- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Examining Cocaine Conditioning Place Preference in Mice
Published: Vol 10, Iss 8, Apr 20, 2020 DOI: 10.21769/BioProtoc.3595 Views: 5538
Reviewed by: Soyun KimAlexandra GrosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Binging from Food to Alcohol: A Sequential Interaction Between Binging Behaviors in Male Wistar Rats
Sergio Cuesta-Martínez [...] Cruz Miguel Cendán
Aug 5, 2023 1352 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1850 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 171 Views
Abstract
A key component of combating substance use disorders is understanding the neural mechanisms that support drug reward. Tasks such as self-administration assess the reinforcing properties of a drug using a learned behavior but require numerous training sessions and surgery. In comparison, the conditioned place preference (CPP) task assesses reward with little training, without costly surgeries, and confounds that accompany the use of anesthesia or pain-relieving drugs. The CPP task contains three phases: pretest, conditioning, and posttest. During the pretest, mice are allowed to explore a three-compartment apparatus. The two outer compartments contain unique olfactory, tactile, and visual cues whereas the middle compartment is used as an entrance and exit for the mice on test days. During conditioning, mice receive cocaine before being confined to one of the outer compartments. The following day, mice are given saline then confined to the other outer compartment. These pairings are then repeated once. At posttest, mice are permitted to freely explore all compartments in a drug-free state while the time spent in each compartment is recorded. A CPP score is calculated for both the pretest and posttest by comparing the time spent in the cocaine-paired and saline-paired compartments. Enhancements in the CPP score from the pretest to the posttest serve as a measure of the rewarding property of the cocaine. This task offers several notable advantages: 1) the simultaneous recording of locomotor activity and reward, which may utilize different neural mechanisms, 2) the three-compartment CPP setup removes the bias that can be observed in a two-compartment design, and 3) use of multimodal cues support the acquisition of a robust preference in a variety of mouse strains.
Background
The conditioned place preference (CPP) paradigm has been used for decades to examine the role of genes, histone modifications, signaling pathways, and brain regions in drug-associated memory and reward (see Tzschentke, 1998; Malvaez et al., 2009). It incorporates classical conditioning to assess the rewarding effects of contextual cues that have been associated with a drug (for reviews see Schechter and Calcagnetti, 1993; Everitt et al., 1999; Bardo and Bevin, 2000). There are various CPP chamber designs and configurations, depending on the specific research question being addressed (Bardo and Bevin, 2000). However, the most commonly used designs are the two-compartment or three-compartment chambers. Both chambers types rely on the ability of mice to differentiate between two compartments that contain distinct olfactory, tactile, and visual cues. The three-compartment design contains an additional compartment, between the two larger compartments, that does not contain olfactory or tactile cues, and is used only for the entry and removal of mice at test. Here, we describe the equipment setup, key parameters, and data collection in the three-compartment CPP paradigm using adult mice. The focus of this protocol is on cocaine CPP. However, this paradigm can be used for other drugs of abuse (e.g., other psychostimulants, opioids, and nicotine).
The three-compartment design allows mice to choose whether to enter either the cocaine or saline-paired compartments or remain in the center compartment. Conversely, the two-compartment chambers require mice to be placed in either the saline-paired or the cocaine-paired compartments to begin the test. This has the potential to introduce bias for the compartment in which the mice is place at post-test. Using tactile, olfactory and visual cues allows mice to form a memory for each context that does not rely on one sensory system. Therefore, the likelihood that mice with sensory deficits (e.g., poor vision) will fail to acquire a drug-associated preference is reduced. Other variations of the task (e.g., olfactory only) can require four cocaine pairings, which results in eight total injections when the saline injections are included. Acquisition in our task requires two cocaine pairings (four total injections) for robust cocaine CPP, which reduces the likelihood that stress and irritation at the site of injection will be significant factors in the experiment. Furthermore, if both the control and experimental groups equally acquire a preference, the posttest day of the CPP task can serve as the first day of an extinction experiment. This subsequent extinction experiment would allow the experimenter to examine the persistence of the acquired cocaine-associated memory. Overall, the CPP paradigm provides an adaptable, quick, and inexpensive method of assessing drug reward in adult mice.
Materials and Reagents
- Paper towels
- BD 1 ml Syringe slip tip with BD PrecisionGuideTM needle, 26G x 5/8 (0.45 mm x 16 mm; Fisher Scientific, catalog number: BD 309597 )
- Gloves (to be worn at all times that researchers will come into contact with mice)
- Handling sleeve (Ansell, catalog number: 19-120-3177 )
- Bedding: Pine shaving (P.J. Murphy Forest Products)
- Cedar bedding (PetsPick)
- Mice (8-15 weeks old) of either sex are housed individually beginning a week prior to behavioral testing
Mice have free access to food and water in their homecage. Environmental enrichment such as nesting materials (e.g., nestlets) and a retreat are features of each homecage. Their homecage area is temperature-controlled (23 °C) with a 12:12 h. light/dark cycle. Behavioral testing is performed during the light portion of the cycle.
Note: This protocol has been optimized using C57BL/6J mice but can be used for other strains as well. - 70% (v/v) ethanol diluted from stock using water (Fisher Scientific, catalog number: A4094 )
- Sterile saline (Growcells, catalog number: msdw-1000 , Shelf life: 3 years, stored at room temperature)
- Cocaine hydrochloride (Sigma-Aldrich, CAS number: 53-21-4)
Equipment
- Heavy-Duty Utility Cart (RubbermadeTM, catalog number: 11-926-75 )
- CPP chambers (custom-made; Figure 1)
- Four digital cameras mounted above the CPP chambers (e.g., monochrome IR GigE camera set; Noldus Information Technology)
- Overhead camera-mounting bracket
- Overhead lamps
- Stopwatch with a silent mode
- Lux meter (Fisher Scientific, catalog number: 0 666264 )
- Dell Precision desktop (e.g., Processing Unit Precision T5810)

Figure 1. Two conditioned place preference apparatus. The outer compartments (Floor: 12.5 cm x 17 cm; Height: 32.5 cm) are separated by a smaller inner compartment (Floor: 12.5 cm x 11.5 cm; Height: 32.5 cm). The compartment with checkered contact paper has a grid floor with cedar shaving below. The compartment with white contact paper has bar flooring with pine chips below. The middle compartment has a solid gray floor, two gray walls, and two walls with either checkered or white contact paper to match the adjoining outer compartments. Guillotine doors separate the outer compartments from the middle compartment.
Software
- EthoVision XT tracking system (Noldus Information Technology, Inc. Leesburg, Virginia)
- Microsoft Excel (Microsoft)
- Statistical Software (i.e., SPSS, GraphPad Prism, R)
Procedure
- Experiment setup
- Place the CPP apparatus in a room dedicated to behavioral research. This room should be devoid of extraneous odors or sounds. The room’s house lights should be turned off throughout the experiment, as the overhead lights will be used to track mice during testing (Figure 2). A lux meter is placed in the middle of each chamber (i.e., on top of either the grid or bar floors shown in Figure 1) to ensure that the light intensity (100 lux) from the overhead lights is similar across all chambers of the CPP apparatus.
Note: Rooms that are used for surgical procedures or animal husbandry are not suitable. Similarly, clothes that have fragrances and other strong odors should be changed prior to the start of the experiment.
Figure 2. Conditioned place preference room setup with eight apparatus. Each CPP chamber is assigned a box ID (e.g., Box 1). The orientation of each box is antiparallel (rotated 180°) from the adjacent box. The location of each box is permanently fixed. The overhead lights are positioned to avoid shadows on the floor of the compartments. Each camera is positioned to record from two boxes. - Create a table in Microsoft Excel that includes the mouse ID, Group Assignment, Box #, and Time spent in the checkered and white compartments.
- Assign each mouse to a dedicated behavioral box with assigned cocaine-paired and saline-paired compartments.
Note: During this step, ensure that both control and experimental groups are equally represented during each trial. For each group, assign the white compartment as the cocaine-paired compartment for half of the mice. For the other half, assign the white compartment as the saline-paired compartment. It is best to ensure that across the entire experiment, each group is represented in each research box. Counterbalancing the injection-compartment assignments in this manner prevents chamber bias from confounding the CPP scores. - House mice in individual cages one week prior to the start of the experiment. At this point, unique identification can be assigned to mice.
Note: Experimenters should be blinded to the treatment group of each mouse. - Weigh the mice before the start of the experiment.
- Prepare the desired cocaine dose for the experiment.
- Set recording software to record video as well as live tracking if possible.
- Place the CPP apparatus in a room dedicated to behavioral research. This room should be devoid of extraneous odors or sounds. The room’s house lights should be turned off throughout the experiment, as the overhead lights will be used to track mice during testing (Figure 2). A lux meter is placed in the middle of each chamber (i.e., on top of either the grid or bar floors shown in Figure 1) to ensure that the light intensity (100 lux) from the overhead lights is similar across all chambers of the CPP apparatus.
- Handling the mice
- Turn on the overhead lights in the behavioral room and put on nitrile or latex gloves.
Note: The same type of glove must be worn throughout the experiment. - Remove the cages containing the mice from their animal rack and place them on the utility cart.
- Transfer the mice to the behavioral room using the utility cart.
Note: It is important to avoid excess noise and rattling during this transfer. These sounds and motions can act as stressors for the mice. - Clean gloves and handling sleeve using 70% ethanol but wait until the ethanol evaporates before handling the mice.
- Remove a mouse from its cage by quickly grabbing the base of its tail and placing it on the handling sleeve.
- Allow the mouse to freely explore the handling sleeve for 3 min before returning it to its cage (Figure 3).
- Clean your gloves and the handling sleeve after handling each mouse.
- Repeat the handling and glove cleaning steps until all mice have been handled.
- Use the utility cart to return mice to their housing room and animal rack.
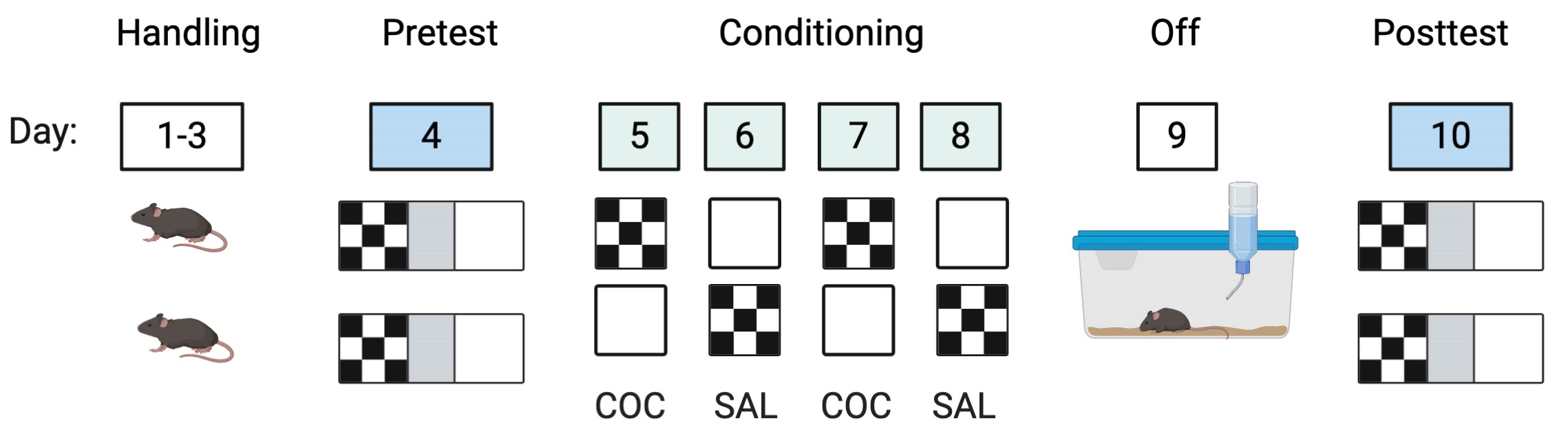
Figure 3. Conditioned place preference schematic. A representative daily sequence for two mice in the same group are illustrated. Mice undergo handling for 3 min on three consecutive days. At pretest, mice are allowed to freely explore the three-compartment apparatus for 15 min. On days 5 and 7, cocaine is administered before the mice are confined to one of the larger compartments. They are then permitted to explore that compartment for 30 min. The experiment is counterbalanced to ensure that half of the mice have cocaine paired with the white compartment while the other half have the cocaine paired with the checkered compartment. On days 6 and 8, saline is administered before the mice are confined to the large compartment that has not been associated with cocaine. They are then permitted to explore that compartment for 30 minutes. Mice are given a day off on day 9 and remain in their home cages. On posttest, mice are allowed to freely explore the three-compartment apparatus in an identical manner to the pretest (15 min duration).
- Turn on the overhead lights in the behavioral room and put on nitrile or latex gloves.
- Pretest
- Prepare the behavioral room by turning on the overhead lights.
- Clean each CPP chamber using 70% ethanol.
- Open the guillotine doors so that mice can freely move between each compartment.
- Turn on the computer and recording software.
- Transfer only the mice that will be run together to the behavioral room using the utility cart.
- Set the recording software to begin tracking when mice enter each chamber and to stop after 15 min have elapsed.
- Pick up each mouse by the base of their tail and transfer them to your palm.
- Place each mouse in the center compartment of their predetermined CPP box.
Note: Ensure that as each mouse is lowered into the CPP apparatus, they are facing one of the gray walls of the center compartment. This will reduce any bias for a particular compartment. - Allow mice to explore the CPP apparatus for 15 min (Figure 3).
- Remove the mice from the apparatus and transfer it to its cage. Start by confining the mice to the center compartment by closing the guillotine doors, then grab it by the base of its tail before immediately placing it in your palm then cage.
- Return all mice to their housing room using the utility cart.
- Open the guillotine doors and clean the CPP apparatus thoroughly using 70% ethanol. Pay special attention to areas that may accumulate pooled urine or defecation.
Note: The bedding (i.e., pine and cedar) can remain in place for the entirety of the experiment unless there is an accumulation of urine or defecation. - Set the computer to record the next set of mice.
- Clean gloves using 70% ethanol before retrieving the next set of mice.
- Repeat Steps C5-C14 until all mice are tested.
- Conditioning
- Prepare the saline and cocaine solutions.
Note: A moderate cocaine dose (e.g., 10 mg/kg) is recommended for preliminary experiments–where either a memory impairment or enhancement may be observed. - Measure out the amount of saline or cocaine to be administered to each mouse from the stock using a syringe.
- Place each filled syringe in front of the CPP chamber in which the injected mouse will be added.
- Clean each CPP chamber using 70% ethanol.
- Close the guillotine doors so that mice will be confined to the outer compartments.
- Transfer only the mice that will be run together to the behavioral room using the utility cart.
- Administer saline or cocaine via intraperitoneal injection before placing the mouse into the assigned outer compartment.
- Set a timer for 30 min. Allow mice to explore the assigned compartment (Figure 3).
Note: Mice do not need to be recorded during conditioning sessions. However, the recording software can be activated if the activity during conditioning is of interest. - After 30 min have elapsed, remove each mouse from their respective CPP box and transfer it to its homecage.
- Return all mice to their housing room using the utility cart.
- Clean gloves using 70% ethanol before retrieving the next set of mice.
- Repeat Steps D1-D10 until all mice are tested.
- Prepare the saline and cocaine solutions.
- Posttest
Repeat the steps outlined in the pretest.
Data analysis
- Use your recording software to calculate the time spent in each compartment during the pretest.
- Calculate the CPP score for each mouse on the pretest. The CPP score is the time spent in the cocaine-paired compartment minus the time spent in the saline-paired compartment (Table 1).
Note: Because the groups are arranged so that each group is represented in each box and that there are an equal number of mice that have cocaine paired with the checkered and white compartments, vigilance is required to accurately calculate the CPP scores of neighboring mice. For example, in Table 1, the CPP scores below for Mouse 1 (CPP Score = Checkered - White) and Mouse 2 (CPP Score = White - Checkered) are calculated differently. - Organize the mice by the independent variable (e.g., genotype, treatment or sex). A representative table of an experiment with two groups is illustrated in Table 2.
- Calculate the CPP score for each mouse on the posttest (Table 3).
- Comparing the total distance traveled across on the pretest and posttest days can also be used as a behavioral control. Observing similar distance traveled between groups suggests that any potential differences in CPP scores from your experimental groups relative to your control group cannot be attributed to differences in locomotion.
- Organize the mice by the independent variable in a similar manner to the pretest (Table 4).
Table 1. Representative CPP Data from the Pretest. Before the experiment, each mouse is assigned a CPP box, cocaine-paired and saline-paired compartments.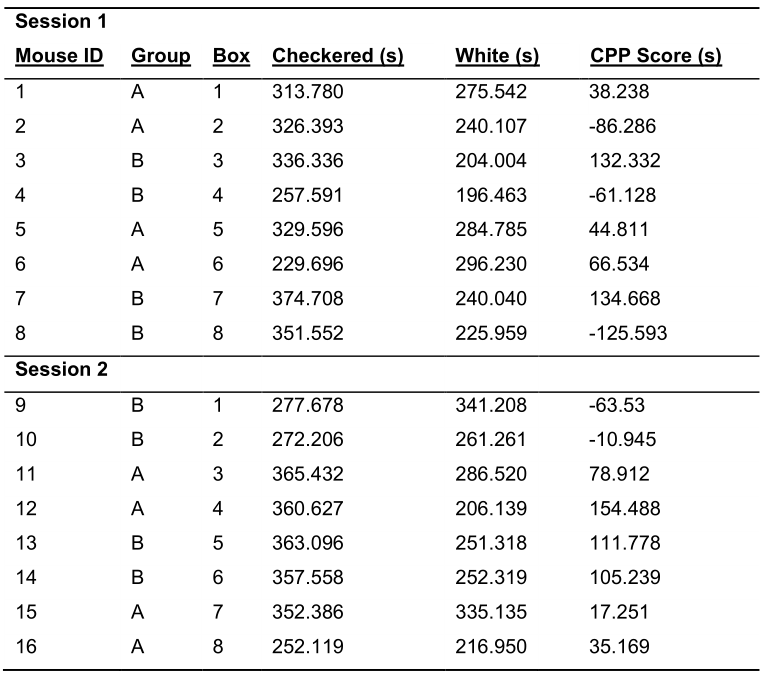
Table 2. Representative Group Data from the Pretest. The mean CPP score of each mouse is reorganized by group to eliminate outliers and enable future analysis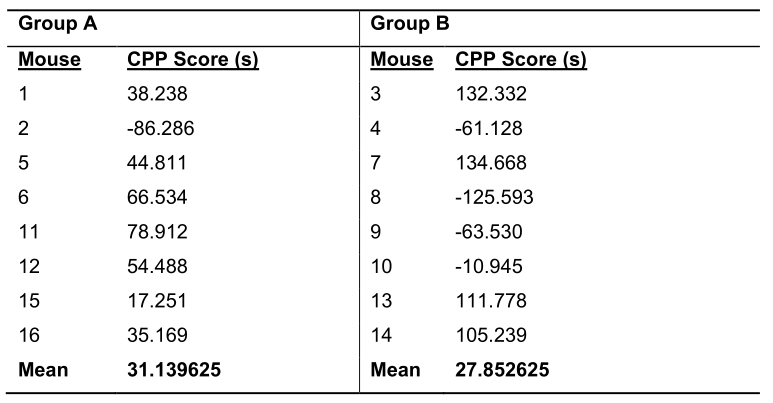
Table 3. Representative CPP Data from the Posttest. Mice that acquire a preference will show a positive CPP score at the posttest. The magnitude of the CPP score illustrates the strength of the learned cocaine association.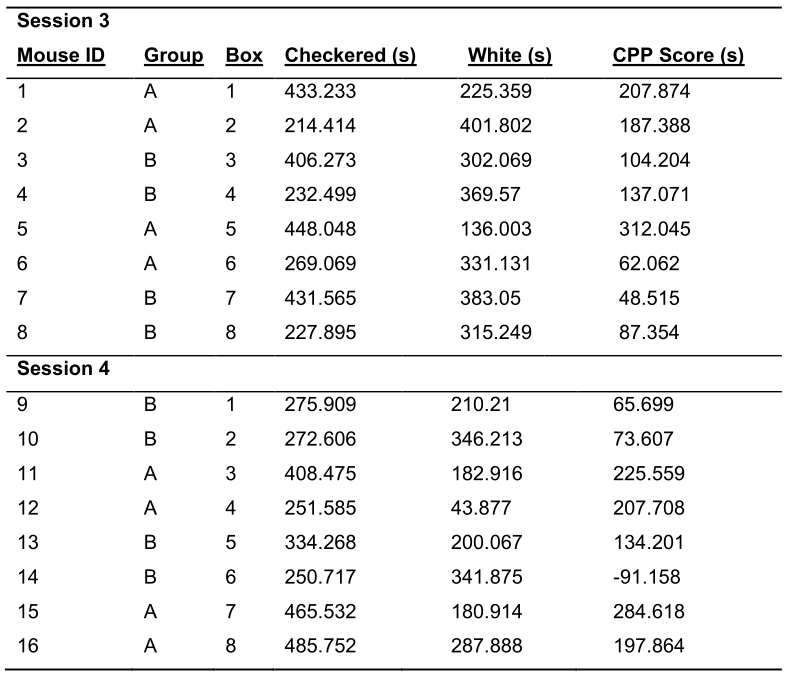
Table 4. Representative Group Data from the Posttest. The mean CPP score of each mouse is reorganized by group to enable future analysis.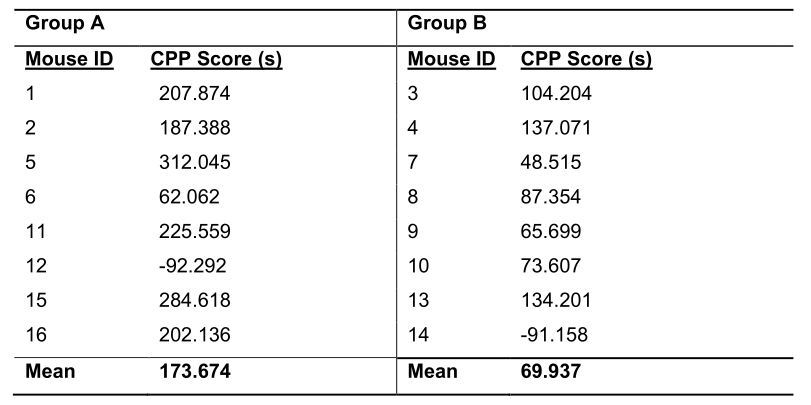
- Use your preferred statistical software (i.e., SPSS, GraphPad Prism, R) to analyze the CPP scores for the pretest and posttest using a two-way repeated-measures analysis of variance. If the data is normally distributed, Bonferroni post-hoc tests can be performed to compare difference in the means of the two groups. Alternatively, if the results are not normally distributed, non-parametric tests such as the Mann-Whitney test can be used. These statistical tests will indicate whether, within each group, significantly more time was spent in the cocaine-assigned chamber following cocaine conditioning. The results from the statistical tests will also indicate whether there are any significant changes in the amount of time in the cocaine paired chamber between groups. These specific comparisons can be made using Student’s t-tests with α-levels held at 0.05. Figure 4 illustrates representative data.
Note: If your groups include both sexes, sex differences should be examined first or as a separate factor.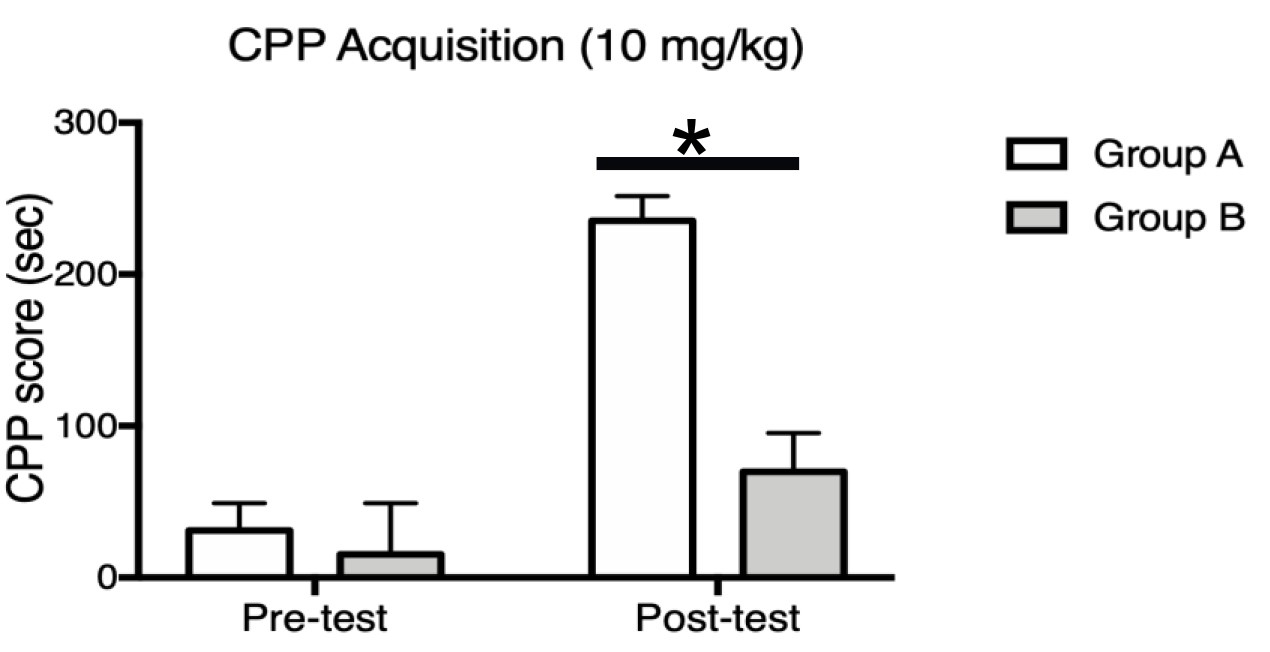
Figure 4. Example graph comparing Groups A and B. Group A represents the control group (e.g., wildtype) whereas Group B represents the experimental group (e.g., mutant). Bars represents the mean CPP score (cocaine-paired minus saline-paired) ± S.E.M. for each group on pretest and posttest. Pretest data illustrates that neither group has a strong preference for either compartment prior to conditioning. Posttest data show that Group A mice developed a robust CPP score, whereas the CPP score of Group B is attenuated. A two-way repeated-measures analysis of variance (ANOVA) revealed main effects of group (F1,14 = 15.31, P = 0.0016) and conditioning (F1,14 = 25.61, P = 0.0002), as well as an interaction (F1,14 = 8.566, P = 0.0110).
Notes
This protocol has been optimized using C57BL/6J mice, which typically yield an individual CPP scores between -150 to 150 s at the pretest. However, this range may vary depending on the strain or treatment etc. Alterations can be made to the lighting and odors intensities to obtain similar results using various strains. Criteria for exclusion should be determined prior to the experiment. The features of the CPP setup (i.e., the odors, wall patterns and light intensity) were selected because they do not lead to CPP scores on the pretest that are significantly different from zero. Furthermore, mice with a CPP score outside of ± 250 s at the pretest are excluded.
Acknowledgments
This protocol was adapted from White et al., 2016. Previous work was supported by grants from the US National Institutes of Health (DA025922, DA036984 and MH101491), the National Institute of General Medical Sciences of the National Institutes of Health (GM055246), the Department of Education GAANN (P200A120165), the US National Institute of Drug Abuse (F31DA038505) and the National Institute on Aging T32 grant (AG000096-31). Recent work has been supported by Mount Holyoke College.
Competing interests
The authors have no conflicts of interest.
Ethics
All experimental procedures were approved by the Institutional Animal Care and Use Committees at the University of California Irvine (IACUC#: 2006-2620) and Mount Holyoke College (IACUC#: BR-56-1117) from the period 2011-2020.
References
- Bardo, M. T. and Bevins, R. A. (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153(1): 31-43.
- Everitt, B. J., Parkinson, J. A., Olmstead, M. C., Arroyo, M., Robledo, P. and Robbins, T. W. (1999). Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci 877: 412-438.
- Schechter, M. D. and Calcagnetti, D. J. (1993). Trends in place preference conditioning with a cross-indexed bibliography; 1957-1991. Neurosci Biobehav Rev 17(1): 21-41.
- Tzschentke, T. M. (1998). Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56(6): 613-672.
- White, A. O., Kramár, E. A., Lopez, A. J., Kwapis, J. L., Doan, J., Saldana, D., Davatolhagh, M. F., Alaghband, Y., Blurton-Jones, M., Matheos, D. P. and Wood, M.A. (2016). The role of neuron-specific nucleosome remodeling in cocaine-associated memories. Nat Commun 7: 11725.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Simkevich, M. J., Campbell, R. R. and White, A. O. (2020). Examining Cocaine Conditioning Place Preference in Mice. Bio-protocol 10(8): e3595. DOI: 10.21769/BioProtoc.3595.
Category
Neuroscience > Behavioral neuroscience > Animal model
Neuroscience > Neuroanatomy and circuitry > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








