- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measuring Breathing Patterns in Mice Using Whole-body Plethysmography
(*contributed equally to this work) Published: Vol 10, Iss 17, Sep 5, 2020 DOI: 10.21769/BioProtoc.3741 Views: 6604
Reviewed by: Arnau Busquets-GarciaAndrew L. EagleAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1644 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 80 Views
Abstract
Respiratory dysfunction is among the main cause of severe and fatal pathologies worldwide. The use of effective experimental models and methodologies for the study of the pulmonary pathophysiology is necessary to prevent, control and cure these diseases. Plethysmography, a technique for the assessment of lung function, has been widely applied in mice for the characterization of respiratory physiology. However, classical plethysmography methods present technical limitations such as the use of anesthesia and animal immobilization. Whole-body plethysmography (WBP) avoids these issues providing a non-invasive approach for the assessment of the respiratory function in conscious animals. WBP relies on the recording of pressure changes that are produced by the spontaneous breathing activity of an animal placed inside an airtight chamber. During normal respiration, pressure variation is directly proportional to the respiratory pattern of the animal allowing the measurement of the respiratory rate and tidal volume. These parameters are commonly used to evaluate pulmonary function in different physiological and disease models. In contrast to classical plethysmography methods, WBP technique allows reproducible serial measurements as it avoids animal restraint or the use of anesthesia. These key features rend WBP a suitable approach for longitudinal studies allowing the assessment of progressive respiratory alterations in physiological and pathological conditions. This protocol describes the procedures for the measurement of the breathing patterns in mice using the WBP method, the data analysis and results interpretation.
Keywords: Whole-body PlethysmographyBackground
Respiratory diseases are leading causes of disability and the third cause of death worldwide. To address the prevention, control and cure of these diseases basic research in the field is needed (Forum of International Respiratory Societies, 2017). Animal models, such as murine models, have been very useful in research due to a well-characterized genome, an improvement in genetically engineered animals, short breeding cycle, cost-efficiency and a well-known immunologic system (Gelfand, 2002; Persson, 2002; Irvin and Bates, 2003). Despite their small size, mice have contributed to the understanding of lung mechanics and functioning in normal conditions as well as in disease (Bates, 2017).
Whole-body plethysmography (WBP) is a technique that allows the analysis of the respiratory function. It is a non-invasive, precise approach that does not required anesthesia, as the mouse is unrestrained and conscious. Therefore, the respiratory parameters obtained with WBP in mice reflect basal physiological values since instrumental restraints and/or anesthesia are not applied (Lim et al., 2014; Quindry et al., 2016). Notably, these experimental conditions are key for the use of WBP in longitudinal studies (Cramer et al., 2015; Flanagan et al., 2019). Moreover, WBP in mice is a well-stablished technique that has been applied for the study of a wide range of biological aspects of the respiratory function providing new insights into neuronal network controlling respiratory rhythm (Crone et al., 2012), sleep-related breathing disorders (Bastianini et al., 2017) or the role of inflammation in the control of breathing (Giannakopoulou et al., 2019).
During WBP, the mouse is placed in an airtight chamber where it can move freely and breathe spontaneously. Inspiration and expiration cycles modify the chamber pressure due to gas compression and expansion within the lungs as well as to changes in temperature and humidity of the air as it enters into the respiratory tract (Lundblad et al., 2002; Lim et al., 2014; Bates, 2017). Breathing frequency and tidal volume can be measured based on the variations in the chamber pressure parameters (period and magnitude) (Lundblad et al., 2002; Adler et al., 2004; Bates, 2017).
Other conventional plethysmography methods require the animal to be immobilized (Double-chamber plethysmography, DCP), or need invasive surgery in an unconscious mouse (Forced oscillation technique, FOT), which does not allow animal recovery. DCP consists of two chambers that separate animal head and thorax in two chambers (Mailhot-Larouche et al., 2018). Habituation is needed prior to acquisition data due to restraint-induced stress. Restraint is well known as a major stressor and it alters respiration parameters (Buynitsky and Mostofsky, 2009), which may confound result interpretation. Another broadly used approach in lung mechanics is FOT, which requires that the subject’s respiratory system remains passive during the mechanical ventilation (McGovern et al., 2013). This is achieved by the administration of anesthetics and tracheotomy. Forced oscillation technique provides reproducible and detailed data due to controlled experimental conditions although these are far from the physiological state (Mailhot-Larouche et al., 2018).
Compared to conventional plethysmography techniques, WBP offers a more versatile and reproducible tool for the assessment of the respiratory function. In addition, body plethysmography is a well-established technique for lung function determination in humans (Criée et al., 2011). Therefore, respiratory diseases in humans, such as asthma and pulmonary fibrosis, can be modeled by whole-body plethysmography in mice (Finkelman, 2008; Vaickus et al., 2010; Milton et al., 2012). WBP also enables lung mechanics studies with hypoxic and hyperoxic models, measurement of infectious agents, allergen sensitization studies and anesthetics function (Detweiler et al., 2018; Hill et al., 2018; Ortega-Sáenz et al., 2018; Receno et al., 2019), which can be progressively monitored. Moreover, WBP can be applied to study alterations in respiratory function that can be a key clinical sign in diseases with secondary respiratory impairment, such as Leigh Syndrome (Quintana et al., 2012; Bolea et al., 2019).
Materials and Reagents
- SilicaGel Orange Indicator 2-4 mm (Bolaseca, catalog number: 8.1.002 )
- Laboratory tissue paper, e.g., Wiping Paper Plus (Tork, catalog number: 130050 )
- C57BL/6J background mice (The Jackson Laboratory, catalog number: 000 664 )
Note: Other laboratory mice strains can be used. Please, see Procedure section. - Ethanol 70%, e.g., Ethanol absolute for analysis (PanReac AppliChem, catalog number: A1613 )
Equipment
- Plethysmograph, Unrestrained Whole Body (90 mm diameter), for Mouse (EMMS, catalog number: PLY310 )
- Pulmonary Flow Transducer (EMMS, catalog number: TPF100 )
- Adaptive Amplifier, strain gauge type; single channel (EMMS, catalog number: AMP110 )
- Large capacity drying column (0.5 kg indicating Silica desiccant, suitable for regeneration) complete with tubing and fitting, including 1ml Lock syringe connect to a plastic tube (EMMS, catalog number: ADR101 )
- 2-channel Bias Flow Generator (EMMS, catalog number: AIR140 )
- Acquisition Unit (laptop)
Software
- USB Data Acquisition (eDacq) Single Subject Version 1.9.0 (Site) only version with Flow Derived Parameters Analyser (EMMS, catalog number: ESS101A)
Note: EMMS company (Electro-Medical Measurement Systems), Bordon, UK, www.electromedsys.com (equipment and eDacq software). - Microsoft Office Excel 2016 or compatible
- Statistical software (e.g., GraphPad Prism 6.0)
Procedure
Several parameters may critically influence WBP outcomes and reproducibility. Therefore, they might be considered in the experimental design. These include time of the day (considering mouse circadian cycle), mouse behavior during recording, and standardization of the habituation period (Quindry et al., 2016; Receno et al., 2019). Depending on the specific research question, strain-specific differences in pulmonary physiology might be evaluated (Cramer et al., 2015; Bates, 2017). Importantly, the parameters described in the following section may be adapted and validated considering the basal/control breathing activity of each mouse strain.
Furthermore, environmental conditions must be kept constant during the entire procedure. Here, we maintained a constant temperature of 22 °C and a relative humidity of 55% (as recommended in the Guide for the Care and Use of Animals, National Research Council, 2011).
- Setting up the equipment
- Install USB Data Acquisition (eDacq) to the computer.
- Connect through the USB connector the Adaptive Amplifier to the acquisition unit.
- Attach Pulmonary Flow Transducer into the transducer entrance from Adaptive Amplifier (Figure 1D).
- Join the Pulmonary Flow Transducer to the Plethysmograph apparatus (Figure 1A).
- Set up Flow 1 and Flow 2 levels at 1cc/min from the Bias Flow Generator (Figure 1C).
- Join Port 1 from Bias Flow Generator with Entrance 1 of Large capacity drying column and Port 2 to the top input from Plethysmograph chamber. Then, connect Entrance 2 of drying column to the lower input from Plethysmograph device (Figures 1A and 1C).
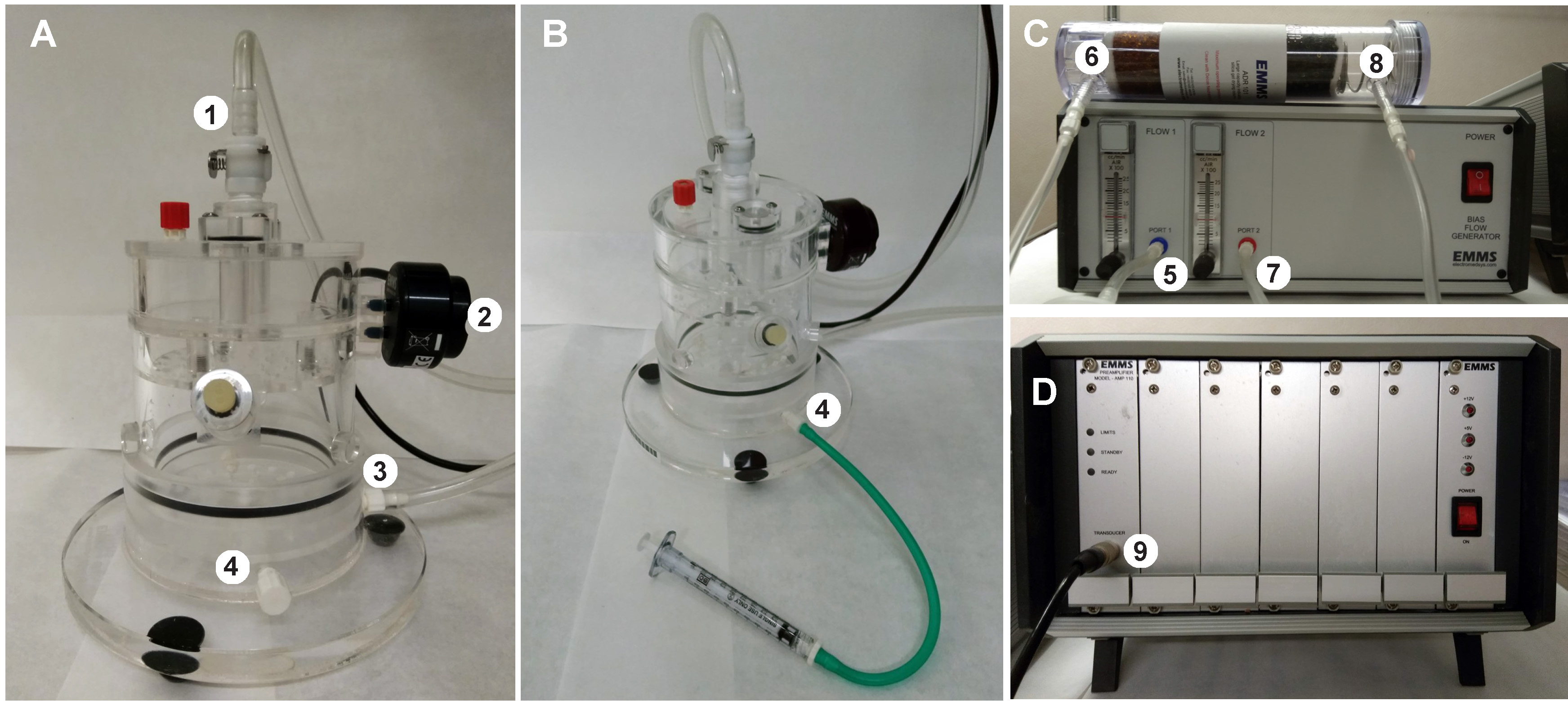
Figure 1. Plethysmograph equipment. A. Plethysmograph chamber. 1: Top input (incoming airflow), 2: Pulmonary Flow Transducer, 3: Lower input (air exit), 4: Alternative lower input). B. Plethysmograph apparatus attached to the 1 ml Lock syringe by the alternative lower input (4). C. Bias Flow generator and drying column containing silica beads. 5: Port 1 (flow1), 6: Entrance 1, 7: Port 2 (flow2, incoming air), 8: Entrance 2 (air extraction). D. Adaptive amplifier connected to the chamber through transducer entrance (9). Fresh air is continuously administrated into the chamber trough the Port2 and released from the chamber trough the lower input (3). Then, the air passes through the drying column and it is extracted from the Bias Flow generator (air dehumidification is required to preserve bias flow generator). - Setting up the protocol (see Video 1)
- Start eDacq program.
- Select Protocol from left panel. A new window will pop up.
- Click Site 1 option and then Edit.
- Add and edit the different steps of the desired protocol:
- Record on all sites
- Wait for user advance
- Pause for 00:03:00 (time to leave the workspace/room)
- Record control period for 00:45:00 (habituation time)
- Record period for 00:15:00 (recording time)
- Record off all sites
- Select Site 1 from left panel. Add the following flow derived parameters (FDP) properties for recording:
- On the General tab: in the Input group select 20 ms of smoothing and in the Output group select 5 s of time based.
- On the Breath Analysis tab: in the Breath Detection group introduce 0.5 ml/s for Row Threshold and 20% TV for Start of Breath Extrapolation. In the Breath Rejection group, do not select Enable Breath Rejection.
- On the Filtering tab: do not select Enable-Pass Filter and Enable Mains Filter.
- Apply all changes.
- Save configuration.
Video 1. Setting up the protocol - Chamber calibration (see Video 2)
- Turn on the Bias Flow Generator and the Adaptive Amplifier (a white light will flash on standby) 15 min before starting calibration.
- Tightly close the plethysmography chamber.
- Start eDacq program.
- Introduce assigned password (select ok).
- Introduce study name (press close).
- Close the configuration file version window that appears.
- Accept calibration option (always recommended).
- Activate DC mode for calibration (AC mode is automatically set during recording).
- Set the high flow value to -10 ml/s.
- Press F8 (Zero button) to adjust basal parameters.
- Press F9 (Low) to adjust low calibration volume.
- Join 1ml lock syringe to the alternative lower stopcock input of plethysmography chamber (Figure1 A-B).
- Gently inject 1 ml air volume into the chamber using the connected syringe and 1.5 s after press F11 (Record volume) to adjust high calibration volume. Proper calibration range should be between -35 and +35 ml/s (repeat the process otherwise).
- Remove the syringe and place back the cap.
- Click Finish button and proceed to respiration recording.
- A white light should flash on ready from the adaptive amplifier machine.
- Calibration must be performed every time the equipment is switched on. There is no need to calibrate between each animal/trial in the same day. Calibration can also be initiated through the U option from toolbar at any time.
- High flow value must be ten times the injected calibration volume (step 13) as a correction factor for FDP analysis.Video 2. System calibration
- Breathing recording (see Video 3)
- Weigh the mouse right before recording.
- Open the plethysmography device and place the animal inside.
Note: It is critical not disturbing the transducer when manipulating the chamber device. Vibrations can affect the calibration. If this occurs recalibrate the system. Avoid installing the equipment in unstable surfaces or close to high-traffic areas. - Close the chamber tightly and make sure the animal moves freely.
- Select Protocol option and then Site 1 choice (the pre-configured protocol will be open).
- Restart the protocol.
- Introduce mouse identification, weight and any other comments of interest. After assessing this step, recording will start, (green flashing window on screen may appear).
- Advance the protocol to wait period (3 min) step. After this period the protocol will automatically proceed to the following steps.
Note: During this interval we recommend that the user leaves the workspace/room without disturbing the equipment to avoid interferences with the analysis. However, mouse must be monitored by visual resources or from outside the room through the whole session to record motor activity periods as well as any other behaviors. - After recording, return the animal to its cage. Clean and wipe the equipment with 70% Ethanol. Recording will be automatically saved in the assigned project folder.
- Record next animal by restart button (start from Step D1) or proceed to data analysis.
- Turn off all the equipment.Video 3. Recording whole-body plethysmography
Data analysis
eDacq software displays the waveforms from the transducers in real-time. Data is finally recorded to a disk file for post experiment replay and analysis. Critically, to avoid background noise during the recording, especially from mouse motor activity, it is crucial to allow sufficient habituation time. Therefore, while the breathing patterns of the animal are recorded for 1 h, only data from the final 15 min are analyzed. Importantly, since no breath rejection parameters are applied, it is necessary to ensure data corresponds to resting periods (Figure 2).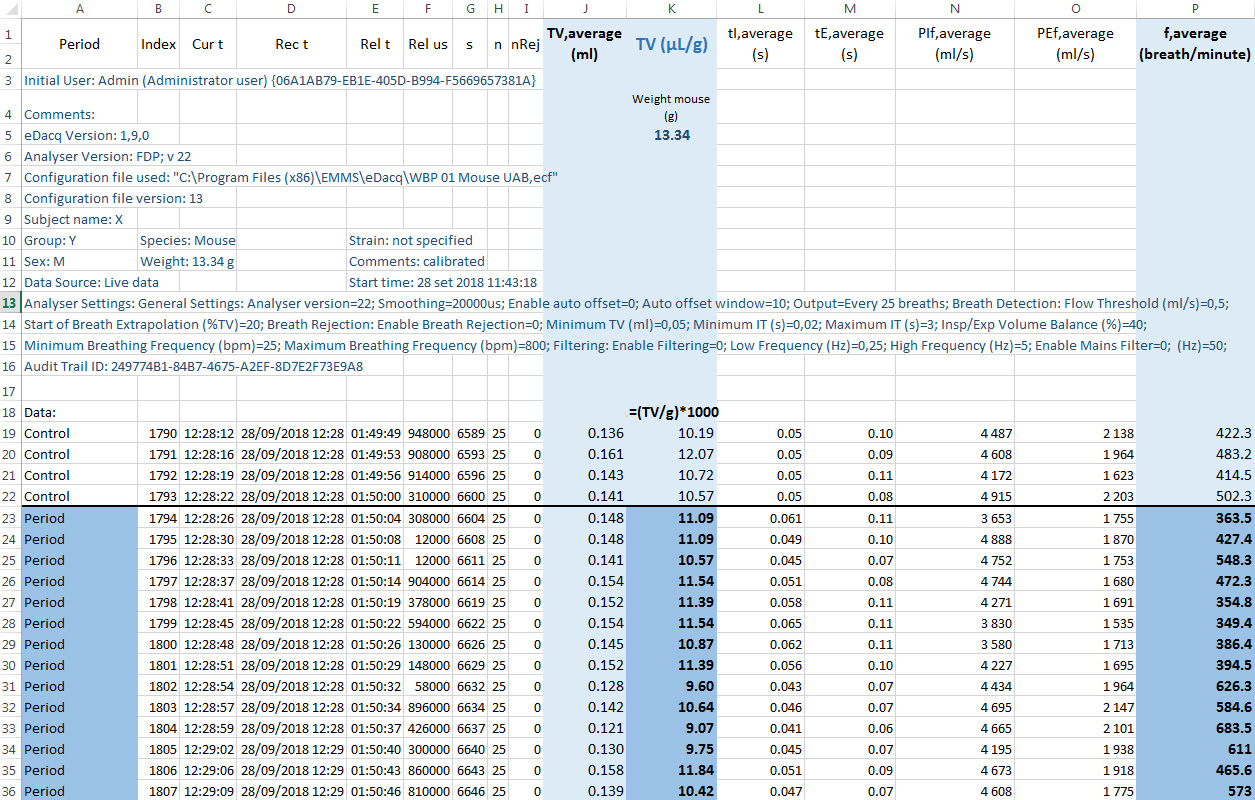
Figure 2. Example of exported raw data from eDacq to Excel. Tidal volume and respiratory frequency values of a resting mouse from an experimental group (male, 33 days, 13.5 g) shown in blue. Corrected tidal volume by the animal weight and respiratory frequency from period values shown in darker blue.
- Export data to an Excel file
- Start Excel with a blank workbook.
- On the Data tab, in the Get External Data group, click From Text.
- Browse your *.csv file (eDacq generates two files, choose the file without the period word) and click Import.
- Text import wizard will appear.
- Select Delimited option.
- Choose Comma as delimiter.
- In the Column data format section, select General and click finish.
- Data will appear in the workbook with column headings in the top row.
- Correct tidal volume values (ml) by the mouse weight (g).
- Average repose respiratory data (final 15 min values, named Period in exported Excel file) to calculate tidal volume (μl/g) and respiratory frequency (breaths/min) (Figure 2).
- Perform proper statistical test according to your experimental conditions.
- Values obtained by the eDacq software once exported in Excel file are reported in Table 1:
Table 1. Breathing values obtained by eDacq software
- (Optional) Represent breathing recording
- Initiate eDacq program.
- Select Experiment option from the toolbar.
- Press Data source.
- Select Recorded data file option from the available options.
- Choose the file of interest.
- Play recording using the toolbar (Play button).
- Pause at the frame time you want to represent (Pause button).
- Mouse right click on Site 1 window recording.
- Select Properties and freely change them.
- Save the file as .jpg selecting Save Chart from mouse right click menu (Figure 3).
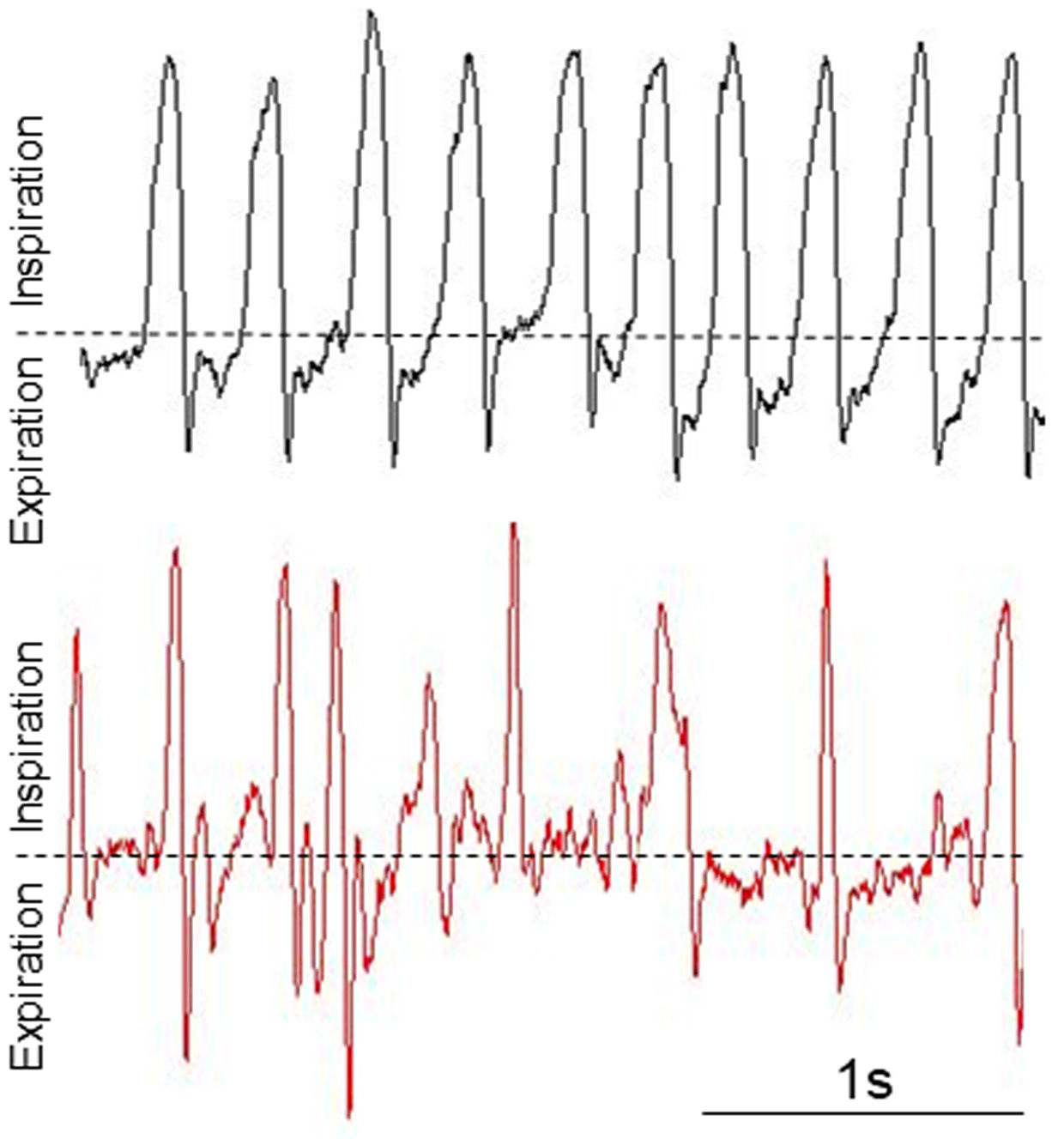
Figure 3. Representative breathing waveforms in a healthy resting animal (Top panel: Male, 62 days, 27 g) and in a resting experimental mouse presenting breathing alterations (Bottom panel: Male, 63 days, 12 g). These waveforms are not normalized by weight.
Acknowledgments
This work was supported by several pre-doctoral fellowships (BES-2015-073041; PPD, 2018FI_B 00452; AU, PRE2018-083179; LSB), and a Ramón y Cajal fellowship (RyC-2012-11873; AQ). AQ. received funds from the Seattle Children’s Research Institute and Northwest Mitochondrial Guild, and grants from the European Research Council (Starting grant NEUROMITO, ERC-2014-StG-638106), MINECO Proyectos I+D de Excelencia (SAF2014-57981P), MINECO Proyectos I+D “Retos Investigación” (SAF2017-88108-R), and AGAUR (2017SGR-323). This protocol was originally used in our previous study by Bolea et al., 2019.
Competing interests
Authors declare no conflict of interest.
Ethics
Original experimental data (Bolea et al., 2019) was collected following the recommendations in the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Seattle Children´s Research Institute and the Universitat Autònoma de Barcelona (Protocol P10127/CEEAH UAB 4155, valid until 10-2023).
References
- Adler, A., Cieslewicz, G. and Irvin, C. G. (2004). Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol (1985) 97(1): 286-292.
- Bastianini, S., Alvente, S., Berteotti, C., Lo Martire, V., Silvani, A., Swoap, S. J., Valli, A., Zoccoli, G. and Cohen, G. (2017). Accurate discrimination of the wake-sleep states of mice using non-invasive whole-body plethysmography. Sci Rep 7: 41698.
- Bates, J. H. T. (2017). CORP: Measurement of lung function in small animals. J Appl Physiol (1985) 123(5): 1039-1046.
- Bolea, I., Gella, A., Sanz, E., Prada-Dacasa, P., Menardy, F., Bard, A. M., Machuca-Marquez, P., Eraso-Pichot, A., Modol-Caballero, G., Navarro, X., Kalume, F. and Quintana, A. (2019). Defined neuronal populations drive fatal phenotype in a mouse model of Leigh syndrome. Elife 8: e47163.
- Buynitsky, T. and Mostofsky, D. I. (2009). Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev 33(7): 1089-1098.
- Cramer, N. P., Xu, X., Christensen, C., Bierman, A., Tankersley, C. G. and Galdzicki, Z. (2015). Strain variation in the adaptation of C57Bl6 and BALBc mice to chronic hypobaric hypoxia. Physiol Behav 143: 158-165.
- Criée, C. P., Sorichter, S., Smith, H. J., Kardos, P., Merget, R., Heise, D., Berdel, D., Köhler, D., Magnussen, H., Marek, W., Mitfessel, H., Rasche, K., Rolke, M., Worth, H., Jörres, R. A., Working Group for Body Plethysmography of the German Society for, P. and Respiratory, C. (2011). Body plethysmography--its principles and clinical use. Respir Med 105(7): 959-971.
- Crone, S. A., Viemari, J. C., Droho, S., Mrejeru, A., Ramirez, J. M. and Sharma, K. (2012). Irregular Breathing in Mice following Genetic Ablation of V2a Neurons. J Neurosci 32(23): 7895-7906.
- Detweiler, N. D., Vigil, K. G., Resta, T. C., Walker, B. R. and Jernigan, N. L. (2018). Role of acid-sensing ion channels in hypoxia- and hypercapnia-induced ventilatory responses. PLoS One 13(2): e0192724.
- Finkelman, F. D. (2008). Use of unrestrained, single-chamber barometric plethysmography to evaluate sensitivity to cholinergic stimulation in mouse models of allergic airway disease. J Allergy Clin Immunol 121(2): 334-335.
- Flanagan, T. W., Sebastian, M. N., Battaglia, D. M., Foster, T. P., Cormier, S. A. and Nichols, C. D. (2019). 5-HT2 receptor activation alleviates airway inflammation and structural remodeling in a chronic mouse asthma model. Life Sci 236: 116790.
- Forum of International Respiratory Societies. (2017). The Global Impact of Respiratory Disease. Second Edition. Sheffield, European Respiratory Society.
- Gelfand, E. W. (2002). Pro: mice are a good model of human airway disease. Am J Respir Crit Care Med 166(1): 5-6; discussion 7-8.
- Giannakopoulou, C. E., Sotiriou, A., Dettoraki, M., Yang, M., Perlikos, F., Toumpanakis, D., Prezerakos, G., Koutsourelakis, I., Kastis, G. A., Vassilakopoulou, V., Mizi, E., Papalois, A., Greer, J. J. and Vassilakopoulos, T. (2019). Regulation of breathing pattern by IL-10. Am J Physiol Regul Integr Comp Physiol 317(1): R190-R202.
- Hill, R., Disney, A., Conibear, A., Sutcliffe, K., Dewey, W., Husbands, S., Bailey, C., Kelly, E. and Henderson, G. (2018). The Novel μ-Opioid Receptor Agonist PZM21 Depresses Respiration and Induces Tolerance to Antinociception. Br J Pharmacol 175(13): 2653-61.
- Irvin, C. G. and Bates, J. H. (2003). Measuring the lung function in the mouse: the challenge of size. Respir Res 4: 4.
- Lim, R., Zavou, M. J., Milton, P. L., Chan, S. T., Tan, J. L., Dickinson, H., Murphy, S. V., Jenkin, G. and Wallace, E. M. (2014). Measuring respiratory function in mice using unrestrained whole-body plethysmography. J Vis Exp (90): e51755.
- Lundblad, L. K., Irvin, C. G., Adler, A. and Bates, J. H. (2002). A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol (1985) 93(4): 1198-1207.
- Mailhot-Larouche, S., Deschênes, L., Lortie, K., Gazzola, M., Marsolais, D., Brunet, D., Robichaud, A. and Bossé, Y. (2018). Assessment of Respiratory Function in Conscious Mice by Double-chamber Plethysmography. J Vis Exp (137): 57778.
- McGovern, T. K., Robichaud, A., Fereydoonzad, L., Schuessler, T. F. and Martin, J. G. (2013). Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp 15(75): e50172.
- Milton, P. L., Dickinson, H., Jenkin, G. and Lim, R. (2012). Assessment of respiratory physiology of C57BL/6 mice following bleomycin administration using barometric plethysmography. Respiration 83(3): 253-266.
- National Research Council. (2011). Guide for the Care and Use of Laboratory Animals. Eighth Edition. Washington, DC: The National Academies Press.
- Ortega-Sáenz, P., Caballero, C., Gao, L. and López-Barneo, J. (2018). Testing acute oxygen sensing in genetically modified mice: plethysmography and amperometry. Methods Mol Biol 1742: 139-153.
- Persson, C. G. (2002). Con: mice are not a good model of human airway disease. Am J Respir Crit Care Med 166(1): 6-7; discussion 8.
- Quindry, J. C., Ballmann, G. C., Epstein, E. E. and Selsby, J. T. (2016). Plethysmography Measurements of Respiratory Function in Conscious Unrestrained Mice. J Physiol Sci 66(2): 157-64.
- Quintana, A., Zanella, S., Koch, H., Kruse, S. E., Lee, D., Ramirez, J. M. and Palmiter, R. D. (2012). Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 122(7): 2359-2368.
- Receno, C. N., Eassa, B. E., Cunningham, C. M. and DeRuisseau, L. R. (2019). Young and middle-aged mouse breathing behavior during the light and dark cycles. Physiol Rep 7(8): e14060.
- Vaickus, L. J., Bouchard, J., Kim, J., Natarajan, S. and Remick, D. G. (2010). Assessing pulmonary pathology by detailed examination of respiratory function. Am J Pathol 177(4): 1861-1869.
Article Information
Copyright
Prada-Dacasa et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Prada-Dacasa, P., Urpi, A., Sánchez-Benito, L., Bianchi, P. and Quintana, A. (2020). Measuring Breathing Patterns in Mice Using Whole-body Plethysmography. Bio-protocol 10(17): e3741. DOI: 10.21769/BioProtoc.3741.
- Bolea, I., Gella, A., Sanz, E., Prada-Dacasa, P., Menardy, F., Bard, A. M., Machuca-Marquez, P., Eraso-Pichot, A., Modol-Caballero, G., Navarro, X., Kalume, F. and Quintana, A. (2019). Defined neuronal populations drive fatal phenotype in a mouse model of Leigh syndrome. Elife 8: e47163.
Category
Neuroscience > Nervous system disorders > Animal model
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










