- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
High-level Production of Recombinant Membrane Proteins Using the Engineered Escherichia coli Strains SuptoxD and SuptoxR
Published: Vol 10, Iss 15, Aug 5, 2020 DOI: 10.21769/BioProtoc.3710 Views: 5202
Reviewed by: Ali Asghar KermaniThibaud T. RenaultAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Prokaryotic Expression and Purification of the hSox2-HMG Domain
Lijie Yang [...] Jingjun Hong
Aug 20, 2025 2369 Views

An Optimized Enzyme-Coupled Spectrophotometric Method for Measuring Pyruvate Kinase Kinetics
Saurabh Upadhyay
Aug 20, 2025 2446 Views

On-Column Dual-Gradient Refolding for Efficient Recovery of Insoluble Affinity-Tagged Recombinant Proteins
Anna Vlaskina [...] Maxim Patrushev
Feb 5, 2026 42 Views
Abstract
We have previously described the development of two specialized Escherichia coli strains for high-level recombinant membrane protein (MP) production. These engineered strains, termed SuptoxD and SuptoxR, are capable of suppressing the cytotoxicity caused by MP overexpression and of producing greatly enhanced MP yields. Here, we present a Bio-protocol that describes gene overexpression and culturing conditions that maximize the accumulation of membrane-integrated and well-folded recombinant MPs in these strains.
Keywords: Recombinant protein productionBackground
MPs perform a variety of critical functions in the cells of all living organisms (Wagner et al., 2006; Schlegel et al., 2010) and constitute major targets for current and future pharmaceuticals (Yildirim et al., 2007). Acquiring sufficient amounts of isolated protein is a prerequisite for their biochemical and structural studies, which in turn can lead to a deeper understanding of their functions and the discovery of new MP-targeting drugs.
Because MPs are typically encountered in their native environments at very low abundances, heterologous hosts have been routinely used for their recombinant overexpression and subsequent purification. Many different systems have been utilized as overexpression hosts for a large variety of MPs of both prokaryotic and eukaryotic origin (Wagner et al., 2006). Among these, Escherichia coli has been one of the most popular ones, owing to its very low cost and ease of use (Makino et al., 2011). Indeed, this bacterium has been successfully utilized for the production of approximately 20% of all recombinantly produced MP structures that are deposited in the Protein Data Bank (Dilworth et al., 2018). Despite these advantages and successes, the use of E. coli as a heterologous host for MP production is often accompanied by severe toxicity, low levels of final biomass and minute final yields (Miroux and Walker, 1996; Wagner et al., 2007; Link et al., 2008; Gubellini et al., 2011).
In order to address these challenges, we have recently developed two specialized E. coli strains, named SuptoxD and SuptoxR, which enable high-level production of recombinant MPs (Gialama et al., 2017a and 2017b). When used as expression hosts, these strains exert a dual positive effect compared to wild-type bacteria:
(1)They suppress the toxicity that frequently accompanies the MP overexpression process, thus enabling enhanced levels of final bacterial biomass, and
(2)They markedly increase the cellular accumulation of membrane-incorporated and properly folded protein.
Combined, these two positive effects result in dramatically enhanced volumetric yields for various recombinant MPs (Gialama et al., 2017a and 2017b). Importantly, these strains have been optimized to enable the production of high-quality recombinant MPs at quantities sufficient for functional and structural studies (Michou et al., 2019). Up to now, we have tested a broad panel of recombinant MPs of both prokaryotic and eukaryotic origin and with different characteristics, all of which are described in Gialama et al. (2017a and 2017b) and in Michou et al. (2019).
The toxicity-suppressing and cellular production-promoting capabilities of SuptoxD and SuptoxR are based on the overexpression of either one of the effector genes djlA or rraA, respectively (Gialama et al., 2017a). DjlA (DnaJ-like protein A) is a single-pass integral MP that functions mainly as a co-chaperone for the central bacterial molecular chaperone DnaK (Clarke et al., 1996). On the other hand, RraA (Regulator of ribonuclease activity A) is known to act as a regulator of the mRNA-degrading activity of RNase E, and rraA overexpression has been found to affect the levels of more than 2,000 different mRNAs in E. coli (Lee et al., 2003). We have previously found that (i) DjlA and RraA act independently, i.e., the beneficial effects of each protein on recombinant MP production occur through a mechanism that does not involve the other, and in a non-additive manner; (ii) full-length and membrane-bound DjlA is required for exerting its beneficial effects on recombinant MP production in E. coli SuptoxD; (iii) the MP production-promoting properties of DjlA in SuptoxD are mediated through the action of the molecular chaperone DnaK; (iv) the observed RraA-mediated effects in E. coli SuptoxR involve the ribonucleolytic activity of RNase E; and (v) DjlA and RraA are unique among similar E. coli proteins in their ability to promote bacterial recombinant MP production (Gialama et al., 2017b). The exact molecular mechanism with which DjlA and RraA suppress MP-induced toxicity and enhance recombinant MP production in E. coli SuptoxD and SuptoxR, respectively, is still under investigation.
Here, we present a protocol that describes gene overexpression and culturing conditions that maximize the accumulation of membrane-integrated and well-folded recombinant MPs when using SuptoxD and SuptoxR. As structural biology of MPs has entered a new era, we believe that these specialized strains will be broadly utilized to address some of the important challenges of MP production and will facilitate the acquirement of sufficient quantities of high-quality recombinant MPs.
Materials and Reagents
- Nylon-membrane Syringe filter 0.2 μm pore size, 25 mm diameter, sterile (Corning, catalog number: CLS431224 )
- HiLoad Superdex 200 16/600 column (GE Healthcare, catalog number: GE28-9893-35 )
- Sterile pipette tips, 10-200 µl (Greiner Bio, catalog number: 739290 )
- Sterile pipette tips, 200-1,000 µl (Greiner Bio, catalog number: 740290 )
- Sterile culture tubes (Sigma-Aldrich, catalog number: C1048-72EA )
- Sterile closures for culture tubes (Sigma-Aldrich, catalog number: C1298-100EA )
- Sterile centrifugation tubes 1.5 ml (Eppendorf, catalog number: 616201 )
- Sterile Falcon tubes 15 ml (Greiner Bio, catalog number: 188271 )
- Sterile Falcon tubes 50 ml (Greiner Bio, catalog number: 227261 )
- Semi-micro-cuvettes (Greiner, catalog number: 613101 )
- Polypropylene centrifuge bottles, 500 ml (Celltreat, catalog number: 229468 )
- Polycarbonate ultracentrifuge bottles, 3 ml (Beckman, catalog number: 355618 )
- Polypropylene chromatography columns, 5 ml (Pierce, catalog number: 29922 )
- Amicon® Ultra-15 Centrifugal Filter Unit (Merck, catalog number: UFC901024 )
- 96-well black plates for fluorescence measurements (Greiner, F-bottom, catalog number: 655076 )
- Polyvinylidene fluoride (PVDF) membrane (Merck, catalog number: IPVH00010 )
- E. coli SuptoxD cells
Genotype: F- λ– Δ(ara-leu)7697 [araD139]B/r Δ(codB-lacI)3 galK16 galE15 e14- mcrA0 relA1 rpsL150(StrR) spoT1 mcrB1 hsdR2(r-m+) pSuptoxD
Note: E. coli SuptoxD carry either the pSuptoxD or the pSuptoxD[untagged] plasmid (see below). - E. coli SuptoxR cells
Genotype: F- λ– Δ(ara-leu)7697 [araD139]B/r Δ(codB-lacI)3 galK16 galE15 e14- mcrA0 relA1 rpsL150(StrR) spoT1 mcrB1 hsdR2(r-m+) pSuptoxR
Note: E. coli SuptoxR carry either the pSuptoxR or the pSuptoxR[untagged] plasmid (see below). - E. coli MC1061 cells (Coli Genetic Stock Center, catalog number: 6649 )
Genotype: F- λ– Δ(ara-leu)7697 [araD139]B/r Δ(codB-lacI)3 galK16 galE15 e14- mcrA0 relA1 rpsL150(StrR) spoT1 mcrB1 hsdR2(r-m+)
Note: We have used E. coli MC1061 as a background host for the co-expression the djlA and rraA effector genes and for the evaluation of the performance of SuptoxD and SuptoxR extensively and successfully. However, we have found that the beneficial effects of DjlA and RrraA on MP productivity are independent of the use of MC1061 and that other E. coli K-12 and B strains can also be utilized as background hosts (Gialama et al., 2017a). - Plasmid pASK-MP
Notes:- This plasmid can be generated by cloning a gene encoding a prokaryotic or eukaryotic MP into the pASK75 vector backbone (Biometra, Göttingen) (Skerra, 1994). We recommend flanking the MP’s sequence between the XbaI and anyone of the remaining restriction sites of the plasmid’s multiple cloning site (MCS), in order to remove the OmpA signal sequence.
- In order to facilitate MP purification via immobilized metal affinity chromatography (IMAC), we recommend inserting a poly-Histidine tag in-frame of the expressed MP. However, other tags and means of MP purification can be used according to the user’s particular preferences and needs.
- In order to easily monitor MP production, we recommend fusing the GFP reporter protein downstream of the MP of interest (Drew et al., 2001 and 2008).
- We have used the vector pASK-MP extensively for production of various recombinant MPs in E. coli SuptoxD and SuptoxR. However, we have found that the beneficial effects of these strains on MP productivity are independent of the use of the tet promoter of pASK75 (Gialama et al., 2017a). Thus, recombinant MPs can be overexpressed in these strains using other types of promoters and plasmids as well.
- pSuptoxD[untagged]
Note: This plasmid overexpresses djlA, the gene encoding the E. coli membrane-bound DnaK co-chaperone DjlA, upon induction with L(+)-arabinose. - pSuptoxD
Notes:- This plasmid overexpresses djlA, the gene encoding the E. coli membrane-bound DnaK co-chaperone DjlA, with a poly-histidine tag at its C-terminus, upon induction with L(+)-arabinose.
- Overexpression of the SuptoxD system can be monitored by western blotting using an anti-His antibody, according to standard protocols. The molecular weight of the expressed protein, i.e., DjlA-His6, is ~32 kDa.
- As we have found that the presence of the poly-histidine tag does not affect the activity of DjlA, we recommend using an untagged version of pSuptoxD, i.e., pSuptoxD[untagged], for MP purification via IMAC.
- pSuptoxR[untagged]
Note: This plasmid overexpresses rraA, the gene encoding the inhibitor of the E. coli RNase E RraA, upon induction with L(+)-arabinose. - pSuptoxR
Notes:- This plasmid overexpresses rraA, the gene encoding the inhibitor of the E. coli RNase E RraA, with a poly-histidine tag at its C-terminus upon induction with L(+)-arabinose.
- Overexpression of the SuptoxR system can be monitored by western blotting using an anti-His antibody, according to standard protocols. The molecular weight of the expressed protein, i.e., RraA-His6, is ~20 kDa.
- As we have found that the presence of the poly-histidine tag does not affect the activity of RraA, we recommend using an untagged version of pSuptoxR, i.e., pSuptoxR[untagged], for MP purification via IMAC.
- Sodium chloride for analysis, ACS, ISO (Applichem, catalog number: 131659 )
- Tryptone BioChemica BC (Applichem, catalog number: A1553 )
- Yeast extract BioChemica BC (Applichem, catalog number: A1552 )
- Agar bacteriology grade BC (Applichem, catalog number: A0949 )
- Ampicillin sodium salt (Sigma-Aldrich, catalog number: A9518 )
- Chloramplenicol (Sigma-Aldrich, catalog number: C0378 )
- L(+)-Arabinose (Applichem, catalog number: A9728 )
- Anhydrotetracycline hydrochloride (Sigma-Aldrich, catalog number: 37919 )
- Sodium hydroxide (NaOH)
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S7907 )
- Sodium dihydrogen phosphate, anhydrous (Chemlab, catalog number: CL00.1496 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Tris Base (Fisher, catalog number: BP154-1 )
- Tween 20 (Fisher, catalog number: BP337 )
- Mini-PROTEAN TGX Precast 4-20% resolving gels (Bio-Rad, catalog number: 456-1094 )
- Non-fat dry milk (Sigma-Aldrich, catalog number: M7409 )
- Monoclonal anti-polyhistidine-peroxidase antibody produced in mouse (Sigma-Aldrich, catalog number: A7058 )
- Pierce ECL western blotting substrate kit (Thermo Fisher Scientific, catalog number: 32106 )
- n-dodecyl β-D-maltoside (DDM) (Glycon Biochemicals, catalog number: D97002 )
- Glycerol (Fisher, catalog number: G/0650/21 )
- Imidazole (Applichem, catalog number: A1073 )
- β-mercaptoethanol (Sigma, catalog number: M6250 )
- Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626 )
- Ni-NTA Agarose (Qiagen, catalog number: 30230 )
- Ethanol absolute (ACROS Organics, catalog number: 448450025 )
- Luria-Bertani broth (LB) (see Recipes)
- Antibiotics (see Recipes)
- Inducers of protein production (see Recipes)
- Lysis buffer (see Recipes)
- Wash buffer (see Recipes)
- Elution buffer (see Recipes)
- Sodium dodecyl sulphate (SDS) sample buffer (6x) (see Recipes)
- Tris-buffered saline with Tween-20 (TBST) (see Recipes)
- Phosphate-buffered saline (PBS) (see Recipes)
- DDM solubilization buffer (see Recipes)
- Size exclusion buffer (see Recipes)
Equipment
- Centrifuge MiniSpin (Eppendorf, Mini Spin, catalog number: 5452000018 )
- High-speed refrigerated centrifuge (Kubota, catalog number: 7780 )
- Ultracentrifuge (Beckman Coulter, model: Optima LE-80K )
- Shaking incubator (Eppendorf, model: New BrunswickTM Innova® 44, catalog number: M1282-0006 )
- Roller mixer (Kisker, catalog number: L005-SLN )
- Microplate reader (Tecan, model: Safire II )
- Electrophoresis power supply (Consort, catalog number: EV231 )
- Imaging system for DNA and protein analysis (ChemiDoc-It2 Imaging System (UVP))
- Sonicator equipped with a 3 mm diameter probe (Qsonica, catalog number: Q125-110 )
- Spectrophotometer UV-VIS (Hitachi, model: U2000 )
- Mini PROTEAN Tetra Vertical Electrophoresis Cell (Bio-Rad, catalog number: 1658005 )
- Type 70Ti fixed-angle titanium rotor (Beckman, catalog number: 337922 )
- Homogenizer (Heidolph, model: RΖR1 )
Procedure
The use of the engineered E. coli strains SuptoxD and SuptoxR for the high-level production of recombinant MPs is outlined in Figure 1.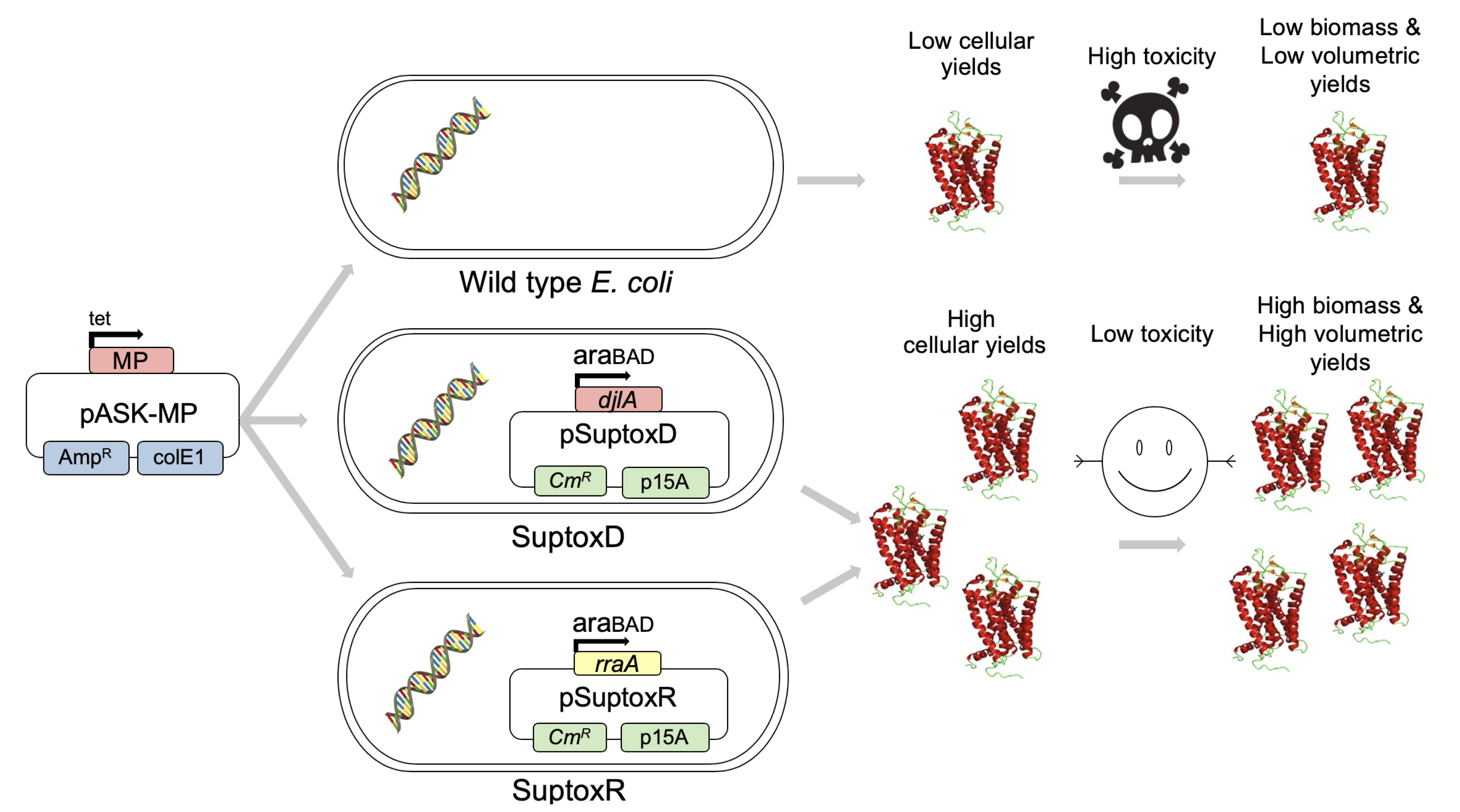
Figure 1. Overview of the E. coli SuptoxD and SuptoxR systems. The toxicity-suppressing and cellular production-promoting capabilities of E. coli SuptoxD and SuptoxR that result in dramatically enhanced volumetric yields for various recombinant MPs, are based on the overexpression of the E. coli genes djlA or rraA, respectively. The effector genes are overexpressed from the vectors pSuptoxD and pSuptoxR under the control of the araBAD promoter and its inducer L(+)-arabinose. For the production of recombinant MPs in these strains, we typically use pASK75-based plasmids under the control of a tet promoter and its inducer aTc (pASK-MP vector).
The procedure of the recombinant MP production and purification is outlined in Figure 2.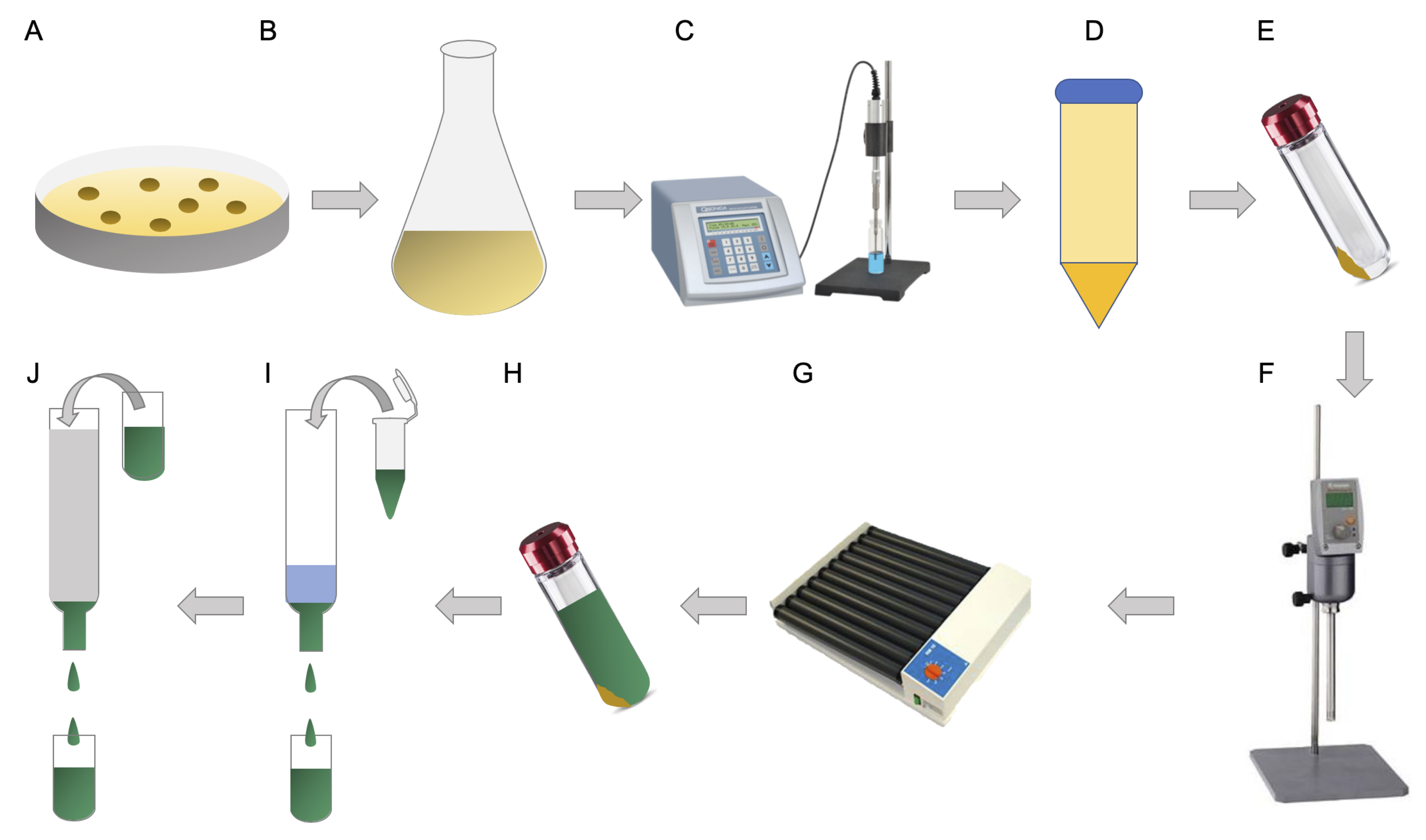
Figure 2. Overview of the recombinant MP production and purification procedure using the E. coli SuptoxD and SuptoxR strains. A. Transform either one of the SuptoxD or SuptoxR strains with the plasmid encoding the MP of interest. B. Overexpress the MP of interest together with the pSuptoxD or pSuptoxR system, at 25 °C for 16 h and using the appropriate inducers. C. Lyse cells by sonication. D. Centrifuge the total cell lysates and transfer the soluble supernatant into an ultracentrifugation tube. E. Ultracentrifuge the soluble cell lysate and collect the pelleted membranes. F. Mechanically resuspend the membranes in DDM-solubilization buffer using a homogenizer. G. Rotate for at least 1 h at 4 °C. H. Ultracentrifuge and collect the supernatant containing the solubilized membranes. I-J. Purify the solubilized membranes using IMAC and SEC.
- MP overexpression in shake flasks (Under sterile conditions)
- Transform (chemically or electro-) competent SuptoxD or SuptoxR cells with the pASK-MP vector and plate on LB agar containing 100 μg/ml ampicillin and 40 μg/ml chloramphenicol.
Notes:- When performing recombinant production of a specific MP of interest for the first time, we recommend using both the SuptoxD and SuptoxR strains, in order to determine the particular strain that yields higher levels of MP production.
- For the expression of highly toxic or otherwise difficult-to-express MPs that accumulate at very low yields, we highly recommend to use the pSuptoxD or pSuptoxR plasmid in isolated form and perform double transformation of pSuptoxD or pSuptoxR with the pASK-MP plasmid in fresh competent E. coli MC1061 cells. Other E. coli strains can also be used as hosts for the pSuptox vectors.
- We have used the pASK75 vector backbone for the expression of the MP of interest extensively. However, other expression vectors can also be used, provided that they are compatible with the pSuptoxD/R system (chloramphenicol resistance, p15A origin of replication, araBAD promoter) and preferably contain an inducible promoter with tight regulation.
- Pick a single transformed bacterial colony and inoculate liquid LB cultures supplemented with the selection antibiotics 100 μg/ml ampicillin and 40 μg/ml chloramphenicol for pASK-MP and SuptoxD/R system maintenance. Incubate at 37 °C for 16 h with constant shaking at 200-220 rpm.
Notes:- We highly recommend using freshly transformed E. coli cells for all MP production experiments in order to obtain maximum protein yields.
- The volume of the LB cultures should be enough for next-day’s inoculation.
- The following day prepare a 2% sub-culture in fresh LB supplemented with the appropriate antibiotics, as well as 0.01% or 0.2% (w/v) L(+)-arabinose for the induction of the SuptoxD and SuptoxR system respectively. Incubate at 30 °C with shaking until an optical density at 600 nm (OD600) of 0.5.
Notes:- The recommended L(+)-arabinose concentrations have been determined following expression optimization in SuptoxD/R using certain model recombinant MPs (Michou et al., 2019). Other MPs, however, may require other concentrations for their optimal accumulation.
- Keep working stock solution of arabinose (20% w/v) in aliquots to avoid contamination.
- Initiate overexpression of the target MP by adding 0.2 μg/ml anhydrotetracycline (aTc) and incubate overnight at 25 °C with shaking.
Notes:- We highly recommend using freshly prepared and light-protected aTc.
- We highly recommend initiating MP overexpression at an OD600 of precisely 0.5 as we have found that deviations result in lower protein yields.
- We recommend to not exceed 16 h of overexpression in order to minimize the accumulation of genomic/plasmid mutations that suppress MP production.
- The recommended aTc concentration has been determined following expression optimization in SuptoxD/R using certain model recombinant MPs (Michou et al., 2019). Other MPs, however, may require other concentrations for their optimal accumulation.
- Harvest cells by centrifugation at 4,000 x g for 10 min at 4 °C.
- Check for MP production by western blotting or activity assay according to standard protocols.
Notes:- Due to the MP-induced toxicity, it is not unusual for genomic/plasmid mutations to accumulate and bypass the overproduction of MP. This will result in a high increase of the culture’s OD600 and low yield of MP production. For this reason, MP production should always be checked before continuing with MP purification.
- When overexpressing an MP-GFP fusion, the bacterial fluorescence can be used as an indicator of protein production. In this case, cells corresponding to an OD600 = 1 should be resuspended in 100 μl PBS and fluorescence should be measured:
- Using a 96-well plate reader at 510 nm after excitation at 488.
- Using a flow cytometer equipped with a 488 nm solid state laser for excitation and a 530/30 nm band pass filter for detection. In this case, events should be first gated in a forward scatter (FSC) vs. side scatter (SSC) plot in order to eliminate non-cellular events and then plotted in a histogram of fluorescence intensity detected by the FITC (fluorescein isothiocyanate) channel photomultiplier tube (PMT) vs. number of events, in order to estimate the mean fluorescence of the population.
- Transform (chemically or electro-) competent SuptoxD or SuptoxR cells with the pASK-MP vector and plate on LB agar containing 100 μg/ml ampicillin and 40 μg/ml chloramphenicol.
- Bacterial membrane isolation and MP extraction
- Resuspend a cell pellet from a 1 L culture in 10 ml ice-cold lysis buffer.
- Lyse cells by sonication for 4 x 25 s on ice, with a 1 min interval between each sonication.
- Centrifuge the total cell lysates (10,000 x g, 15 min, 4 °C) and transfer carefully the soluble supernatant into an ultracentrifugation tube.
- Ultracentrifuge the soluble fraction at 130,000 x g for 1 h at 4 °C using a 70-Ti rotor, in order to pellet the bacterial membranes.
- Collect the pelleted membranes using a spatula and mechanically resuspend them in 5 ml ice-cold DDM-solubilisation buffer using a homogenizer. The homogenate should contain no visible particles.
Note: Care should be taken when collecting the membranes as they form a very sticky and difficult to handle pellet. - Rotate the resuspension at 180 rpm for at least 1 h at 4 °C, in order for MP extraction to take place.
Note: A range of different DDM concentrations and incubation periods should be tested in order to determine the optimal conditions for MP extraction. When using MP-GFP fusions or chromophore-containing MPs, such as certain bacteriorhodopsins (Michou et al., 2019), this procedure can be easily optimized by regular centrifugations and monitoring of the fluorescence levels or discoloration respectively, of the resulting pellet. - Ultracentrifuge at 130,000 x g for 1 h at 4 °C and collect the supernatant that contains the extracted MP.
- Check for successful MP extraction by western blotting or activity assay according to standard protocols.
Note: When overexpressing an MP-GFP fusion, we highly recommend using in-gel fluorescence analysis as a quality control of the extraction of well-folded and active MP (Geertsma et al., 2008). In this case, the following procedure should be followed:- Mix 100 μl of the supernatant containing the extracted MP with 20 μl of 6x SDS loading dye.
- Load 50 μl of sample on a 4-20% precast polyacrylamide gel without prior boiling of the sample and run the gel at a constant voltage of 80 V.
- Visualize the MP-GFP fluorescent band on an imaging system, e.g., ChemiDoc-It2 Imaging System, equipped with a CCD camera and a GFP filter, after exposure of about 3 s.
- Western blotting of the analysed proteins according to standard protocols should result in the visualization of the MP’s dual band migration, with the lower band corresponding to the fluorescent and well-folded MP-GFP fusion and the upper band corresponding to non-fluorescent and misfolded MP-GFP fusion (Drew et al., 2008; Geertsma et al., 2008).
- Analyzing the same samples after boiling for 10 min with SDS-PAGE and western blotting results in full denaturation of the fusion and single band migration that lacks fluorescence.
- Store the supernatant on ice until further use or purification.
- Small-scale, two-step MP purification for initial studies using immobilized metal affinity chromatography (IMAC) and size exclusion chromatography (SEC) (All steps should be performed at 4 °C)
- Transfer 1 ml of Ni-NTA agarose resin to a 15 ml tube and remove the supernatant after brief centrifugation at low speed (1,000 x g, 1 min). Resuspend in 2 ml lysis buffer and mix by gently inverting.
- Mix the supernatant containing the extracted MP with the equilibrated resin from the above step at 4 °C for 1 h on a roller mixer.
- Equilibrate a 5 ml polypropylene chromatography column with 1 column volume (CV) of lysis buffer and load the mixture onto the equilibrated column.
- Collect the flow-through, and reload it onto the column. Repeat for a total of 5-6 times.
Note: This step should be repeated multiple times in order to ensure complete binding of the MP to the nickel resin. When using MP-GFP fusions or chromophore-containing MPs, this step should be repeated until a non-fluorescent or colourless flow-through is collected. - Wash the column-bound protein with 40 ml wash buffer.
- Elute the MP with 6 ml elution buffer.
- Concentrate the elution to a final volume of approximately 500 μl using a centrifugal filter.
- Equilibrate a Superdex 200 column with 2 CV of size exclusion buffer at 1 ml/min, ensuring that the pressure does not exceed the maximum limit of 0.3 MPa.
- Inject the concentrated sample onto the column and pass 1 CV of size exclusion buffer through the system while collecting the eluted fractions.
- Pool together the fractions that correspond to the MP’s peak.
- Quantify the amount of purified MP by calculating the absorbance at 280 nm of the integrated peak area and using the MP’s extinction coefficient (ε280).
Note: By following this protocol, and using the SuptoxD strain we have obtained 1 mg per L of bacterial culture of purified human bradykinin receptor 2 (BR2), which under normal conditions exhibits very high levels of toxicity and low yields when overexpressed in E. coli (Michou et al., 2019). This amount was 14-fold higher than the corresponding yield from wild-type E. coli. Similar results were achieved by the use of SuptoxR in overexpressing the large bacterial mechano-sensitive ion channel (MscL), in which case we isolated 0.33 mg per L of bacterial shake flask culture, a > 10-fold increased yield compared to wild-type E. coli (Michou et al., 2019). However, other means of MP purification can be used according to the user’s particular preferences and needs. - Evaluate MP purity by SDS-PAGE analysis and Coomassie staining.
Note: For highly concentrated MP fractions, we recommend running the gel at a very low constant current, such as 12-13 mA.
Recipes
- Luria-Bertani broth (LB)
10 g/L tryptone
5 g/L yeast extract
10 g/L NaCl
Adjust pH to 7.0 with 5 N NaOH
Autoclave for sterilization for 20 min at 15 psi - Antibiotics
- 40 mg/ml chloramphenicol in ethanol 99.8%, store at -20 °C
- 100 mg/ml ampicillin in MilliQ water, sterilize the solution using a 0.22 μm syringe filter, store at -20 °C
- Inducers of protein production
- 20% w/v L(+)-arabinose in MilliQ water, sterilize the solution using a 0.22 μm syringe filter, store at room temperature
- 20 mg/L anhydrotetracycline (aTc) in ethanol 99.8%, store at -20 °C
- Lysis buffer
300 mM NaCl
50 mM NaH2PO4
15% glycerol
5 mM dithiothreitol (add immediately before use)
0.1 mM PMSF (add immediately before use)
Adjust pH to 7.5
Store at 4 °C - Wash buffer
20 mM imidazole
300 mM NaCl
50 mM NaH2PO4
Adjust pH to 8
Add 0.1% w/v DDM (add immediately before use)
Store at 4 °C - Elution buffer
250 mM imidazole
300 mM NaCl
50 mM NaH2PO4
Adjust pH to 8
Add 0.1% w/v DDM (add immediately before use)
Store at 4 °C - Sodium dodecyl sulphate (SDS) sample buffer (6x)
6% w/v SDS
300 mM Tris
15% v/v glycerol
0.01% w/v bromophenol blue
10% v/v β-mercaptoethanol
Adjust pH to 6.8 - Tris-buffered saline with Tween-20 (TBST)
20 mM Tris base
150 mM NaCl
Adjust pH to 7.5
0.1% v/v Tween-20 - Phosphate-buffered saline (PBS)
137 mM NaCl
2.7 mM KCl
10 mM Na2HPO4
1.8 mM KH2PO4
Adjust pH to 7.4
Autoclave for sterilization for 20 min at 15 psi - DDM solubilization buffer
Lysis buffer
2.5% w/v DDM (add immediately before use) - Size exclusion buffer
This buffer should be selected according to the characteristics of the MP of interest. Some examples include:
PBS supplemented with 0.1% w/v DDM or 10 mM HEPES, 0.4 M NaCl, 0.05% w/v DDM, pH 7.2
Acknowledgments
This work has received funding from the following: (i) the Greek State Scholarships Foundation (Idryma Kratikon Ypotrofion–IKY) scholarship, funded by the action “Strengthening human research potential through doctoral research” of the Partnership Agreement “Development of human potential, education and lifelong learning” 2014-2020 (Grant number: MIS 5000432), which is co-financed by the European Structural and Investment Fund (ESIF) and the Greek State, (ii) the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Project “ProMiDis”; grant agreement no. 819934).
Competing interests
GS is inventor on a patent application for E. coli SuptoxD and SuptoxR (PCT/EP2017/025168). The authors declare no other competing interests.
References
- Clarke, D. J., Jacq, A. and Holland, I. B. (1996). A novel DnaJ-like protein in Escherichia coli inserts into the cytoplasmic membrane with a type III topology. Mol Microbiol 20(6): 1273-1286.
- Dilworth, M. V., Piel, M. S., Bettaney, K. E., Ma, P., Luo, J., Sharples, D., Poyner, D. R., Gross, S. R., Moncoq, K. and Henderson, P. J. (2018). Microbial expression systems for membrane proteins. Methods 147: 3-39.
- Drew, D., Newstead, S., Sonoda, Y., Kim, H., Von Heijne, G. and Iwata, S. (2008). GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nature protocols 3(5): 784.
- Drew, D. E., von Heijne, G., Nordlund, P. and de Gier, J. W. (2001). Green fluorescent protein as an indicator to monitor membrane protein overexpression in Escherichia coli. FEBS Lett 507(2): 220-224.
- Geertsma, E. R., Groeneveld, M., Slotboom, D. J. and Poolman, B. (2008). Quality control of overexpressed membrane proteins. Proc Natl Acad Sci U S A 105(15): 5722-5727.
- Gialama, D., Kostelidou, K., Michou, M., Delivoria, D. C., Kolisis, F. N. and Skretas, G. (2017a). Development of Escherichia coli strains that withstand membrane protein-induced toxicity and achieve high-level recombinant membrane protein production. ACS Synth Biol 6(2): 284-300.
- Gialama, D., Delivoria, D. C., Michou, M., Giannakopoulou, A. and Skretas, G. (2017b). Functional requirements for DjlA- and RraA-mediated enhancement of recombinant membrane protein production in the engineered Escherichia coli strains SuptoxD and SuptoxR. J Mol Biol 429(12): 1800-1816.
- Gubellini, F., Verdon, G., Karpowich, N. K., Luff, J. D., Boel, G., Gauthier, N., Handelman, S. K., Ades, S. E. and Hunt, J. F. (2011). Physiological response to membrane protein overexpression in E. coli. Mol Cell Proteomics 10(10): M111 007930.
- Lee, K., Zhan, X., Gao, J., Qiu, J., Feng, Y., Meganathan, R., Cohen, S. N. and Georgiou, G. (2003). RraA. a protein inhibitor of RNase E activity that globally modulates RNA abundance in E. coli. Cell 114(5): 623-634.
- Link, A. J., Skretas, G., Strauch, E. M., Chari, N. S. and Georgiou, G. (2008). Efficient production of membrane-integrated and detergent-soluble G protein-coupled receptors in Escherichia coli. Protein Sci 17(10): 1857-1863.
- Makino, T., Skretas, G. and Georgiou, G. (2011). Strain engineering for improved expression of recombinant proteins in bacteria. Microb Cell Fact 10: 32.
- Michou, M., Kapsalis, C., Pliotas, C. and Skretas, G. (2019). Optimization of recombinant membrane protein production in the engineered Escherichia coli strains SuptoxD and SuptoxR. ACS Synth Biol 8(7): 1631-1641.
- Miroux, B. and Walker, J. E. (1996). Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol 260(3): 289-298.
- Schlegel, S., Klepsch, M., Gialama, D., Wickstrom, D., Slotboom, D. J. and de Gier, J. W. (2010). Revolutionizing membrane protein overexpression in bacteria. Microb Biotechnol 3(4): 403-411.
- Skerra, A. (1994). Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene 151(1-2): 131-135.
- Wagner, S., Baars, L., Ytterberg, A. J., Klussmeier, A., Wagner, C. S., Nord, O., Nygren, P. A., van Wijk, K. J. and de Gier, J. W. (2007). Consequences of membrane protein overexpression in Escherichia coli. Mol Cell Proteomics 6(9): 1527-1550.
- Wagner, S., Bader, M. L., Drew, D. and de Gier, J. W. (2006). Rationalizing membrane protein overexpression. Trends Biotechnol 24(8): 364-371.
- Yildirim, M. A., Goh, K. I., Cusick, M. E., Barabasi, A. L. and Vidal, M. (2007). Drug-target network. Nat Biotechnol 25(10): 1119-1126.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Michou, M., Delivoria, D. C. and Skretas, G. (2020). High-level Production of Recombinant Membrane Proteins Using the Engineered Escherichia coli Strains SuptoxD and SuptoxR. Bio-protocol 10(15): e3710. DOI: 10.21769/BioProtoc.3710.
Category
Microbiology > Heterologous expression system > Escherichia coli
Microbiology > Microbial physiology > Membrane property
Molecular Biology > Protein > Expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








