal I. JoVE, Cambridge, MA.
- Yokozawa, T., Oura, H. and Okada, T. (1982). Metabolic effects of dietary purine in rats. J Nutr Sci Vitaminol (Tokyo) 28(5): 519-526.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kieswich, J. E., Chen, J., Alliouachene, S., Caton, P. W., McCafferty, K., Thiemermann, C. and Yaqoob, M. M. (2020). Immunohistochemistry of Kidney a-SMA, Collagen 1, and Collagen 3, in A Novel Mouse Model of Reno-cardiac Syndrome. Bio-protocol 10(18): e3751. DOI: 10.21769/BioProtoc.3751.
Category
Immunology > Animal model > Mouse
Molecular Biology > Protein > Phosphorylation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









