- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of ATPase Activity of Valosin-containing Protein/p97
Published: Vol 10, Iss 3, Feb 5, 2020 DOI: 10.21769/BioProtoc.3516 Views: 5094
Reviewed by: Gal HaimovichSeda EkiciAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2310 Views

An Optimized Enzyme-Coupled Spectrophotometric Method for Measuring Pyruvate Kinase Kinetics
Saurabh Upadhyay
Aug 20, 2025 2489 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 522 Views
Abstract
Valosin-containing protein (VCP; also known as p97) is a type II ATPase regulating several cellular processes. Using proteomic techniques, we identified a chemical compound that binds to the D1 ATPase domain of VCP. The protocol described here was to study the effect of the compound on ATPase activity in vitro of purified VCP protein. ATPases are enzymes that hydrolyze ATP in a reaction resulting the release of an inorganic phosphate. This reaction can be measured using several methods, such as colorimetric, fluorescence, and radiometric assays, in addition to the bioluminescence assay mentioned here. Since the remaining ATP level after the reaction was detected using a luciferase assay, the luminescent signal indicates the ATPase activity inversely. This protocol is sensitive, rapid, and can be used for high-throughput screening assays to study the effect of compounds on ATPase function.
Keywords: ATPaseBackground
Valosin-containing protein (VCP) belongs to the AAA+ (adenosine triphosphate-hydrolyzing enzymes [ATPases] associated with a variety of cellular activities) family of proteins. It is a 96 kDa protein containing three regions: the N-terminal domain, and the ATPase domains D1 and D2. The D2 domain of VCP is known to show major ATPase activity when compared to the D1 domain (Song et al., 2003). Since D1 domain of human VCP ends at 459th serine, the truncated mutant S459* (459th serine was changed to stop codon) contains only the D1 domain but not D2 domain. Thus we used this mutant to examine the effect of our compound on D1 domain of VCP (Suvarna et al., 2019). NPD8733, a compound from the RIKEN Natural Products Depository (NPDepo) was identified as an inhibitor of cancer cell-enhanced fibroblast migration. Pull-down assay and proteomic techniques revealed that this compound specifically binds to the D1 domain of VCP (Suvarna et al., 2019). The bioluminescence assay reported here was used to study the effect of NPD8733 on the ATPase activity of VCP. ATPases hydrolyze adenosine triphosphate (ATP) in a reaction, which results in the release of an inorganic phosphate (Rule et al., 2016). Most of the methods used for evaluating the ATPase activity measure phosphate release using radioactive (Sanghera et al., 2009), fluorescent (Bastola et al., 2017; Magnaghi et al., 2013), or colorimetric substrates (Song et al., 2003; Rule et al., 2016). All these assays have some drawbacks that include the use of hazardous substrates, long detection time, low sensitivity, and are difficult to be used in high-throughput screening (HTS) assays (Tanega et al., 2009; Sanghera et al., 2009). Here, we applied a bioluminescence assay (Promega) for the detection and measurement of the ATPase activity of purified recombinant VCP protein. This assay measures the remaining ATP levels after ATPase reaction using a thermostable luciferase reagent. Luciferase catalyzes the mono-oxygenation of luciferin using ATP as the substrate. The luciferase reaction generates one photon of light per turnover. The luminescence measured is inversely related to the ATPase activity.
This simple, rapid, and sensitive assay overcomes the limitations of previous techniques. The luminescence signal produced by the luciferase reaction has a half-life greater than five hours (h), which allows processing of multiple assay plates. This technique is less susceptible to interference from NPDepo compounds and, thus, can be used for HTS assays to evaluate the roles of inhibitors (Tanega et al., 2009; Sasazawa et al., 2012). It can also be used to study and characterize the functions of ATPase domains and their specific residues. In addition, the assay can be modified to study other enzymes, such as kinases.
Materials and Reagents
- Solid white multiwell plates (SUMILON, catalog number: MS-8096W)
- Pipette tips (Gilson)
- Aluminum foil
- 1.5 ml Eppendorf tubes
- Spreader (Sarstedt, catalog number: 86.1569.005)
- Petri dish (IWAKI, catalog number: SH90-15E)
- Glutathione S-transferase (GST)-fused VCP and its deletion mutants in a pGEX-2T vector plasmid DNA (gifts from Drs. Masaya Imoto and Etsu Tashiro at Keio University (Sasazawa et al., 2012).Truncated mutant that lacks D2 domain of VCP was constructed by ourselves (Suvarna et al., 2019).
- BL21 Star (DE3) competent E. coli cells (Thermo Fisher Sientific, catalog number: C6010-03)
- Ethylenediaminetetraacetic acid (EDTA) (FUJIFILM Wako Pure Chemical, catalog number: 345-01865, CAS number: 6381-92-6)
- ATP (GE Healthcare, catalog number: 27-2056-01, CAS number: 56-65-5)
- MgCl2 (FUJIFILM Wako Pure Chemical, catalog number: 136-03995, CAS number: 7786-30-3)
- MgSO4 (FUJIFILM Wako Pure Chemical, catalog number: 137-12335, CAS number: 7487-88-9)
- Glucose (FUJIFILM Wako Pure Chemical, catalog number: 049-31165, CAS number: 50-99-7)
- Tris (Sigma-Aldrich, catalog number: 252859, CAS number: 77-86-1)
- NaCl (FUJIFILM Wako Pure Chemical, catalog number: 191-01665, CAS number:7647-14-5)
- 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS) (Sigma-Aldrich, catalog number: C3023, CAS number: 33171745-4)
- KCl (FUJIFILM Wako Pure Chemical, catalog number: 163-03545, CAS number: 7447-40-7)
- Na2HPO4 (FUJIFILM Wako Pure Chemical, catalog number: 196-02835, CAS number: 10039-32-4)
- KH2PO4 (FUJIFILM Wako Pure Chemical, catalog number: 169-04245, CAS number: 7778-77-0)
- Tryptone (Becton Dickinson, catalog number: 211701)
- Yeast extract (Becton Dickinson, catalog number: 212750)
- Agar powder (Becton Dickinson, catalog number: 156783)
- Milli-Q water (Purified water produced by MilliQ Q-POD, Millipore)
- Ampicillin (Sigma-Aldrich, catalog number: A0166, CAS number: 69-52-3)
- Protease inhibitor tablets (ROCHE cOmplete, catalog number: 4693159001)
- Isopropyl-β-D-thiogalactoside (IPTG) (Sigma-Aldrich, catalog number: 10724815001, CAS number: 367-93-1)
- Glutathione Sepharose 4B (GE Healthcare, catalog number: 17075605)
- Reduced glutathione (Sigma-Aldrich, catalog number: G4251, CAS number: 70-18-8)
- Column for purification (Econo-Column, Bio-Rad, catalog number: 7371512)
- N,N'-Dibenzylquinazoline-2,4-diamine (DBeQ; known inhibitor of ATPase activity of VCP; Sigma-Aldrich, catalog number: SML0031, CAS number: 177355-84-9)
- Kinase Glo Plus reagent (Promega, catalog number: V3771, Reference 8)
- Assay buffer (1x; see Recipes)
- ATP solution (see Recipes)
- Kinase-glo reagent (see Recipes)
- DBeQ solution (see Recipes)
- LB agar (250 ml) (see Recipes)
- Cell lysis buffer (see Recipes)
- Elution buffer (see Recipes)
- Phosphate-buffered saline (PBS 10x; 1 L; see Recipes)
- Super optimal broth (SOC) (see Recipes)
Equipment
- Multi-channel pipettes (Gilson)
- Flask (500 ml glass conical flask)
- pH meter (HORIBA, model: D-21)
- Microplate reader (Thermo Fisher Scientific, VarioSkan Lux)
- Sonicator (Tomy, model: UR-20P)
- Vortex (Scientific Industries, Genie 2)
- Standard refrigerated centrifuge for 15/50 ml and Eppendorf tubes (Tomy, model: MX-301)
- Heat block (Scinics, model: ALB-121)
- Air incubator for plate incubation (EYELA, model: MTI-203)
- Water bath (EYELA, model: NTT)
- Shaker (TAITEC, model: BR-15)
Software
- R (R Foundation for Statistical Computing; https://www.r-project.org)
Procedure
- Expression and purification of GST-VCP fusion protein
- Expression of GST-fused VCP and GST-fused VCP deletion mutants
Transformation- Thaw competent Escherichia coli BL21 star (DE3) cells on ice.
- Take 50 µl of competent cells in a 1.5 ml Eppendorf tube.
- Add 5 ng (2 µl) of the plasmid DNA to the competent cells. Mix gently by flicking the tube 4 to 5 times to mix the cells and DNA. Do not vortex.
- Place the mixture on ice for 30 min. Do not mix.
- Heat shock at 42 °C for 45 s using water bath. Do not mix.
- Keep on ice for 2 min.
- Add 500 µl SOC (pre-warmed to 37 °C) to the tube. Mix.
- Keep the cells at 37 °C for 45-60 min to allow expression of the Ampr gene.
- Warm the LB agar plates containing 100 µg/ml ampicillin to 37 °C in the plate incubator.
- Spread 50-100 µl of the cell mixture onto the agar plates.
- Incubate overnight at 37 °C.
- Pick up one isolated colony and pre-culture in 5 ml LB medium containing 100 µg/ml of ampicillin (15 h at 37 °C, 180 rpm).
- Make glycerol stock (500 µl broth and 500 µl autoclaved 40% glycerol) for backup and store at -80 °C (for future use if required).
- Next day add 1 ml of pre culture in 100 ml LB medium containing 100 µg/ml of ampicillin and grow at 37 °C 180 rpm until OD is 0.6 (flask should only be filled to 20-30% of their capacity).
- Check OD initially after 2 h, then after every 1 h.
- At OD 0.6, cool the flask on ice.
- Take 1 ml sample before induction. Centrifuge at 10,000 x g at 4 °C for 15 min. Wash the pellet with 1x PBS and store at -80 °C after snap freezing with liquid nitrogen. (Use this sample as “before induction run” for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis).
- For induction, add 0.4 mM IPTG to the flask and allow the bacteria to grow at 20 °C for 15 h at 180 rpm.
- Take 1 ml of the culture, centrifuge at 10,000 x g at 4 °C for 15 min, discard the supernatant, wash remaining cell pellet with 1x PBS, snap freezing with liquid nitrogen and store at
-80 °C as a sample of “after induction”. Centrifuge the remaining induced bacterial culture at 10,000 x g at 4 °C for 15 min. - Wash the pellet with 10 ml 1x PBS. Centrifuge again at 10,000 x g at 4 °C 15 min. (The cell pellet can be snap frozen and stored at -80 °C if not immediately used).
- Add 10 ml of cell lysis buffer to the cell pellet.
- Vortex it to get clear cell suspension.
- Sonicate using pulse sonicator for 3 min (5 s pulse with 10 s break).
- Centrifuge again at 10,000 x g at 4 °C for 20 min.
- Use the supernatant for purification.
- Purification of GST-fused VCP protein
Note: Perform all the steps at 4 °C.- Add 500 µl of 50% glutathione Sepharose 4B slurry resin into a column (column size 18 ml). (500 µl of 50% glutathione sepharose slurry will give 250 µl of bed volume.)
- Thoroughly wash the glutathione Sepharose with 10 ml 1x PBS three times to remove the ethanol storage solution.
- Load 10 ml of the supernatant from previous Step A1n to the equilibrated glutathione Sepharose column gently. Allow the column to drain (collect the 50 µl sample here for SDS analysis if required).
- Wash the column thrice with 10 ml 1x PBS.
- Elute the protein with 0.5 ml elution buffer. Repeat this step 3 times and collect at least 3 elution samples.
- Estimate the amount of purified protein using bicinchoninic acid (BCA) reagent and also run the sample on SDS-PAGE (10% acrylamide) to check the purity of the fraction.
- Expression of GST-fused VCP and GST-fused VCP deletion mutants
- ATP hydrolysis reaction using purified VCP protein
Before starting the reaction, thaw the purified and aliquoted VCP protein, Kinase-Glo Plus reagent and ATP on ice. Other reagents, such as buffers, should be allowed to reach room temperature. The amount of purified protein, ATP, and inhibitor used in the reaction may vary and should be standardized upon the requirement.- Prepare and label 96-well solid white multi-well plates. Dispense 20 µl of assay buffer (2.5x) to each well carefully using multi-channel pipettes.
- Add 10 µl of purified VCP protein or elution buffer (blank) of required concentration to each well.
- Add 10 µl test compound, or dimethyl sulfoxide (DMSO) or DBeQ (positive control) to each well.
- Incubate at room temperature for 60 min.
- Add 10 µl of 0.5 µM ATP to the solution and mix.
- Incubate at room temperature for 60 min.
- Measure the remaining amount of ATP after the reaction by adding 10 µl of Kinase-Glo Plus reagent to each well.
- Incubate for 10 min at room temperature in the dark. The plate should be kept in a dark room or covered using aluminum foil to protect from the light.
- Read the luminescence using the micro-plate reader at luminescence mode (1 s).
- Subtract the obtained values from the value of blank reaction (without ATPase) to calculate the amount of ATP consumed, which indicates the ATPase activity of the reaction (Figure 1).
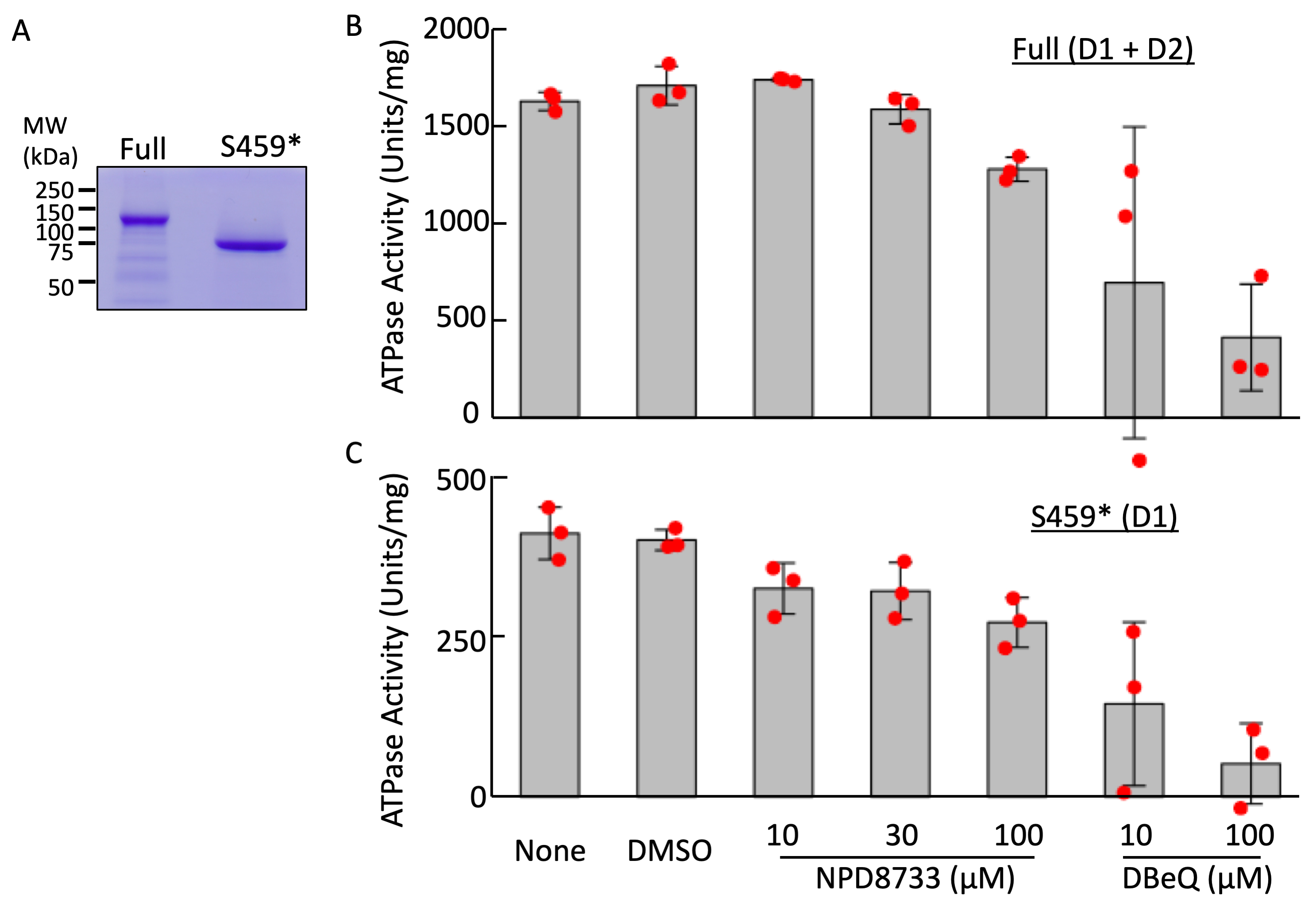
Figure 1. Effect of NPD8733 on ATPase activity of VCP. A. Purified GST-VCP (Full) and its truncated mutant (S459*; Ser 459 was changed to stop codon) proteins (6 µg each) were analyzed using SDS PAGE and CBB staining. B and C. ATPase activities of purified Full and S459* proteins were measured in the presence of NPD8733 or N,N'-Dibenzylquinazoline-2,4-diamine (DBeQ) at concentrations shown in the figures. The red dots indicate the individual measurements.
Data analysis
An example of the assay is indicated in Figure 1 together with the SDS-PAGE of purified enzymes. The values indicated in B show the ATPase activity of the full length VCP, while those in C indicated the ATPase activity of the D1 domain of VCP, since truncated VCP mutant contains only the D1 domain. Since NPD8733 did not significantly affect the activity of either full length or truncated VCP, we concluded that it binds to the D1 domain of VCP without affecting its ATPase activity, while DBeQ inhibited ATPase activity of both D1 and D2 domains of VCP. Experiments were performed in triplicate, and statistical analysis was done using R. Data (Supplement file: R script) were subjected to one-way analysis of variance and Tukey’s Honestly Significant Difference post-hoc test using the functions of ANOVA and Tukey HSD in R, respectively.
Recipes
- Assay buffer (1x)
- Prepare 1x assay buffer
50 mM Tris (pH 7.4)
20 mM MgCl2
1 mM EDTA - Use only MiiliQ water for buffer preparation
- Store at 4 °C. Warm to room temperature prior to use
- Prepare 1x assay buffer
- ATP solution
- Prepare 100 mM ATP in 200 mM Tris base (pH 7.4)
- Aliquot and store the ATP stock at -20 °C
- Donot freeze thaw as ATP will break down over time
- Dilute using MilliQ water
- Kinase-Glo reagent
- Equilibrate the buffer and substrate at room temperature before use
- Transfer the entire volume of the buffer into the amber colored bottle containing lyophilized luciferase substrate
- Mix by gently vortexing or swirling
- The Kinase-Glo reagent should be used fresh or dispensed into aliquots and stored at -20 °C
- DBeQ solution
- Prepare 10 mM DBeQ stock solution using DMSO
- Dispense into several aliquots and store at -20 °C
- LB agar (250 ml)
2.5 g Tryptone
1.25 g Yeast extract
2.5 g NaCl
3.75 g Agar - Cell lysis buffer
150 mM NaCl
5 mM EDTA
50 mM Tris pH 7.5
0.5% CHAPS
Protease/phosphatase Inhibitor (added fresh) - Elution buffer
50 mM Tris pH 8.0
20 mM Reduced glutathione
Prepare freshly - Phosphate-buffered saline (PBS 10 x) (1 L)
80 g NaCl
2 g KCl
14.4 g Na2HPO4
2.4 g KH2PO4
pH 7.5 - SOC (200 ml)
4 g Trypton
1 g Yeast extract
0.1 g NaCl
37.2 mg KCl
Autocrave
1 ml 1 M MgCl2
1 ml 1M MgSO4
2 ml 1 M glucose
Stored at -20 °C after aliquoting
Acknowledgments
We thank Dr. Masaya Imoto and Dr. Etsu Tashiro (Faculty of Science and Technology, Keio University, Japan) for providing the VCP expression vectors. We thank the members of RIKEN NPDepo for providing the chemical libraries, Dr. Naoki Kato (RIKEN) for discussions and Harumi Aono (RIKEN) and Emiko Sanada (RIKEN) for continuous technical help during the study. This research was partially supported by the research grant from JSPS KAKENHI (Grant Number JP16H06276, JP17H06412, JP17K07783, JP18H05503, JP18K05366, JP18H02555, JP19H05302) and AMED (Grant number JP19cm0106112). This protocol was adapted from a previously described work (Sasazawa et al., 2012).
Competing interests
The authors have no competing interest to declare.
References
- Song, C., Wang, Q. and Li, C. C. (2003). ATPase activity of p97-valosin-containing protein (VCP). D2 mediates the major enzyme activity, and D1 contributes to the heat-induced activity. J Biol Chem 278(6): 3648-3655.
- Suvarna, K., Honda, K., Muroi, M., Kondoh, Y., Osada, H. and Watanabe, N. (2019). A small-molecule ligand of valosin-containing protein/p97 inhibits cancer cell-accelerated fibroblast migration. J Biol Chem 294(9): 2988-2996.
- Rule, C. S., Patrick, M. and Sandkvist, M. (2016). Measuring in vitro atpase activity for enzymatic characterization. J Vis Exp(114).
- Sanghera, J., Li, R. and Yan, J. (2009). Comparison of the luminescent ADP-Glo assay to a standard radiometric assay for measurement of protein kinase activity. Assay Drug Dev Technol 7(6): 615-622.
- Bastola, P., Wang, F., Schaich, M. A., Gan, T., Freudenthal, B. D., Chou, T. F. and Chien, J. (2017). Specific mutations in the D1-D2 linker region of VCP/p97 enhance ATPase activity and confer resistance to VCP inhibitors. Cell Death Discov 3: 17065.
- Magnaghi, P., D'Alessio, R., Valsasina, B., Avanzi, N., Rizzi, S., Asa, D., Gasparri, F., Cozzi, L., Cucchi, U., Orrenius, C., Polucci, P., Ballinari, D., Perrera, C., Leone, A., Cervi, G., Casale, E., Xiao, Y., Wong, C., Anderson, D. J., Galvani, A., Donati, D., O'Brien, T., Jackson, P. K. and Isacchi, A. (2013). Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol 9(9): 548-556.
- Tanega, C., Shen, M., Mott, B. T., Thomas, C. J., MacArthur, R., Inglese, J. and Auld, D. S. (2009). Comparison of bioluminescent kinase assays using substrate depletion and product formation. Assay Drug Dev Technol 7(6): 606-614.
- Promega Corporation. Kinase-Glo® Luminescent Kinase Assay Platform. Technical Bulletin #TB372. (Accessed on Jan 6, 2020)
- Sasazawa, Y., Kanagaki, S., Tashiro, E., Nogawa, T., Muroi, M., Kondoh, Y., Osada, H. and Imoto, M. (2012). Xanthohumol impairs autophagosome maturation through direct inhibition of valosin-containing protein. ACS Chem Biol 7(5): 892-900.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Suvarna, K., Honda, K., Muroi, M., Kondoh, Y., Osada, H. and Watanabe, N. (2020). Measurement of ATPase Activity of Valosin-containing Protein/p97. Bio-protocol 10(3): e3516. DOI: 10.21769/BioProtoc.3516.
- Suvarna, K., Honda, K., Muroi, M., Kondoh, Y., Osada, H. and Watanabe, N. (2019). A small-molecule ligand of valosin-containing protein/p97 inhibits cancer cell-accelerated fibroblast migration. J Biol Chem 294(9): 2988-2996.
Category
Cancer Biology > Cancer biochemistry > Protein
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









