- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Acute Isolation of Cells from Murine Sino-atrial Node
Published: Vol 10, Iss 1, Jan 5, 2020 DOI: 10.21769/BioProtoc.3477 Views: 5395
Reviewed by: Gal HaimovichAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2234 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1462 Views
Abstract
The cardiac conduction system allows the synchronized propagation of electrical activity through heart muscle. This is initiated by the spontaneous activity of the specialized pacemaker cells of the sino-atrial node (SAN). The SAN region underlies automaticity in mammals and therefore has a crucial role in the pathogenesis of cardiac disorders such as arrhythmia. Isolation of SAN tissue and SAN cells is critical to advance our understanding of SAN structure and function in health and disease. Initially, isolation of SAN tissue and SAN cells was carried out in the rabbit owing to its larger size and similar electrical properties to human. This protocol was optimized by Mangoni and Nargeot (2001) for use in mice to take advantage of advancements in transgenic models. Here, we provide a step-by-step guide to dissecting the SAN tissue and isolating pacemaker cardiomyocytes from mouse hearts using an enzyme digestion approach.
Keywords: Conduction systemBackground
Cardiovascular diseases and abnormalities are a major cause of morbidity and mortality and are major burden on healthcare systems. Arrhythmias as result of changes in the structure, rate and rhythm of the cardiac conduction system underlie many cardiac disorders. In the past, a lack of availability of homogenous conduction system cell lines has proven to be a hindrance to investigators wishing to study the function of conduction system cells in health and disease. The emergence of transgenic animal models of cardiac abnormalities provides an even greater impetus to reliably isolate healthy conduction system cells. Intact nodes allow investigations into expression profiles of important signaling proteins and genes using immunohistochemistry and qPCR approaches. More detailed functional analysis is possible on isolated single nodal cells using patch-clamp for example. The SAN tissue and the cells it consists of were first isolated many decades ago (Noble and Tsien, 1968). However, the cell population isolated was generally a mixed population. SAN specific cells were first isolated from rabbit SAN by DiFranscesco et al. (1986) in the mid-1980s to allow study of ion channel expression. More recently, the protocol was adapted for isolation of SAN cells from mice by Mangoni and Nargeot (2001). This has allowed transgenic mouse models specific to the SAN to be studied in greater detail. We describe the procedure for isolating the SAN tissue and single SAN cells. The intact node can be used for investigation of gene and protein expression as well as for tissue histology, whilst acutely isolated cells can be interrogated in detail for their electrophysiological properties.
Materials and Reagents
- 15 ml Falcon tubes (Sarstedt, catalog number: 65.554.001)
- Petri Dish 100 x 21 mm (Thermo Fisher Scientific, NuncTM, catalog number: 172931)
- Sylgard 170-Silicone Elastomer (Dow Corning, Farnell, catalog number: 101693)
- 10 ml Glass vials with snap cap (VWR international, catalog number: 548-0555)
- 1 ml Syringe (Terumo, Scientific laboratory supplies, catalog number: SYR6200)
- 26 G needle (VWR International, catalog number: 613-3896)
- Insect pins (Fine Science Tools, catalog number: 260002-13)
- Pyrex disposable borosilicate culture tubes, Corning 99445-10 (Thermo Fisher Scientific, catalog number: 13023073)
- Multi-purpose parafilm M (Sigma-Aldrich, catalog number: P6543)
- Glass or plastic Beaker, 100 ml and 200 ml (Laboratory specific)
- Borosilicate Glass Pasteur Pipettes, autoclaved (Fisher Scientific, catalog number: 13-678-20C)
- 13 mm glass coverslips (VWR, catalog number: 631-0148)
- 12-well cell culture plates, Cellstar (Greiner Bio-One, catalog number: 665 180)
- Adult mice, 6-12 weeks, e.g., C57/Bl6 (The Jackson Laboratory, catalog number: 000664)
- Bovine Serum Albumin (Sigma-Aldrich, catalog number: A6003)
- Collagenase Type II (Worthington Biochemical Corp, catalog number: 4176)
- Elastase (Worthington Biochemical Corp, catalog number: 2292)
- Protease XIV (Sigma-Aldrich, catalog number: P5147)
- Laminin (Sigma-Aldrich, catalog number: L2020)
- Deionized, filtered water (dH2O) (Merck Millipore, Milli-Q)
- Sodium Chloride (NaCl) (Sigma-Aldrich, catalog number: S7653)
- Potassium Chloride (KCl) (Sigma-Aldrich, catalog number: P9333)
- Potassium Phosphate (KH2PO4) (Sigma-Aldrich, catalog number: P0662)
- L-Glutamic Acid (C5H9NO4) (Sigma-Aldrich, catalog number: 09581)
- Potassium Glutamate (C5H8KNO4·H2O) (Sigma-Aldrich, catalog number: G1501)
- Potassium Hydroxide pellets (KOH) (Sigma-Aldrich, catalog number: P6310)
- Sodium Hydroxide pellets (NaOH) (Thermo Fisher Scientific, catalog number: S/4920/53)
- HEPES-NaOH (Sigma-Aldrich, catalog number: H7006)
- HEPES-KOH (Sigma-Aldrich, catalog number: H0527)
- D-Glucose (Sigma-Aldrich, catalog number: G5767)
- Taurine (Sigma-Aldrich, catalog number: T8691)
- Calcium Chloride (CaCl2) 1 M solution (Fluka, catalog number: 21114)
- Magnesium Chloride (MgCl2) 1 M solution (Fluka, catalog number: 63026)
- DL-β-Hydroxybutyric acid sodium salt (Sigma-Aldrich, catalog number: H6501)
- Ketamine (Narketen-10) (Vetoquinox, procured via biological services unit)
- Xylazine (Rompun 2% w/v) (Bayer, procured via biological services unit)
- Heparin Sodium Salt (1,000 U/ml) (Wockhardt, procured via biological services unit)
- Tyrode solution (see Recipes)
- Tyrode-BSA solution (see Recipes)
- Low Ca2+/Mg2+ solution (see Recipes)
- Kraftbruhe (KB) solution (see Recipes)
- SAN storage solution (see Recipes)
- Enzyme digestion solution (see Recipes)
- Re-adaptation solution (see Recipes)
- Storage solution (mM) (see Recipes)
- Heparin/Ketamine/Xylazine Injection (see Recipes)
- Sylgard dissection dishes (see Recipes)
Equipment
- Scale and weighing (Laboratory specific)
- Dissecting scissors (World Precision Instruments, catalog number: 14192)
- Student Vannas scissors (World Precision Instruments, catalog number: 501777)
- Fine Vannas Scissors (World Precision Instruments, catalog number: 500086)
- Fine Vannas Scissors curved (World Precision Instruments, catalog number: 501232)
- 2x Dumont No.5 forceps (World Precision Instruments, catalog number: 500085)
- Zeiss Stereomicroscope Stemi2000 (Zeiss, catalog number: 455052)
- Grant Water Bath (Scientific Laboratory Supplies, catalog number: BAT3310)
- Stuart SSL4 See-Saw Rocker (Scientific Laboratory Supplies, catalog number: MIX2066)
- Cell Culture Incubator (Laboratory-specific)
- PIPETMAN Classic P10 (Gilson, catalog number: F144802)
- PIPETMAN Classic P100 (Gilson, catalog number: F144802)
- PIPETMAN Classic P200 (Gilson, catalog number: F144802)
- PIPETMAN Classic P1000 (Gilson, catalog number: F144802)
- Centrifuge 5810 R swing bucket for conical tubes (Eppendorf, model: 5810 R)
- Diamond cutting tool for glass (Amazon)
- Culture hood
Procedure
The procedure is illustrated in Figure 1.
Note: Stock solutions for dissection and isolation of the SAN can be prepared the day before isolation (Recipes 1, 2, 4, 5 and 7) as it can take ~1 h to make them. Solutions should be kept in the fridge (~4-8 °C) and can be used for isolation for up to a week. For working solutions, such as the enzyme solution it is advisable to make on the day. All working solutions need to be pre-warmed (in a water bath) at 37 °C before use (~15-20 min). It is critical that CaCl2 (10 µl of 100 mM stock to 2.5 ml-final concentration 400 µM) is added to the enzyme solution, otherwise tissue digestion will be compromised.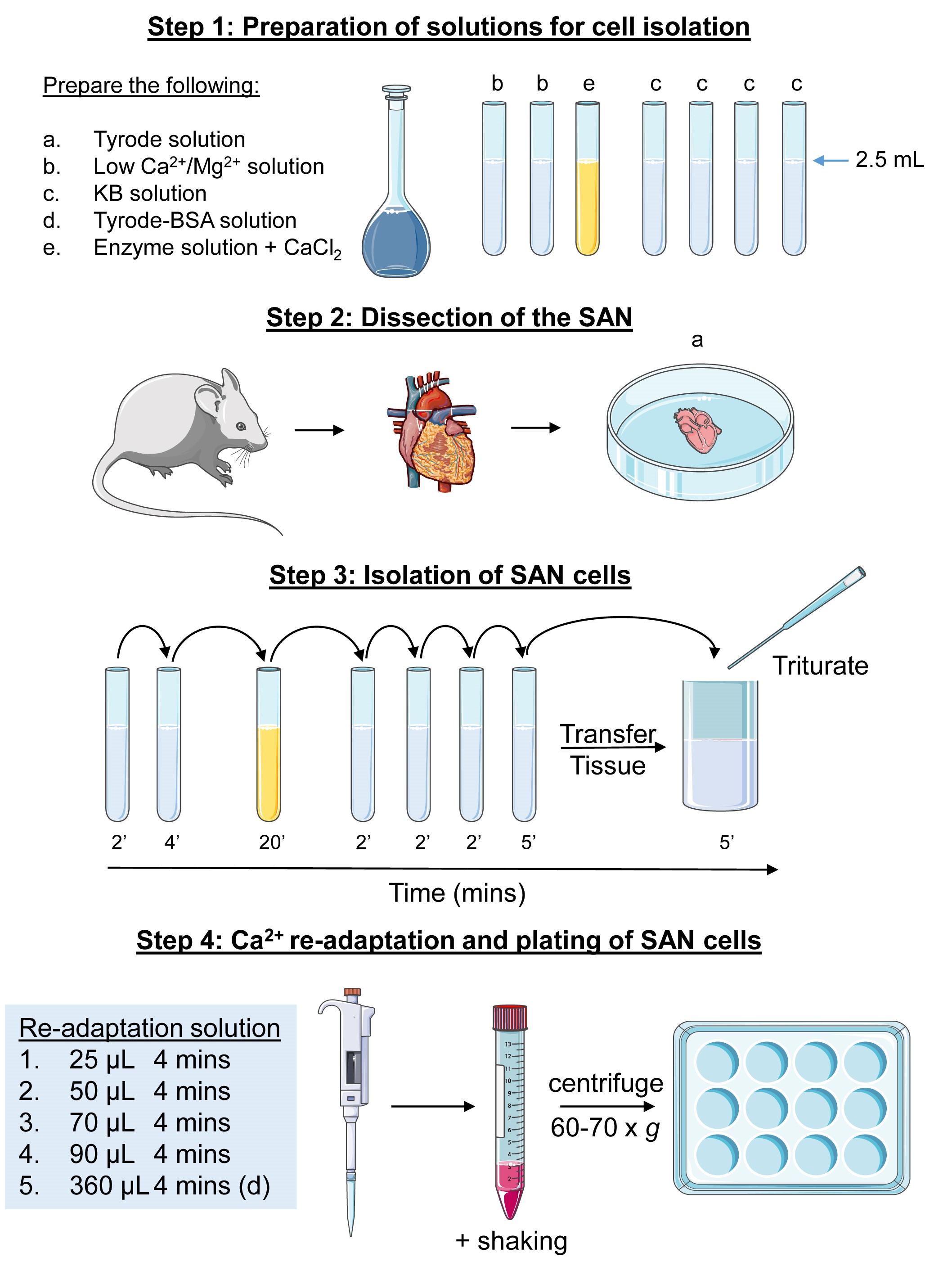
Figure 1. Schematic of the protocol for the isolation of SAN cells from a mouse heart
- Preparation
- Before dissection of the SAN, prepare the following Pyrex glass tubes:
- 2 x 2.5 ml of low Ca2+/Mg2+ solution (Recipe 2);
- 1 x 2.5 ml of enzyme solution (Recipe 3 and Note above);
- 4 x 2.5 ml of KB solution (Recipe 4).
- Beaker with pre-warmed Tyrode solution (Recipe 1).
- 2 dissection dishes with pre-warmed Tyrode solution.
- Heparin/Xylazine/Ketamine injection (Recipe 8).
- Assemble tools required for dissection (dissecting scissors, fine forceps, Vannas scissors).
- Prepare a 12-well plate with laminin-coated coverslips. Coat coverslips with 1 µl of Laminin per coverslip and spread using a P200 tip and allow to dry in the culture hood with the lid off.
- Before dissection of the SAN, prepare the following Pyrex glass tubes:
- Heart removal from chest cavity
- Anesthetize mice with an intraperitoneal injection of Xylazine/Ketamine/Heparin mix (adhere to Institute guidelines).
- Lay the mouse in the supine position (Figure 2) and spray the chest area with 70% ethanol. Open the chest cavity by cutting through the rib cage using dissecting scissors and remove the intact heart by holding it at the aortic arch with forceps and cutting just above the forceps using the student Vannas spring scissors. Do not worry about taking extra-cardiac tissue as this can be cleaned later. Rinse the heart in a beaker filled with Tyrode solution. Be careful not to cut/damage atrial appendage regions of the heart.
- Place the heart, pinned at the apex with an insect pin on a dissection dish submerged with Tyrode solution. The heart should be placed as it would be in the mouse chest cavity (see Figure 2). Remove non-cardiac tissue such as lungs, thymus, connective tissue and fat under a microscope. Make a small incision in the ventricles using Vannas scissors and flush the heart of blood using a Pasteur pipette filled with Tyrode solution. Make sure to avoid air bubbles.

Figure 2. Dissection of the mouse heart and isolation of the SAN. A. The anesthetized mouse is placed in the supine position and incisions made where indicated (dotted lines) to access the cardiac cavity. B. Removal of the rib cage shows the position of the heart. The heart is dissected by cutting at the aortic arch. The lungs can be removed before or after excision. C. Excised heart showing the right and left atrial appendage (RA and LA), right and left ventricles (RV and LV) and the aorta (Ao). D. The heart is pinned to the dissection dish- the dotted line shows where to cut to separate the atrial/nodal tissue from the ventricles without damaging the SAN. E. Intact SAN, RA and LA after separation from the ventricles. The incision seen on the left ventricle was used to flush out the blood from the heart before separation of the upper chambers from the ventricles. F and G. The SAN (within dotted area) and surrounding tissue after the fat and connective tissue have been removed. CT, cristae terminalis. IAS, intra-atrial septum. - SAN dissection
- Under the microscope, carefully make a horizontal incision using students Vannas scissors at the junction between the ventricles and atria (see Figure 2). The ventricles should stop beating and can be discarded. The upper chambers with the atrial appendages and conduction tissue should continue to beat.
- Flip the atrial/conduction tissue so that the aorta is facing you with the right atria on your left side.
- Using insect pins carefully pin the atrial/conduction tissue in a clean Sylgard dissection dish (Recipe 9) containing Tyrode solution (see Figure 2). Place pins strategically on the left and right atria, on the edges of the aorta (see Figure 2) or superior vena cava and inferior vena cava (if present). Do not over-stretch the tissue. Carefully remove the remaining ventricular tissue, if necessary, using fine Vannas scissors.
- Once the tissue is free of ventricular muscle, cut open the right atrial appendage pocket and re-pin to reveal the Cristae terminalis (CT). The SAN (transparent) will be clearly visible between the inter-atrial septum and the cristae terminalis (see Figure 2).
- Carefully remove any fat/connective tissue from beneath the SAN.
- Finally, carefully cut the SAN with fine Vannas scissors and, transfer to a Pyrex tube containing pre-warmed low Ca2+/Mg2+ solution with fine forceps.
- Enzyme digestion of SAN tissue (see the schematic Figure 1)
All solutions should be pre-warmed at 37 °C in a water bath.- Place the SAN tissue into a pre-prepared Pyrex tube containing low Ca2+/Mg2+ solution. Incubate for 2 min at 37 °C (in the water bath). Using a fire-polished Pasteur pipette carefully transfer the tissue in to the second low Ca2+/Mg2+ tube for a further 4 min.
- Transfer the SAN tissue in to the enzyme solution for 10-15 min at 37 °C. Agitate manually every 2-3 min with a fire polished Pasteur pipette. The tissue should start to look less structurally rigid.
- Wash the tissue 4 times in the KB solution. The first 3 washes should be for 2 min followed by a fourth and final wash for 4 min.
- Transfer the tissue and KB solution from the final wash to a 5 or 10 ml glass vial. Gently triturate (pipette up and down) the tissue with an appropriately sized (see Notes) fire-polished glass Pasteur pipette for ~30-60 s, avoiding air bubbles. Allow the cell suspension to rest at room temperature for 5 min.
- Re-adaptation of SAN cells
- Transfer the triturated cell suspension to a 15 ml Falcon tube.
- Cumulatively add the following volumes of the NaCl/CaCl2 solution (Recipe 5) to the Falcon tube containing the cell suspension and place on the rocking platform.
25 µl for 4 min
50 µl for 4 min
70 µl for 4 min
90 µl for 4 min - Add 360 µl of Tyrode-BSA (Recipe 6) to the cell suspension and place on the rocker for 4 min.
- Centrifuge the cell suspension at ~60-70 x g for 7 min. There should be a small pellet of cells.
- Carefully remove 2-2.2 ml of the supernatant using a 1 ml Gilson Pipetman (~0.8-1 ml supernatant should remain).
- Gently re-suspend the pellet of cells in the remaining supernatant using 1 ml Gilson Pipetman and place roughly an equal amount (~60-80 µl) on the pre-prepared laminin coated coverslip (within a 12-well culture plate). Incubate the plate at 37 °C (95% O2/5% CO2) cell culture incubator for ~30-45 min to allow cells to adhere to the coverslips.
- Check cells under the microscope (using the 20x objective) to see if cells are viable–cells should. Add 1 ml/well of pre-warmed storage solution (Recipe 7) or Tyrode-BSA. Cells are now ready to use.
Morphologically SAN cells consist of 3 types: Spindle-shaped, spider-like and elongated (see Figure 3).
Note: We found that placing the cells in Tyrode-BSA was just as effective as placing them in storage solution. The number of total cells can be very variable depending on the success of the preparation, however on average we expect to see ~100-120 (5-10 per coverslip) viable cells per preparation.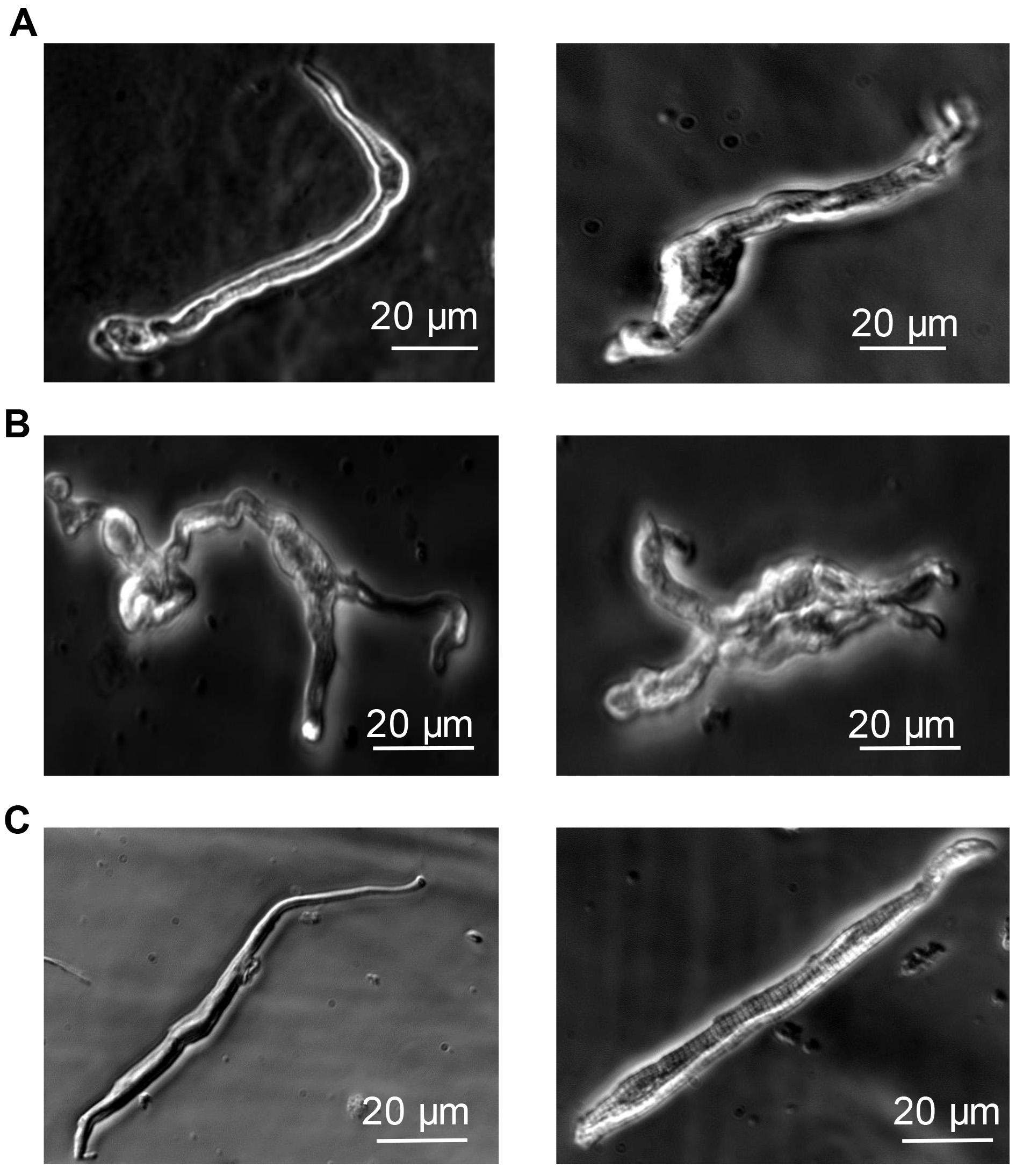
Figure 3. Morphologies of the three types of acutely isolated single SAN cells from mouse heart. A. Spindle-shaped. B. Spider-like. These cells have 1 or more processes. C. Elongated spindle-shaped. Healthy cells beat spontaneously. Scale bar: 20 µm.
Recipes
- Tyrode solution
140 mM NaCl
5.4 mM KCI
1.2 mM KH2PO4
1.8 mM CaCI2
1.2 mM MgCI2
5 mM HEPES-NaOH
5.5 mM D-Glucose
Adjust the pH to 7.4 with NaOH or HCI if pH basic - Low ‘Ca2+/Mg2+’ solution
140 mM NaCl
5.4 mM KCI
1.2 mM KH2PO4
0.2 mM CaCI2
0.5 mM MgCI2
50 mM Taurine
5 mM HEPES-NaOH
5.5 mM D-Glucose
1 mg/ml BSA
Adjust the pH to 6.9 with NaOH or HCI if pH if basic - Enzyme solution (made in 2.5 ml of low Ca2+/Mg2+ solution [Recipe 2])
1 mg/ml Collagenase Type II
1 mg/ml Protease XIV
0.5 mg/ml Elastase
10 μl of 100 mM CaCl2 (400 µM final concentration) - Kraftsbuhe ‘KB’ medium
70 mM L-glutamic acid
20 mM KCI
80 mM KOH
10 mM KH2PO4
10 mM Taurine
10 mM HEPES-KOH
1 mg/ml BSA
Adjust the pH to 7.4 with KOH - Re-adaptation solution
10 mM NaCl
1.8 mM CaCI2 - Tyrode-BSA
Tyrode solution (Recipe 1)
1 mg/ml BSA - Storage solution
100 mM NaCl
35 mM KCI
4 mM KH2PO4
1.3 mM CaCI2
0.7 mM MgCI2
2 mM Taurine
14 mM Potassium glutamate
0.2 mM (±) D-β-butyric acid
1 mg/ml BSA
Adjust the pH to 7.4 with NaOH - Heparin/Ketamine/Xylazine Injection
- Heparin
Dilute Heparin stock (e.g., 1,000 IU/ml) to 200 IU/ml in sterile saline–e.g., 1 ml of Heparin in 4 ml of saline. This solution can be kept in the fridge for up to 4 weeks. Use ~1 IU/g (mouse weight). For example, 100 µl of 200 IU/ml heparin for a 20 g mouse - Ketamine
Use 0.5 mg/g. For example, 100 µl of 100 mg/ml stock for 20 g mouse - Xylazine
Use 0.1 mg/g. For example, 100 µl of 20 mg/ml stock for 20 g mouse - For injection make a cocktail of heparin/ketamine/xylazine of between 300 µl to 500 µl per mouse
- Heparin
- Sylgard dissection dishes
- Dissection dishes were made using Sylgard 170-Silicone Elastomer (Dow Corning) as per manufacturer’s instructions
- Parts A and B were mixed in equal amounts in a Petri Dish (100 x 21 mm). The amounts used are dependent on the thickness required (we recommend enough for 50% depth)
- The Sylgard mix allowed to set at 37 °C for at least 24 h (this can be done at room temperature but it will take longer to set)
Acknowledgments
We would like to thank the BHF for funding (RG/15/15/311742). The work was facilitated by the NIHR Biomedical Research Centre at Barts. Special thanks to Dr Mangoni for allowing Dr Nobles to visit his laboratory to learn the procedure for isolation of murine SAN cardiomyocytes. This protocol was adapted/modified from the protocol of Mangoni and Nargeot (2001).
Competing interests
We have no conflicts of interest.
Ethics
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the British Home Office regulations (covered by project license PE9055EAD) and by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
References
- DiFrancesco, D., Ferroni, A., Mazzanti, M. and Tromba, C. (1986). Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol 377: 61-88.
- Mangoni, M. E. and Nargeot, J. (2001). Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells. Cardiovasc Res 52(1): 51-64.
- Noble, D. and Tsien, R. W. (1968). The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol 195(1): 185-214.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Aziz, Q., Nobles, M. and Tinker, A. (2020). Acute Isolation of Cells from Murine Sino-atrial Node. Bio-protocol 10(1): e3477. DOI: 10.21769/BioProtoc.3477.
- Aziz, Q., Finlay, M., Montaigne, D., Ojake, L., Li, Y., Anderson, N., Ludwig, A. and Tinker, A. (2018). ATP-sensitive potassium channels in the sinoatrial node contribute to heart rate control and adaptation to hypoxia. J Biol Chem 293(23): 8912-8921.
Category
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link