- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Induction and Detection of Acrosomal Exocytosis in Human Spermatozoa
(*contributed equally to this work) Published: Vol 10, Iss 14, Jul 20, 2020 DOI: 10.21769/BioProtoc.3689 Views: 4795
Reviewed by: Alexandros AlexandratosShweta Mayank BhagwatAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Imaging VIPER-labeled Cellular Proteins by Correlative Light and Electron Microscopy
Julia K. Doh [...] Kimberly E. Beatty
Nov 5, 2019 6652 Views

A New Method for Studying RNA-binding Proteins on Specific RNAs
Weiping Sun [...] Min Zhuang
May 20, 2021 10608 Views

Flow Cytometry Analysis of Microglial Phenotypes in the Murine Brain During Aging and Disease
Jillian E. J. Cox [...] Sarah R. Ocañas
Jun 20, 2024 3617 Views
Abstract
The acrosome reaction is a highly regulated exocytotic event that primes spermatozoa for successful fertilization. Upon induction, acrosomal exocytosis proceeds via a wave of vesiculation that radiates across the sperm head, destabilizing the acrosomal vesicle and resulting in the release of the acrosomal contents. Having shed their acrosome, spermatozoa are then capable of penetrating the outer vestments of the oocyte and initiating fertilization. Accordingly, the failure of spermatozoa to complete an acrosome reaction represents a relatively common etiology in male infertility patients, and the ability to induce acrosomal exocytosis has found clinical utility in the evaluation of sperm fertilizing capacity. Here, we firstly describe protocols for driving the capacitation of human spermatozoa in vitro using chemically defined media in order to prime the cells for completion of acrosomal exocytosis. We then describe methodology routinely used for the induction of acrosomal exocytosis incorporating either a physiological agonist (i.e., the steroidal hormone, progesterone) or pharmacological reagent (i.e., the divalent cation ionophore, A23187). Finally, we describe the application of histochemical and immunofluorescence techniques that can be applied to study the completion of the acrosome reaction. Such protocols have important diagnostic utility for sperm function testing in both clinical and andrological research laboratories.
Keywords: AcrosomeBackground
Unique to the male gamete, the acrosome is a lysosome-like membranous organelle that adorns the anterior region of the sperm head and is delineated by both inner and outer acrosomal membranes (Hermo et al., 2010a and 2010b). The acrosomal vesicle, so formed, encapsulates a myriad of structural and enzymatic components that are compartmentalized into either soluble or insoluble fractions (Guyonnet et al., 2012; Guyonnet et al., 2014). The latter of these, termed the acrosomal matrix, functions as a stable scaffold that allows for the controlled release of matrix associated proteins necessary for fertilization during the acrosome reaction (Guyonnet et al., 2014). This reaction is a distinctive exocytotic event that is initiated by the formation of multiple sites of fusion between the outer acrosomal membrane and the overlying plasma membrane. As this vesiculation process propagates across the anterior region of the sperm head, it destabilizes the acrosomal vesicle leading to the formation of hybrid membrane vesicles and the concomitant release of acrosomal contents (Barros et al., 1967). This, in turn, renders the fertilizing spermatozoon competent to penetrate the formidable outer vestments of the oocyte and access the underlying oocyte plasma membrane (Buffone et al., 2008).
Recent work suggests that the acrosome reaction proceeds with relatively slow kinetics through several intermediary steps (Buffone et al., 2012; Stival et al., 2016). These distinctive features underscore the highly specialized nature of this secretory process and the likelihood that it is governed by complex molecular mechanisms. Accordingly, the ability to complete a physiological acrosome reaction necessitates that spermatozoa must have first undergone a functional maturation process known as capacitation (Aitken and Nixon, 2013). Capacitation occurs during sperm ascent of the female reproductive tract and incorporates numerous biochemical and biophysical changes, including the modulation of intracellular ion concentrations, hyperpolarization of the sperm plasma membrane, and the induction of signaling cascades, which culminate in increased protein phosphorylation (Nixon and Bromfield, 2018). While these collective processes prime the sperm cell for completion of an acrosome reaction, the nature of the physiological stimulus responsible for initiating this exocytotic event remains a matter of some controversy (Buffone et al., 2014); with candidate agonists including zona pellucida ligands, follicular fluid and/or progesterone (a steroidal hormone that sperm encounter within the oviduct prior to zona pellucida adhesion) (Tesarik, 1985). Alternatively, acrosomal exocytosis can be recapitulated in vitro using non-physiological pharmacological reagents, such as the divalent cation ionophore, A23187 (Jamil and White, 1981). Indeed, by directly facilitating Ca2+ influx, the A23187 ionophore is relatively forgiving of the need for intracellular signaling mechanisms and effector pathways that otherwise exert strict control over the progression of the acrosome reaction (Reid et al., 2012; Zhou et al., 2017).
Notably, the characteristics of acrosomal exocytosis driven by calcium ionophore differ from those elicited in response to physiological stimuli, such as solubilized zonae pellucidae (Liu and Baker, 1996). Specifically, ionophore induction leads to random vesiculation across the sperm head. By contrast, solubilized zonae pellucidae stimulates a more orderly loss of acrosomal components that begins at the posterior acrosome before proceeding in an anterograde direction (Buffone et al., 2009). Whilst these data lend support to the concept of receptor-mediated events controlling membrane fusion and release of acrosomal components, the limited availability of human zonae pellucidae prevents this resource being widely used as a physiological stimulus in assisted reproductive settings (Liu et al., 2003). Thus, despite its limitations, monitoring of the ability of spermatozoa to undergo an ionophore-induced acrosome reaction has become an important diagnostic criterion of human sperm function testing in clinical laboratories (Nixon and Bromfield, 2020).
Within the clinical setting, the impetus to study acrosomal exocytosis stems from evidence that a failure to complete this event represents a relatively common etiology associated with the defective spermatozoa of male infertility patients; potentially accounting for up to one-third of all cases of failed in vitro fertilization in couples taking recourse to assisted reproductive programs due to male factor infertility (Liu et al., 2001; Liu and Baker, 2003). However, in addition to prospective evaluation of semen quality, assaying the ability spermatozoa to complete an acrosome reaction has utility in the context of andrological research for development of male contraceptives and/or detection of the cytotoxic effects of various environmental insults (Katen et al., 2016; Houston et al., 2018 and 2019). Accordingly, here we describe optimized protocols for the induction and monitoring of human sperm acrosomal exocytosis in an in vitro setting.
Materials and Reagents
- 12-well glass microscope slides (Thermo Fisher Scientific, catalog number: MENX2XER202W )
- Ammonium acetate (Merck, catalog number: A7330 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Merck, catalog number: C5080 )
- Calcium ionophore A23187 (Merck, catalog number: C7522 ), powder stored at 4 °C; stock solution aliquoted and stored at -20 °C
- Coomassie Brilliant Blue G-250 (Merck, catalog number: B0770 )
- D-glucose (Ajax, catalog number: 783 )
- DABCO (Merck, catalog number: D27802 )
- Dibutyryl cyclic adenosine monophosphate (Merck, catalog number: D0627 )
- Diff-Quik Differential Staining Kit (Dade Behring, catalog number: 564-850-02 )
- DPX Mountant (Merck, catalog number: 06522 )
- Eosin Y (Merck, catalog number: E4382 )
- Fluorescein isothiocyanate (FITC) conjugated PSA lectin from Pisum sativum (pea) (Merck, catalog number: L0770 ), stored at 4 °C
- Formaldehyde 37% v/v (Merck, catalog number: 252549 )
- Fructose (Merck, catalog number: F0127 )
- Glacial acetic acid (Biostrategy, catalog number: 20104.332 )
- Glycine (Merck, catalog number: G8898 )
- Ham’s F-10 Nutrient Mix (MP Biomedicals, catalog number: 091440049 )
- HEPES buffer (GE Healthcare, catalog number: SH30237.01 )
- Lectin from Pisum sativum (pea) (Merck, catalog number: L5380 ), stored at 4 °C
- Magnesium sulfate heptahydrate (MgSO4·7H2O) (Merck, catalog number: M5921 )
- Methanol (POCD Scientific, catalog number: Meth25P )
- Mowiol 4-88 (Merck, catalog number: 81381 ), powder stored at room temperature, solution aliquoted and stored at -20 °C
- Paraformaldehyde aqueous solution (Proscitech, catalog number: 15710 )
- Penicillin-Streptomycin (Merck, catalog number: P4458 )
- Pentoxifylline (Merck, catalog number: P1784 )
- Percoll (GE Healthcare, catalog number: 17-0891-01 ), stored at 4 °C
- Phosphate buffered saline (Merck, catalog number: P4417 )
- Polyvinyl alcohol (PVA) (Merck, catalog number: A8136 )
- Potassium chloride (KCl) (Merck, catalog number: P9333 )
- Potassium phosphate monobasic (KH2PO4) (Merck, catalog number: P5655 )
- Progesterone (Merck, catalog number: P0130 )
- Sodium bicarbonate (NaHCO3) (Merck, catalog number: S6297 )
- Sodium chloride (NaCl) (Merck, catalog number: S7653 )
- Sodium citrate (Merck, catalog number: S1804 )
- Sodium pyruvate (Merck, catalog number: P3662 )
- Sodium lactate (Merck, catalog number: L1375 )
- Tris (MP Biomedicals, catalog number: 152176 )
- Water, sterile-filtered, suitable for cell culture (Merck, catalog number: W3500 )
- Capacitating BWW (see Recipes)
- Coomassie Blue stain (see Recipes)
- HOS medium (see Recipes)
- Fixative for diluting sperm (see Recipes)
- Mowiol mounting solution (see Recipes)
- Non capacitating BWW (see Recipes)
- Percoll working solution (see Recipes)
- BWW stock solution (see Recipes)
Equipment
- Finnpipette F1 Variable Volume Pipettes (Thermo Fisher Scientific, 1 to 10 µl, 20 to 200 µl, 100 to 1,000 µl)
- Fluorescence Microscope (Zeiss, model: Axio Imager A1 )
- Biological Phase Contrast Microscope (Olympus, model: CX41 )
- Micro-Osmometer (Fiske, Model 210)
Procedure
- Isolation of good quality human spermatozoa
For assessment of the acrosome reaction it is preferable to use a highly motile sperm preparation free from contaminants such as leukocytes, germ cells and dead spermatozoa. Accordingly, below we describe a density-gradient isolation protocol compatible with the fractionation of high-quality human spermatozoa from within an ejaculate.- Allow 30-60 min for complete liquefaction of the fresh semen, before preparing samples for routine semen analysis in accordance with WHO criteria (World Health Organization, 2010). Score at least 100 cells in each of two duplicates for cell motility, viability and morphology (with at least 5 microscope fields of view being examined in each duplicate count).
- Assess sperm morphology in accordance with WHO criteria in the presence of Diff-Quik staining using 400× magnification and bright field microscopy (World Health Organization, 2010).
- Assess sperm motility using phase contrast microscope optics (400× magnification), and classify cells as either motile (i.e., sperm displaying any form of motility ranging from rapid progressive to non-progressive) or immotile (World Health Organization, 2010).
- Assess sperm viability by Eosin Y exclusion method (dead cells stain red with Eosin Y) (World Health Organization, 2010).
- Assess sperm morphology in accordance with WHO criteria in the presence of Diff-Quik staining using 400× magnification and bright field microscopy (World Health Organization, 2010).
- Fractionate human semen samples over a discontinuous Percoll density gradient (comprising 40% and 80% Percoll suspensions; Recipe 7).
- Assemble the gradient by using a transfer pipette to add 3 ml 40% Percoll to a 15 ml centrifuge tube. Then underlay the 40% fraction with 3 ml 80% Percoll (one gradient is required per semen sample and should be prepared and maintained at 37 °C for no more than 30 min prior to use to avoid loss of gradient).
- Layer semen sample atop the 40% Percoll suspension
- Centrifuge gradient at 500 × g for 30 min at room temperature using a swing-out rotor.
- Assemble the gradient by using a transfer pipette to add 3 ml 40% Percoll to a 15 ml centrifuge tube. Then underlay the 40% fraction with 3 ml 80% Percoll (one gradient is required per semen sample and should be prepared and maintained at 37 °C for no more than 30 min prior to use to avoid loss of gradient).
- Sperm cells collected from the base of the 80% Percoll suspension are nominally referred as ‘good quality’ or ‘mature’ spermatozoa, whereas those partitioning at the 40/80% Percoll interface are referred to as ‘poor quality’ spermatozoa. The latter population of spermatozoa are characterized by reduced functionality, including compromised ability to complete acrosomal exocytosis (Zhou et al., 2017). Thus, recover the former population of good quality cells from the pellet and discard the remainder of the sample partitioning into the supernatant.
- Gently resuspend the sperm pellet in 5 ml of a non-capacitating formulation of Biggers, Whitten and Whittingham medium (BWW) (Biggers, 1971) (Recipes 6 and 8).
- Re-assess sperm motility as described above and then wash the remainder of the cells by centrifugation at 500 × g for 15 min at room temperature in a swing-out rotor. Resuspend the good quality spermatozoa in medium appropriate to experimental requirements.
- Assess the total number of good quality spermatozoa recovered after Percoll isolation by diluting an aliquot (1:20) in fixative (Recipe 4) and loading into the counting chambers of an improved Neubauer hemocytometer. Assess the samples within 10 min and ensure a minimum of 200 spermatozoa are counted per replicate. Calculate the total number of spermatozoa per sample (World Health Organization, 2010).
Note: If, the total number good quality sperm recovered after Percoll isolation is below a threshold of 2 × 106 cells, the experiment should not proceed.
- Allow 30-60 min for complete liquefaction of the fresh semen, before preparing samples for routine semen analysis in accordance with WHO criteria (World Health Organization, 2010). Score at least 100 cells in each of two duplicates for cell motility, viability and morphology (with at least 5 microscope fields of view being examined in each duplicate count).
- Induction of human sperm capacitation
- To induce capacitation in vitro, incubate spermatozoa in a formulation of BWW optimized for the induction of human sperm capacitation (Capacitating BWW; Recipes 1 and 8) as assessed via tyrosine phosphorylation status, zona pellucida binding competence and, importantly in the context of these protocols, the ability to complete an acrosome reaction (Mitchell et al., 2007; Redgrove et al., 2011). Vehicle control and replicate experimental tubes should each contain approximately 10 × 106 spermatozoa suspended in 1 ml of pre-warmed Capacitating BWW medium.
- Incubate spermatozoa at 37 °C under an atmosphere of 5% CO2:95% air for 3 h with a gentle inversion every 30 min to prevent settling of the cells.
Note: The cap of each should be loosened during incubation to facilitate gas exchange. - After incubation, prepare sperm for assessment of their ability to undergo an acrosome reaction utilizing the assay protocols outlined below.
- Stimulation of acrosomal exocytosis in vitro
Here, we describe optimized methodology for the induction of acrosomal exocytosis in capacitated populations of human spermatozoa using either a calcium ionophore (A23187) or progesterone challenge based on studies conducted by Zhou et al. (2017). Whilst both A23187 and progesterone are effective agonists, capable of modulating an increase in cytosolic Ca2+ levels needed to initiate this event, we note that these two stimuli elicit acrosomal exocytosis via distinct mechanisms. Thus, exposure to progesterone reportedly leads to stimulation of ion channels and the initiation of intracellular signaling mechanisms and effector pathways that regulate the progression of the acrosome reaction. In contrast, by directly facilitating Ca2+ influx, the A23187 ionophore has the potential to bypass the downstream regulatory pathways and is thus relatively forgiving of the functional status of exposed spermatozoa (Tateno et al., 2013). It follows that A23187 challenge tends to elicit higher rates of acrosome reaction (~40-50%) than that of progesterone (~30-40%) (Zhou et al., 2017). From a clinical perspective, this variability in agonist response is reflected in acrosome reaction rates following A23187 challenge offering lower discriminative power for predicting in vitro fertilization (IVF) success rate than those achieved using progesterone (Krausz et al., 1996). Moreover, even at relatively high concentrations, progesterone is less cytotoxic than A23187 and thus better suited to the preparation of live acrosome-reacted spermatozoa compatible with downstream clinical applications such as micromanipulation (Parinaud et al., 1992).- To induce acrosomal exocytosis, incubate capacitated human spermatozoa at 37 °C with 1 ml of Capacitating BWW supplemented with either:
- 2.5 µM A23187 for 30 min. Note that a 10 mM stock solution is first diluted 1:2,000 in Capacitating BWW, then mixed 1:1 (v/v) with spermatozoa.
- 15 µM progesterone* for 2 h. Note that a 75 mM stock solution is first diluted 1:500 in Capacitating BWW, then mixed 1:10 (v/v) with spermatozoa.
- DMSO vehicle control (diluted to an equivalent concentration to that used in the preparation of either stimulant).
- 2.5 µM A23187 for 30 min. Note that a 10 mM stock solution is first diluted 1:2,000 in Capacitating BWW, then mixed 1:1 (v/v) with spermatozoa.
- At the completion of the appropriate induction period, remove 10 µl from each tube for assessment of sperm motility and viability to ensure neither are adversely affected by any of the treatments.
- Pellet the remainder of the sperm suspension by centrifugation at 500 × g for 5 min at room temperature in a fixed rotor before resuspension in 500 µl of pre-warmed hypo-osmotic swelling (HOS) medium (Recipe 3) and incubation for an additional 30 min at 37 °C.
Note: The HOS test is used to differentiate those cells that were viable at the time of acrosome reaction induction (i.e., swollen curled tails) as opposed moribund/dead cells (i.e., non-swollen straight tails) that may have experienced premature/spontaneous loss of the acrosomal contents independent of having completed a physiological acrosome reaction (Jeyendran et al., 1984). - After completion of incubation in HOS medium, pellet the sperm suspension by centrifugation at 500 × g for 5 min in a fixed rotor, before fixing spermatozoa by resuspending the pellet in 500 µl of 4% (v/v) paraformaldehyde (PFA) solution for 10 min at room temperature.
- As outlined below, resuspend and wash PFA fixed spermatozoa in a medium appropriate for detection of acrosomal exocytosis.
- To induce acrosomal exocytosis, incubate capacitated human spermatozoa at 37 °C with 1 ml of Capacitating BWW supplemented with either:
- Assessment of sperm acrosome status
The completion of acrosomal exocytosis may be assessed using either histochemical or immunofluorescence techniques (Nixon et al., 2020), the latter of which relies on labeling of permeabilized cells with fluorescently labeled lectins [e.g., Pisum sativum agglutinin (PSA), peanut agglutinin (PNA), Concanavalin A lectin (ConA)] or antibodies directed against acrosome-associated antigens (e.g., anti-CD46, anti-acrosin antibodies) (Aitken and Brindle, 1993; Carver-Ward et al., 1997; Larson and Miller, 1999). Both approaches are relatively simple, reproducible and produce clearly differentiated staining patterns (Figures 1 and 2).- Histochemical labeling of the sperm acrosome (Figure 1):
Below we describe a simple and rapid single staining protocol based on the application of Coomassie Blue G-250 to produce intense labeling of the protein-rich acrosomal contents (Larson and Miller, 1999). Although this single step protocol is not capable of discriminating live spermatozoa (and therefore completion of a physiological acrosome reaction), this objective can be achieved by performing Coomassie Blue labeling in concert with the hypo-osmotic swelling test (Jeyendran et al., 1984), the principles of which are described above. Although histochemical techniques may lack the inherent sensitivity achieved using fluorophore conjugated lectins, they nonetheless have several advantages, including that they are relatively inexpensive and easy to perform, utilize stains that are stable (thus avoiding issues of photo-bleaching and deterioration of signal intensity that are common to fluorescent reagents), and the labeled spermatozoa can be readily visualized using standard light microscopy; infrastructure that is commonly available in clinical laboratories (as opposed to expensive specialist fluorescence instrumentation).- Wash PFA fixed spermatozoa twice by centrifugation at room temperature and resuspension in 100 mM ammonium acetate (pH 9.0). Briefly, centrifuge the sperm suspension at 500 × g for 5 min in a fixed rotor. Carefully aspirate and discard all but 20-40 µl of the supernatant. Resuspend the sperm pellet and the remaining supernatant in 1.5 ml 100 mM ammonium acetate by gentle pipetting. Repeat the washing procedure.
- Resuspend the resultant sperm pellet in 1 ml of 100 mM ammonium acetate and then smear 50 μl of this sperm suspension onto a glass microscope slide using another glass slide. Briefly, using a clean slide, pull the sperm suspension droplet across the surface of the slide at a 45° angle. This technique should be completed within 1 s, ensuring that the smear will be thin and evenly distributed across the slide surface.
- Inspect the wet smears by phase-contrast microscopy (×400) to ensure that the spermatozoa are evenly distributed on the slides without clumping.
- Allow the slides to air-dry thoroughly. Approximately 15 min should be sufficient, but slides may otherwise be left overnight at this step.
- Immerse sperm smears in freshly prepared Coomassie Blue stain (Recipe 2) and incubate for 2 min at room temperature.
- Thoroughly wash slides using distilled water to remove excess stain prior to air-drying (~15 min). Mount a coverslip on the dried, stained slide with 20 µl of DPX Mountant. Allow the DPX Mountant medium to dry completely (~30 min), excess can be removed by blotting the slide with tissue.
- Observe the slide under oil-immersion brightfield optics at 1,000 × magnification and assess stained spermatozoa for the percentage of viable acrosome reacted cells; evaluating a minimum of 200 cells in randomly selected fields in order to achieve an acceptably low sampling error (see Data analysis below).
- Wash PFA fixed spermatozoa twice by centrifugation at room temperature and resuspension in 100 mM ammonium acetate (pH 9.0). Briefly, centrifuge the sperm suspension at 500 × g for 5 min in a fixed rotor. Carefully aspirate and discard all but 20-40 µl of the supernatant. Resuspend the sperm pellet and the remaining supernatant in 1.5 ml 100 mM ammonium acetate by gentle pipetting. Repeat the washing procedure.
- Immunofluorescent labeling of the sperm acrosome (Figure 2):
Glycoconjugates residing in the acrosomal matrix and outer acrosomal membranes of human spermatozoa can be reliably and sensitively detected via labeling with fluorescently conjugated lectins [i.e., Pisum sativum agglutinin (PSA) and Arachis hypogaea (peanut) agglutinin (PNA), respectively] (Aitken and Brindle, 1993). Although neither lectin is capable of discriminating live spermatozoa (and therefore completion of a physiological acrosome reaction), this objective can be achieved by performing immunofluorescent labeling in concert with the hypo-osmotic swelling test (Jeyendran et al., 1984), the principles of which are described above. Then main advantages of using fluorescently conjugated probes for the detection of acrosome status rest with their inherent specificity and sensitivity even at low concentrations; with the latter being attributed to a high signal to noise ratio. Furthermore, the availability of multiple different fluorophores presents the opportunity to multiplex analyses such that several molecules can be simultaneous observed in a single sperm sample (Zhou et al., 2017; Gaikwad et al., 2019).- Wash PFA fixed spermatozoa twice by centrifugation (500 × g for 5 min at room temperature in a fixed rotor) and resuspension in 0.05 M glycine prepared in 1× phosphate buffered saline (PBS; pH 7.4) at room temperature to eliminate unreduced aldehydes. Spermatozoa may be stored at this step at 4 °C or processed immediately.
- When appropriate, aliquot 10 μl of the fixed sperm suspension into the wells of a 12-well glass microscope slide.
- Inspect the wet slides by phase-contrast microscopy (×400) to ensure that the spermatozoa are evenly distributed in each well without clumping.
- Let the slides air-dry slides thoroughly. Approximately 15 min should be sufficient, but slides may otherwise be left overnight.
- Permeabilize sperm by immersion of samples in a droplet of ice-cold methanol and incubation for 10 min at room temperature. Place slides in a sealed humidified chamber (created by lining the bottom of a plastic container with a wet tissue) for the duration of permeabilization to avoid drying out.
- Incubate slides with fluorescein isothiocyanate (FITC)-conjugated PSA (10 µg/µl) at 37 °C for 30 min. To control for the possibility of sperm and/or lectin auto-fluorescence, include a negative control treatment in which spermatozoa are incubated with non-conjugated PSA (10 µg/µl) at 37 °C for 30 min. This treatment should yield no/minimal fluorescent labeling.
- Thoroughly wash slides three times using 1× PBS at room temperature to remove excess stain prior to mounting in Mowiol (Recipe 5) and applying coverslips.
- Observe the slide using fluorescence microscopy (Zeiss Axio Imager A1 ) (see Data analysis below). Noting that the wavelengths of the microscopic filters to be used for excitation and emission are 475 nm and ~524 nm, respectively. Assess stained spermatozoa for the percentage of viable acrosome reacted cells; evaluating a minimum of 200 cells in randomly selected fields in order to achieve an acceptably low sampling error (see Data analysis below).
- Wash PFA fixed spermatozoa twice by centrifugation (500 × g for 5 min at room temperature in a fixed rotor) and resuspension in 0.05 M glycine prepared in 1× phosphate buffered saline (PBS; pH 7.4) at room temperature to eliminate unreduced aldehydes. Spermatozoa may be stored at this step at 4 °C or processed immediately.
- Histochemical labeling of the sperm acrosome (Figure 1):
Data analysis
Assessment of sperm acrosome status: The acrosomal status of human spermatozoa can be classified on the basis of staining of the glyoconjugates encapsulated within the cap-like structure, which overlays the anterior two-thirds of the sperm head. Specifically, those non-reacted spermatozoa in which the acrosome is retained display intense, uniform labeling throughout the entire acrosomal cap domain.
Assessment of sperm vitality: The inclusion of a HOS test enables the discrimination of spermatozoa with intact membranes (i.e., vital) at the time of acrosome reaction induction from those with compromised membranes (i.e., moribund/dead cells) that may have experienced premature/spontaneous loss of the acrosomal contents independent of having completed a physiological acrosome reaction (Jeyendran et al., 1984). Specifically, incubation in HOS medium causes substantial intracellular swelling, which changes the shape of vital cells with intact membranes leading to coiling of the tail. In this assay, all forms of coiled tails are scored as live spermatozoa.
Tally the number of vital spermatozoa in each acrosomal category (acrosome intact and acrosome reacted) with the aid of a laboratory counter. As indicated above, the evaluation of 200 spermatozoa in each replicate is recommended in order to achieve an acceptably low sampling error. Data are then reported as the average percentage of live acrosome reacted spermatozoa. Noting that overall rates of acrosome reaction are expected to vary in accordance with sperm quality, WHO endorsed criteria provide valuable references to discriminate normal rates of acrosomal exocytosis (World Health Organization, 2010). Thus, the difference expected between vehicle control (DMSO) and A23187/progesterone induced acrosome reaction rates in a normal sperm sample is approximately 15%. By contrast, differences in acrosome reaction rates between vehicle control and A23187/progesterone treated cells of under 10% are considered abnormal and values of between 10%-15% indicate that sperm function may be abnormal. In a similar context, vehicle control values above 15% are generally indicative of spontaneous and/or premature acrosome reaction.
- Histochemical labeling of the sperm acrosome: Four patterns of sperm labeling are anticipated (Figures 1B, C), with their interpretation being:
- Vital spermatozoa with reacted acrosome = light blue/clear acrosomal domain and curled tail.
- Vital spermatozoa with intact acrosome = dark blue acrosomal domain and curled tail.
- Dead spermatozoa with reacted acrosome = light blue/clear acrosomal domain and straight tail.
- Dead spermatozoa with intact acrosome = dark blue acrosomal domain and straight tail.
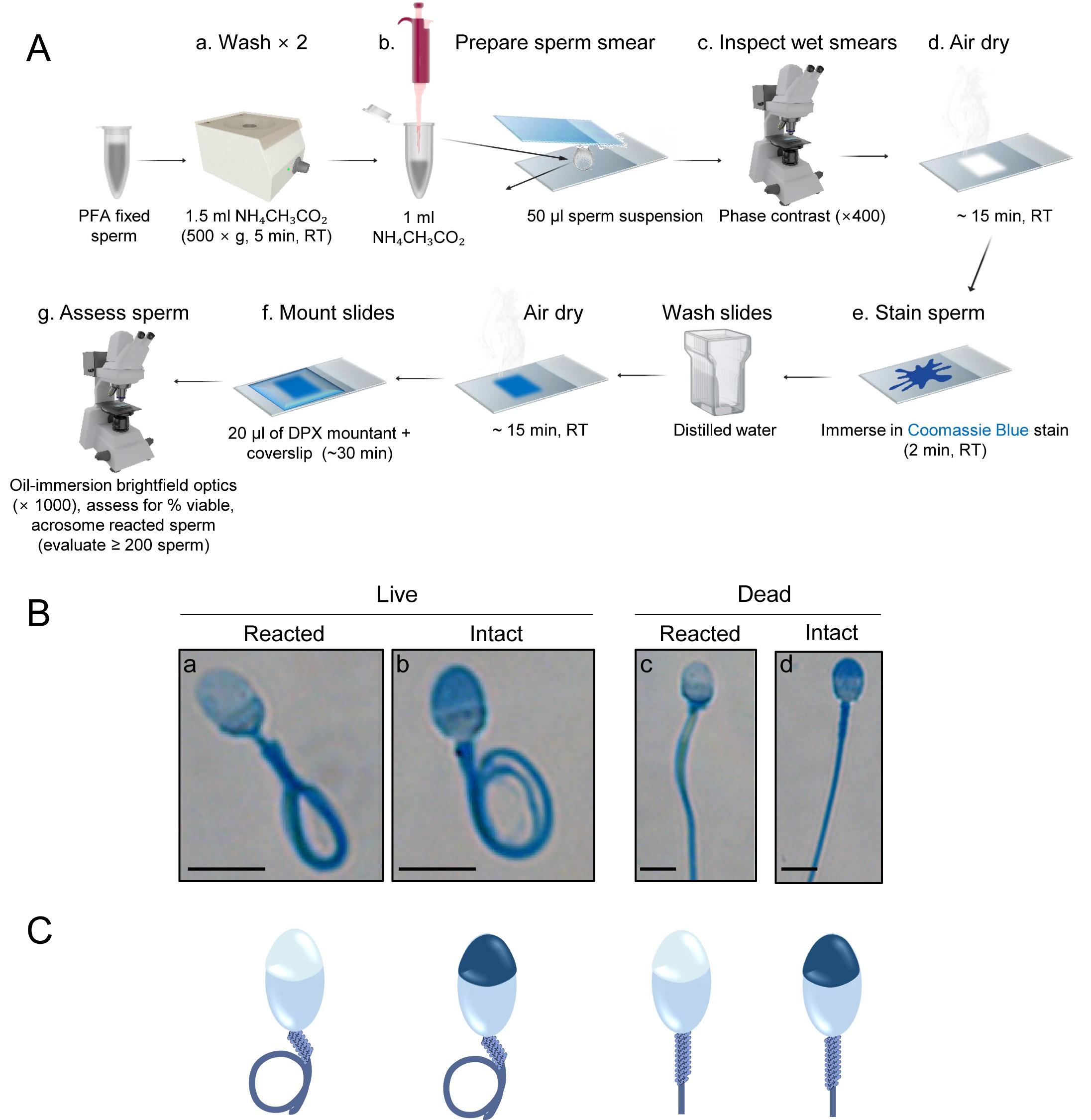
Figure 1. Histochemical labeling of the sperm acrosome. A. Schematic diagram illustrating the workflow utilized for Coomassie Blue G-250 labeling of the sperm acrosome. B. Representative images. C. Corresponding schematic diagrams of human spermatozoa depicting the four categories detected after Coomassie Blue G-250 labeling of cells subjected to a hypo-osmotic swelling test. Vital (live) spermatozoa are identified on the basis of a tightly curled tail, whereas non-vital (dead) cells possess a straight tail. Spermatozoa with an intact acrosome feature intense dark blue labeling within the apical region of the sperm head, whereas those that have lost their acrosomal contents, either through physiological (live cells) or degenerative (dead cells) acrosome reaction, are distinguished by a light blue/unstained apical region. Scale bars = 10 µm. - Vital spermatozoa with reacted acrosome = light blue/clear acrosomal domain and curled tail.
- Immunofluorescent labeling of the sperm acrosome: Four patterns of sperm labeling are anticipated (Figure 2), with their interpretation being:
- Vital spermatozoa with reacted acrosome = spermatozoa with no fluorescent staining within the acrosome region, or a green fluorescent band restricted to the equatorial segment, and curled tail.
- Vital spermatozoa with intact acrosome = spermatozoa in which more than half the head is brightly and uniformly displaying green fluorescence and curled tail.
- Dead spermatozoa with reacted acrosome = spermatozoa with no fluorescent staining within the acrosome region, or a green fluorescent band restricted to the equatorial segment, and straight tail.
- Dead spermatozoa with intact acrosome = spermatozoa in which more than half the head is brightly and uniformly displaying green fluorescence and straight tail.
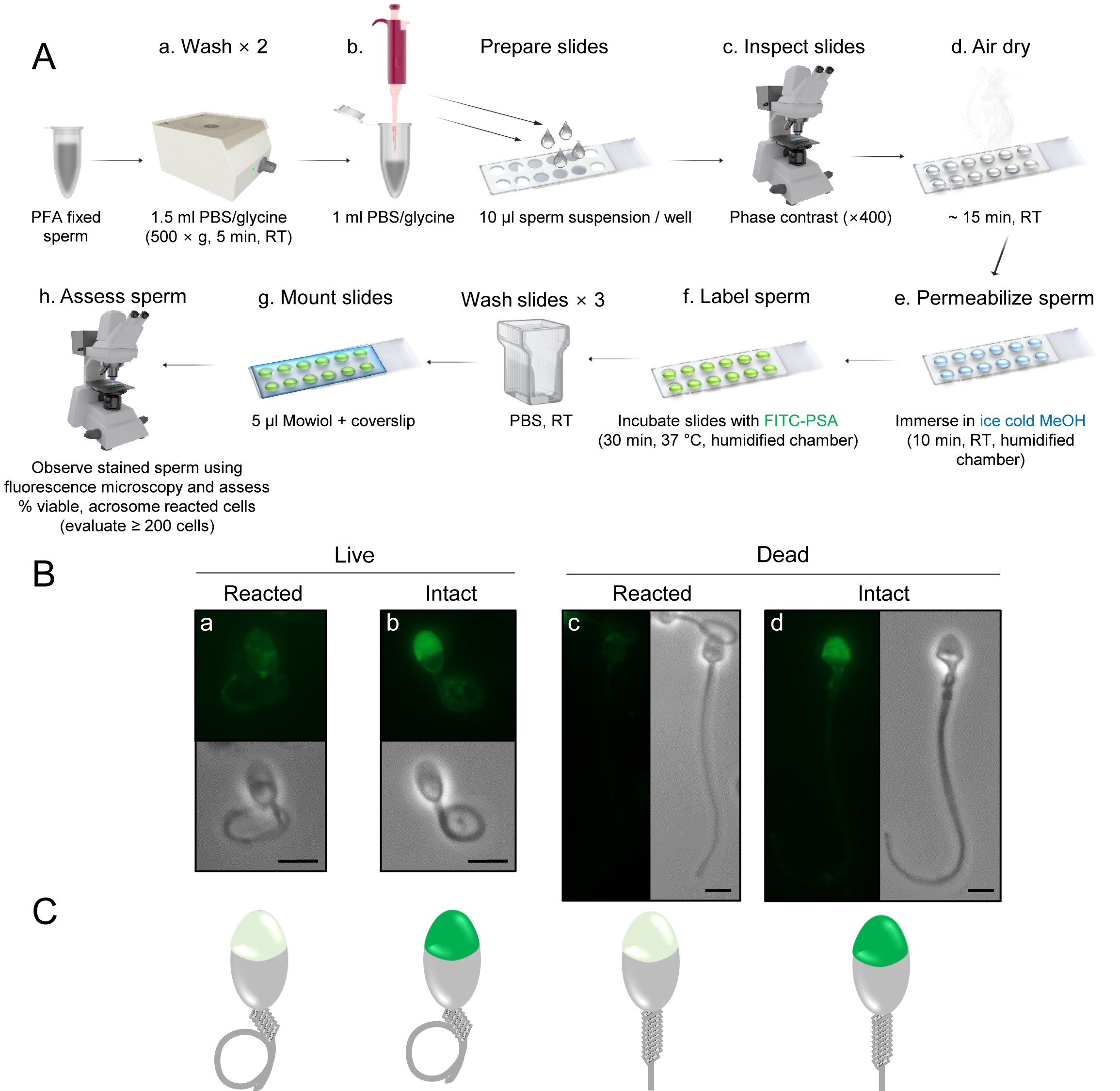
Figure 2. Immunofluorescent labeling of the sperm acrosome. A. Schematic diagram illustrating the workflow utilized for FITC-conjugated PSA labeling of the sperm acrosome. B. Representative images. C. Corresponding schematic diagrams of human spermatozoa depicting the four categories detected after FITC-conjugated PSA labeling of cells subjected to a hypo-osmotic swelling test. Vital (live) spermatozoa are identified on the basis of a tightly curled tail, whereas non-vital (dead) cells possess a straight tail. Spermatozoa with an intact acrosome feature bright and uniform green fluorescence labeling within the apical region of the sperm head, whereas those that have lost their acrosomal contents, either through physiological (live cells) or degenerative (dead cells) acrosome reaction, are distinguished by either no fluorescent staining within the acrosome region, or a green fluorescent band restricted to the equatorial segment (not shown). Scale bars = 10 µm. - Vital spermatozoa with reacted acrosome = spermatozoa with no fluorescent staining within the acrosome region, or a green fluorescent band restricted to the equatorial segment, and curled tail.
Notes
We note that whilst comparable results can be obtained using both FITC-conjugated PSA and PNA lectins to assess human sperm acrosome status, in our experience the latter yields somewhat more variable results including inconsistencies in both the intensity and uniformity of acrosomal labeling. We therefore cautiously recommend the use of FITC-conjugated PSA, over that of PNA, since the robust labeling patterns PSA yields lend themselves to more consistent interpretation, particularly for those unfamiliar with assessment of acrosome labeling.
Recipes
- Capacitating BWW (for 50 ml of working solution)
- Weigh out dry ingredients:
105 mg NaHCO3
50 mg D-glucose
50 mg PVA (substituted for BSA) - Make up to 45 ml with BWW stock solution (Recipe 8)
- Add liquids:
0.5 ml penicillin-streptomycin
1 ml HEPES buffer
50 µl sodium pyruvate
180 µl sodium lactate (this solution is very viscous; to ensure accurate dispensing, add this ingredient using a 20 to 200 µl Finnpipette with the tip cut midway to increase bore diameter and aspirate liquid slowly) - Adjust final volume to 50 ml using BWW stock
- Check final osmolarity is within the range of 290-310 mOsm/kg
- Add 3 mM pentoxifylline (ptx) and 5 mM dibutyryl cyclic adenosine monophosphate (dcAMP)
- Weigh out dry ingredients:
- Coomassie Blue stain
0.22% (w/v) Coomassie Brilliant Blue G-250
50% (v/v) methanol
10% (v/v) glacial acetic acid
40% (v/v) sterile-filtered water - HOS medium
0.735 g sodium citrate
1.351 g fructose
Made up to 100 ml with sterile-filtered water (osmolality should approximate 155 mosmol/kg). Aliquot and stored at -20 °C. Pre-warm to 37 °C before use. - Fixative for diluting semen
- Dissolve 5 g of sodium bicarbonate (NaHCO3) and 1 ml of 37% (v/v) formaldehyde in 100 ml of purified water
- Store at 4 °C. If crystals form in the solution, pass it through a 0.45 µm filter before use
- Dissolve 5 g of sodium bicarbonate (NaHCO3) and 1 ml of 37% (v/v) formaldehyde in 100 ml of purified water
- Mowiol mounting solution
- 6 g glycerol, 2.4 g mowiol, stir to mix
- Add 6 ml H2O while stirring at room temperature for 2 h
- Add 12 ml 0.2 M Tris (pH 8.5) then heat at 60 °C for 10 min to dissolve mowiol (may be repeated if necessary until mowiol is completely dissolved)
- Add 2.5% (w/v) DABCO and centrifuge at 5,000 × g for 15 min in a swing-out rotor to pellet any undissolved solids
- Aspirate supernatant and freeze in 1 ml aliquots
- 6 g glycerol, 2.4 g mowiol, stir to mix
- Non capacitating BWW (for 50 ml of working solution)
- Weigh out dry ingredients:
73 mg NaCl
50 mg D-glucose
50 mg PVA (substituted for BSA) - Make up to 45 ml with BWW stock solution (Recipe 8)
- Add liquids:
0.5 ml penicillin-streptomycin
1 ml HEPES
50 µl sodium pyruvate
180 µl sodium lactate (this solution is very viscous; to ensure accurate dispensing, add this ingredient using a 20 to 200 µl Finnpipette with the tip cut midway to increase bore diameter and aspirate liquid slowly) - Adjust final volume to 50 ml using BWW stock
- Check final osmolarity is within the range of 290-310 mOsm/kg
Note: Non capacitating and capacitating BWW solutions should be made fresh daily.
- Weigh out dry ingredients:
- Percoll working solution (for 50 ml of 100% stock solution)
- 5 ml 10× Ham’s F-10 Nutrient Mix, 50 µl sodium pyruvate, 180 µl sodium lactate, 50 mg PVA, 105 mg NaHCO3, make up to 50 ml with Percoll
- Dilute with Non capacitating BWW to working solutions comprising either 40% or 80% Percoll
- Prepare and use fresh
- 5 ml 10× Ham’s F-10 Nutrient Mix, 50 µl sodium pyruvate, 180 µl sodium lactate, 50 mg PVA, 105 mg NaHCO3, make up to 50 ml with Percoll
- BWW stock solution (for 1 L)
- Weigh out dry ingredients:
5.54 g NaCl
356 mg KCl
250 mg CaCl2·2H2O
162 mg KH2PO4
294 mg MgSO4·7H2O - Make up to 1 L with sterile-filtered water and wait for powder to dissolve
- Check osmolarity approximates 190 mOsm/kg
- Weigh out dry ingredients:
Acknowledgments
The optimization of the experimental procedures described in this manuscript was originally described in Zhou et al. (2017). This research was supported by a National Health and Medical Research Council of Australia (NHMRC) Project Grant (APP1103176) awarded to B.N. S.L.C. is the recipient of a University of Newcastle Postgraduate Research Scholarship and B.N. is the recipient of an NHMRC Senior Research Fellowship (APP1154837).
Competing interests
The authors declare no financial or non-financial competing interests.
Ethics
The experimental procedures described in this manuscript were optimized on human semen samples obtained with informed written consent from a panel of healthy normozoospermic donors assembled for the Reproductive Science Group at the University of Newcastle, NSW, Australia. All experiments were performed in accordance with protocols approved by the University of Newcastle Human Research and Ethics Committee (Approved protocol number: H-2013-0139; validity period: 2013-2020).
References
- Aitken, R. J. and Brindle, J. P. (1993). Analysis of the ability of three probes targeting the outer acrosomal membrane or acrosomal contents to detect the acrosome reaction in human spermatozoa. Hum Reprod 8(10): 1663-1669.
- Aitken, R. J. and Nixon, B. (2013). Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod 19(12): 785-793.
- Barros, C., Bedford, J. M., Franklin, L. E. and Austin, C. R. (1967). Membrane vesiculation as a feature of the mammalian acrosome reaction. J Cell Biol 34(3): C1-5.
- Biggers, J. D. (1971). Metabolism of mouse embryos. J Reprod Fertil Suppl 14: 41-54.
- Buffone, M. G., Foster, J. A. and Gerton, G. L. (2008). The role of the acrosomal matrix in fertilization. Int J Dev Biol 52(5-6): 511-522.
- Buffone, M. G., Hirohashi, N. and Gerton, G. L. (2014). Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biol Reprod 90(5): 112.
- Buffone, M. G., Ijiri, T. W., Cao, W., Merdiushev, T., Aghajanian, H. K. and Gerton, G. L. (2012). Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev 79(1): 4-18.
- Buffone, M. G., Rodriguez-Miranda, E., Storey, B. T. and Gerton, G. L. (2009). Acrosomal exocytosis of mouse sperm progresses in a consistent direction in response to zona pellucida. J Cell Physiol 220(3): 611-620.
- Carver-Ward, J. A., Moran-Verbeek, I. M. and Hollanders, J. M. (1997). Comparative flow cytometric analysis of the human sperm acrosome reaction using CD46 antibody and lectins. J Assist Reprod Genet 14(2): 111-119.
- Gaikwad, A. S., Anderson, A. L., Merriner, D. J., O’Connor, A. E., Houston, B. J., Aitken, R. J., O’Bryan, M. K. and Nixon. B. (2019). GLIPR1L1 is an IZUMO1 binding protein required for optimal fertilization in the mouse. BMC Biol 17: 86.
- Guyonnet, B., Egge, N. and Cornwall, G. A. (2014). Functional amyloids in the mouse sperm acrosome. Mol Cell Biol 34(14): 2624-2634.
- Guyonnet, B., Zabet-Moghaddam, M., SanFrancisco, S. and Cornwall, G. A. (2012). Isolation and proteomic characterization of the mouse sperm acrosomal matrix. Mol Cell Proteomics 11(9): 758-774.
- Hermo, L., Pelletier, R. M., Cyr, D. G. and Smith, C. E. (2010a). Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 73(4): 241-278.
- Hermo, L., Pelletier, R. M., Cyr, D. G. and Smith, C. E. (2010b). Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech 73(4): 279-319.
- Houston, B. J., Nixon, B., Martin, J. H., De Iuliis, G. N., Trigg, N. A., Bromfield, E. G., McEwan, K. E. and Aitken, R. J. (2018). Heat exposure induces oxidative stress and DNA damage in the male germ line. Biol Reprod 98(4): 593-606.
- Houston, B. J., Nixon, B., McEwan, K. E., Martin, J. H., King, B. V., Aitken, R. J. and De Iuliis, G. N. (2019). Whole-body exposures to radiofrequency-electromagnetic energy can cause DNA damage in mouse spermatozoa via an oxidative mechanism. Sci Rep 9(1): 17478.
- Jamil, K. and White, I. G. (1981). Induction of acrosomal reaction in sperm with ionophore A23187 and calcium. Arch Androl 7(4): 283-292.
- Jeyendran, R. S., Van der Ven, H. H., Perez-Pelaez, M., Crabo, B. G. and Zaneveld, L. J. (1984). Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 70(1): 219-228.
- Krausz, C., Bonaccorsi, L., Maggio, P., Luconi, M., Criscuoli, L., Fuzzi, B., Pellegrini, S., Forti, G. and Baldi, E. (1996). Two functional assays of sperm responsiveness to progesterone and their predictive values in in-vitro fertilization. Human Reprod 11(8): 1661-1667.
- Katen, A. L., Stanger, S. J., Anderson, A. L., Nixon, B. and Roman, S. D. (2016). Chronic acrylamide exposure in male mice induces DNA damage to spermatozoa; Potential for amelioration by resveratrol. Reprod Toxicol 63: 1-12.
- Larson, J. L. and Miller, D. J. (1999). Simple histochemical stain for acrosomes on sperm from several species. Mol Reprod Dev 52(4): 445-449.
- Liu, D. Y. and Baker, H. W. (1996). Relationship between the zona pellucida (ZP) and ionophore A23187-induced acrosome reaction and the ability of sperm to penetrate the ZP in men with normal sperm-ZP binding. Fertil Steril 66(2): 312-315.
- Liu, D. Y. and Baker, H. W. (2003). Disordered zona pellucida-induced acrosome reaction and failure of in vitro fertilization in patients with unexplained infertility. Fertil Steril 79(1): 74-80.
- Liu, D. Y., Clarke, G. N., Martic, M., Garrett, C. and Baker, H. W. (2001). Frequency of disordered zona pellucida (ZP)-induced acrosome reaction in infertile men with normal semen analysis and normal spermatozoa-ZP binding. Hum Reprod 16(6): 1185-1190.
- Liu, D. Y., Stewart, T. and Baker, H. W. (2003). Normal range and variation of the zona pellucida-induced acrosome reaction in fertile men. Fertil Steril 80(2): 384-389.
- Mitchell, L. A., Nixon, B. and Aitken, R. J. (2007). Analysis of chaperone proteins associated with human spermatozoa during capacitation. Mol Hum Reprod 13(9): 605-613.
- Nixon, B. and Bromfield, E. G. (2018). Sperm capacitation. In: The Encyclopedia of Reproduction. Skinner, M. K. (Ed.). Cambridge, MA, USA, Academic Press: Elsevier. 3: 272-278.
- Nixon, B. and Bromfield, E. G. (2020). New horizons in male subfertility and infertility. In: Male and Sperm Factors that Maximize IVF Success. Aitken, R. J., Mortimer, D. and G. Kovacs (Eds.). UK, Cambridge University Press. 1: 15-27.
- Nixon, B., Cafe, S. L., Bromfield, E. G., De Iuliis, G. N. and Dun, M. D. (2020). Capacitation and acrosome reaction: Histochemical techniques to assess the acrosome reaction. In: Manual of Sperm Function Testing in Human Assisted Reproduction. Agarwal, A., Henkel, R. and Majzoub, A. (Eds.). UK, Cambridge University Press.
- Parinaud, J., Labal, B. and Vieitez, G. (1992). High progesterone concentrations induce acrosome reaction with a low cytotoxic effect. Fertil Steril 58(3): 599-602.
- Redgrove, K. A., Anderson, A. L., Dun, M. D., McLaughlin, E. A., O'Bryan, M. K., Aitken, R. J. and Nixon, B. (2011). Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae. Dev Biol 356(2): 460-474.
- Reid, A. T., Lord, T., Stanger, S. J., Roman, S. D., McCluskey, A., Robinson, P. J., Aitken, R. J. and Nixon, B. (2012). Dynamin regulates specific membrane fusion events necessary for acrosomal exocytosis in mouse spermatozoa. J Biol Chem 287(45): 37659-37672.
- Stival, C., Puga Molina Ldel, C., Paudel, B., Buffone, M. G., Visconti, P. E. and Krapf, D. (2016). Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol 220: 93-106.
- Tesarik, J. (1985). Comparison of acrosome reaction-inducing activities of human cumulus oophorus, follicular fluid and ionophore A23187 in human sperm populations of proven fertilizing ability in vitro. J Reprod Fertil 74(2): 383-388.
- Tateno, H., Krapf, D., Hino, T., Sánchez-Cárdenas, C., Darszon, A., Yanagimachi, R. and Visconti, P. E. (2013). Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc Natl Acad Sci U S A 110(46): 18543-18548.
- World Health Organization. (2010). WHO laboratory manual for the examination and processing of human semen. Switzerland, World Health Organization.
- Zhou, W., Anderson, A. L., Turner, A. P., De Iuliis, G. N., McCluskey, A., McLaughlin, E. A. and Nixon, B. (2017). Characterization of a novel role for the dynamin mechanoenzymes in the regulation of human sperm acrosomal exocytosis. Mol Hum Reprod 23(10): 657-673.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cafe, S. L., Anderson, A. L. and Nixon, B. (2020). In vitro Induction and Detection of Acrosomal Exocytosis in Human Spermatozoa. Bio-protocol 10(14): e3689. DOI: 10.21769/BioProtoc.3689.
Category
Developmental Biology > Cell signaling > Fate determination
Cell Biology > Cell-based analysis > Immunocytochemistry
Biochemistry > Protein > Labeling
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









