- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Actinoplanes missouriensis Zoospores and Assay for Their Adherence to Solid Surfaces
Published: Vol 9, Iss 24, Dec 20, 2019 DOI: 10.21769/BioProtoc.3458 Views: 4190
Reviewed by: David A. CisnerosTimo A LehtiJose Antonio Reyes-Darias

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analysis of B Cell Migration by Intravital Microscopy
Michael Schnoor [...] Eduardo Vadillo
Dec 5, 2020 4827 Views

Static Adhesion Assay for Human Peripheral Blood Mononuclear Cells
Giulia Vanoni [...] Sara Trabanelli
Jan 5, 2022 3809 Views

An Unbiased CRISPR-Cas9 Screening Method for the Identification of Positive and Negative Regulatory Proteins of Cell Adhesion
Yvonne J. Thus [...] Marcel Spaargaren
Nov 5, 2022 2792 Views
Abstract
Spherical zoospores of a rare actinomycete, Actinoplanes missouriensis, adhere to various hydrophobic solid surfaces by means of type IV pili. The purpose of this protocol is to provide detailed descriptions of the preparation of A. missouriensis zoospores and an assay for the adhesion of the zoospores to solid surfaces. This adhesion assay, which measures numbers of zoospores that adhered to the dish surface and swimming zoospores in a tunnel chamber by using a phase-contrast microscope, can also be used for swimming cells of other microorganisms.
Keywords: AdhesionBackground
Zoospores are motile asexual cells for reproduction that swim in aquatic environments by means of flagella. Although zoospores are often described as a kind of spore because of their function in the life cycle of producing organisms, attention must be paid to the fact that they are not dormant cells when they are swimming. Both eukaryotic and prokaryotic organisms produce zoospores, but eukaryotic zoospores produced by protists and fungi are more well-known compared with prokaryotic zoospores. Members of a fungal phylum of chytridiomycota, as well as oomycetes (which are pseudomycetes), are known to develop zoospores (Sharma et al., 2015; Letcher and Powell, 2017). Zoospores of these microorganisms swim in aquatic environments and adhere to the surface of organic substances, including parasitism hosts and dead bodies of animals and plants. In bacteria, several rare actinomycetes are known to produce zoospores, which are developed in a sporangium or by the fragmentation of aerial hyphae. A wide variety of bacterial zoospores have been isolated from natural environments by taking advantage of their chemotactic property (Hayakawa et al., 1991). Importantly, bacterial zoospores arise from dormant sporangiospores or arthrospores. A rare actinomycete Actinoplanes missouriensis produces terminal sporangia that contain a few hundred flagellated spores. The spores are dormant in a sporangium and, are activated and released to external environments when the sporangium is immersed in water. Although the presence of pili had not been reported in bacterial zoospores, we recently found a biosynthetic gene cluster for functional type IV pili in the A. missouriensis genome sequence, genetically analyzed the gene cluster, and successfully observed the unprecedented zoospore pili in A. missouriensis (Kimura et al., 2019). Furthermore, we developed an adhesion assay for A. missouriensis zoospores to characterize the function of the zoospore pili to attach the zoospores to solid surfaces. A similar adhesion assay for Mycoplasma has already been published by Kasai and Miyata (2013). The adhesion assay described in this protocol can be used not only for zoospores of other species but also for swimming cells of other microorganisms.
Materials and Reagents
- Coverslips (18 x 18 mm, Matsunami Glass Industry, catalog number: C218181)
- Glass dish (f 35 mm, AGC Techno Glass, catalog number: 3970-035)
- Polystyrene dish (f 35 mm, AGC Techno Glass, catalog number: 1000-035)
- Polystyrene dish (f 90 mm, Sansei Medical, catalog number: 01-013)
- 1.5-ml centrifuge tube (Greiner Bio-One, catalog number: J618201)
- 50-ml centrifuge tube (Corning, catalog number: 430829)
- Shaking (Sakaguchi) flask (Sansyo, catalog number: 82-0317)
- Double-sided tape (width 15 mm, thickness 0.086 mm, NICETACK, Nichiban)
- WhatmanTM Filter paper (GE Healthcare Life Sciences, catalog number: 3030-917)
- Toothpick
- A. missouriensis 431T (NBRC 102363T)
- Yeast extract (Becton, Dickinson and Company, catalog number: 212750)
- Meat extract (Kyokuto, catalog number: 551-01240-8)
- N-Z-Amine® (FUJIFILM Wako Pure Chemical, catalog number: 146-08675)
- D (+)-Maltose monohydrate (FUJIFILM Wako Pure Chemical, catalog number: 130-00615)
- Agar powder (Kokusan Chemical, catalog number: 2111136)
- Peptone (Becton, Dickinson and Company, catalog number: 211677)
- Magnesium sulfate heptahydrate (MgSO4·7H2O) (Kokusan Chemical, catalog number: 2114992)
- Saccharose (Kokusan Chemical, catalog number: 2111624)
- Casamino acids, technical (Becton, Dickinson and Company, catalog number: 223120)
- Dipotassium hydrogen phosphate (K2HPO4) (Kokusan Chemical, catalog number: 2115140)
- Nitrohumic acid (Tokyo Chemical Industry, catalog number: H0161)
- Sodium hydroxide (NaOH) (Kokusan Chemical, catalog number: 2112744)
- Zinc chloride (ZnCl2) (FUJIFILM Wako Pure Chemical, catalog number: 263-00271)
- Iron (III) chloride hexahydrate (FeCl3·6H2O) (FUJIFILM Wako Pure Chemical, catalog number: 091-00872)
- Cupric chloride, dihydrate (CuCl2·2H2O) (Kokusan Chemical, catalog number: 2150417)
- Manganese (II) chloride tetrahydrate (MnCl2·4H2O) (FUJIFILM Wako Pure Chemical, catalog number: 139-00722)
- Sodium tetraborate (Na2B4O7·10H2O) (Kokusan Chemical, catalog number: 2114089)
- Ammonium molybdate ((NH4)6Mo7O24·4H2O) (Kokusan Chemical, catalog number: 2152738)
- Sodium chloride (NaCl) (Kokusan Chemical, catalog number: 2110733)
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9647)
- Ammonium hydrogencarbonate (NH4HCO3) (FUJIFILM Wako Pure Chemical, catalog number: 017-02875)
- YBNM agar medium (see Recipes)
- PYM broth (see Recipes)
- HAT agar medium (see Recipes)
- Nitrohumic acid solution (see Recipes)
- Trace element solution (see Recipes)
Equipment
- Mortar and pestle
- Laminar flow cabinet
- Flask shaker
- Centrifuge (Kubota Corp., model: 5200)
- Incubator (PHC Holdings, model: MIR-154)
- Micropipette
- Phase-contrast microscope (Olympus, model: IX73)
- Objective lens (Olympus, model: UPLFLN20×PH)
- Optical table (JVI, model: HAX-0605)
- High speed recorder system (Digimo, model: LRH1540) with complementary metal-oxide semiconductor (CMOS) camera
Software
- ImageJ (https://imagej.nih.gov/ij/)
Procedure
- Preparation of A. missouriensis zoospores
- Inoculate A. missouriensis cells from a glycerol stock on YBNM agar medium and cultivate them at 30 °C in an incubator for 2 or 3 days (Figure 1).

Figure 1. A YBNM agar plate (diameter, 9 cm) on which A. missouriensis mycelia are grown for 4 days at 30 °C - Using a sterilized toothpick, cut the agar medium to prepare an agar piece (approximately 1 cm square), on the surface of which A. missouriensis mycelium has proliferated (Figure 2). Put the agar piece into PYM broth (100 ml) in a shaking flask to inoculate the mycelium, and cultivate the A. missouriensis cells by shaking at 120 rpm at 30 °C for 2 days (Figure 3).
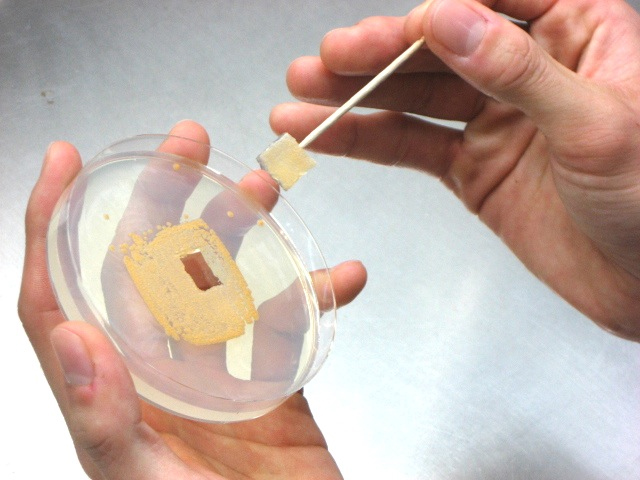
Figure 2. Preparation of an agar piece for the inoculation of A. missouriensis
Figure 3. Liquid culture of A. missouriensis in a shaking flask (at 30 °C for 2 days) - Collect the cells by centrifugation at 2,330 x g for 10 min at RT.
- Suspend the cells in 0.75% NaCl solution (50 ml) and centrifuge them at 2,330 x g for 10 min at RT.
- Resuspend the cells in 0.75% NaCl solution (10 ml; Figure 4) and inoculate a portion (0.1 ml) of the cell suspension on one HAT agar plate.

Figure 4. A. missouriensis mycelia suspended in 0.75% NaCl solution - Spread the cell suspension and completely dry the surface of the HAT agar medium.
- Incubate the plate at 30 °C for at least 7 days (Figure 5).

Figure 5. A HAT agar plate (diameter, 9 cm) on which A. missouriensis are grown for 7 days at 30 °C. Many sporangia are produced on substrate mycelium. - Pour 25 mM NH4HCO3 solution (10 ml) onto the HAT agar plate (Figure 6) and incubate it at 30 °C for 1 h.

Figure 6. Pouring of NH4HCO3 solution onto HAT agar - Collect the poured solution, which contains swimming zoospores (approximately 104-105 cells/μl; Figure 7). Keep the solution at RT until the use.

Figure 7. A. missouriensis zoospore-containing solution
- Inoculate A. missouriensis cells from a glycerol stock on YBNM agar medium and cultivate them at 30 °C in an incubator for 2 or 3 days (Figure 1).
- Zoospore adhesion test
- Attach a coverslip to a hydrophobic polystyrene dish or a hydrophilic glass dish by using two pieces of double-sided tape. Make a tunnel chamber by arranging the tape as parallel lines and making open slits on both sides of the coverslip (Figure 8). This arrangement enables a reproducible distance between the coverslip and dish, ensuring the fixed volume of the tunnel chamber, and also enables a replacement of the solution inside the chamber using a filter paper. The protocol using a similar tunnel chamber has already been published by Kasai and Miyata (2013).
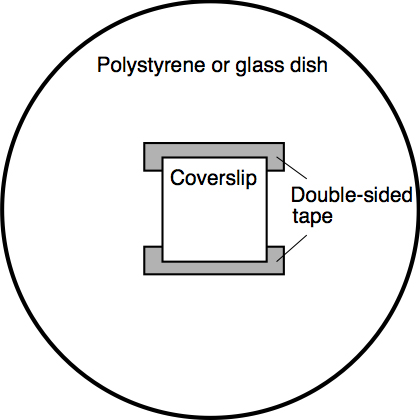
Figure 8. Illustration of a tunnel chamber - (Optional; BSA-coating on the surface of a hydrophobic polystyrene dish) Using a micropipette, pour 1% BSA solution into the space between the dish and coverslip. Keep it at RT for 1 min. Absorb 1% BSA solution from a side of the chamber using a filter paper. By a similar procedure, wash the chamber with 25 mM NH4HCO3 solution. The BSA-coating treatment renders the dish surface hydrophilic. Through the treatment, the average proportion of the zoospores that adhered to the dish surface decreased from 41% (non-treated dish surface) to 0.2% (BSA-treated dish surface; Kimura et al., 2019).
- Using a micropipette, put the zoospore-containing solution (12 µl) carefully into the space between the dish and coverslip without introducing air bubbles. Incubate the chamber at RT for 10 min.
- Record the zoospores that adhered to the dish surface and the swimming zoospores in the tunnel by using a phase-contrast microscope equipped with a 20x objective lens. A lab recorder system and a CMOS camera enable the high-speed (200 frames per second) imaging. Scan the microscopic fields along the vertical direction for the analyzing region of the tunnel chamber in 3 s (600 images in total).
- Using a micropipette, put the 25 mM NH4HCO3 solution (150 μl) on one side of the tunnel and absorb the zoospore-containing solution from the other side using a filter paper to completely exchange the solution for 25 mM NH4HCO3 solution for the removal of the zoospores that did not adhere to the solid surface.
- Photograph the zoospores that adhered to the dish surface by using the microscope.
- Attach a coverslip to a hydrophobic polystyrene dish or a hydrophilic glass dish by using two pieces of double-sided tape. Make a tunnel chamber by arranging the tape as parallel lines and making open slits on both sides of the coverslip (Figure 8). This arrangement enables a reproducible distance between the coverslip and dish, ensuring the fixed volume of the tunnel chamber, and also enables a replacement of the solution inside the chamber using a filter paper. The protocol using a similar tunnel chamber has already been published by Kasai and Miyata (2013).
- Data analysis
- Convert the movie files recorded in Step B4 into AVI files. Convert the 8-bit images recorded in Step B6 into TIF files without compression using ImageJ, an image analysis software.
- Count the total number of swimming zoospores and those adhered to the dish surface in the analyzing region of the tunnel chamber by ImageJ using the microscopic images recorded in Step B4. The Color_FootPrint macro for ImageJ (http://www.jaist.ac.jp/ms/labs/hiratsuka/images/0/09/Color_FootPrint.txt) enables the visualization of the zoospore swimming trajectories (Hiratsuka et al., 2006).
- Open a movie file by using the command “File > Open”. Check off the boxes for “Use Virtual Stack” and “Convert to Grayscale”.
- Start the Color_FootPrint macro by using the command “Plugins > Macros > Color Footprint Rainbow”. The macro visualizes zoospore swimming trajectories (Figure 9B).
- Count the number of the stationary cells on the dish surface (Figure 9A) and the number of the colored trajectories, which represents the number of swimming zoospores, in the analyzing region of the tunnel chamber scanned in Step B4, by visual inspection.
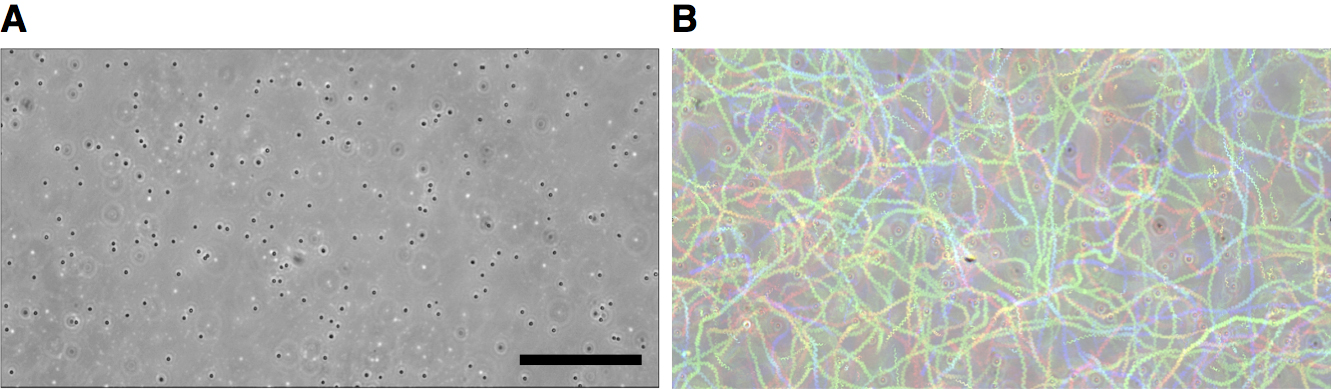
Figure 9. Counting the number of zoospores in the tunnel chamber by ImageJ. A. Input image; the first image of the total 600 scanning images is shown as a representative. B. Output image; colored trajectories are shown. Scale bar = 50 μm.
- Count the number of zoospores that adhered to the dish surface using the microscopic images recorded in Step B6 by ImageJ.
- Open an image by using the command “File > Open”.
- Select the analyzed region of the microscopic field scanned in Step B4 by using the “Rectangle” tool and clip the field by using the command “Image > Crop”.
- Set a threshold level by using the command “Image > Adjust > Threshold”.
- Count the number of zoospores by using the command “Analyze > Analyze Particles”. Set appropriate values of the parameters “Size” and “Circularity” (Figure 10).
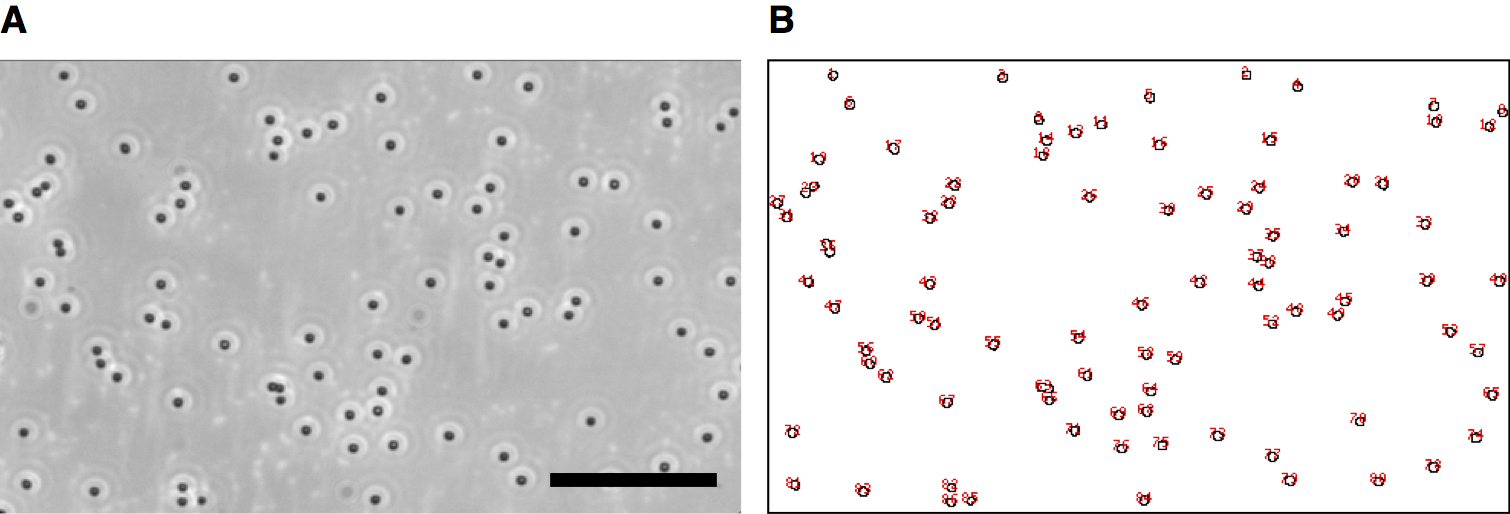
Figure 10. Counting the number of zoospores on the dish surface by ImageJ. A. Input image. Scale bar = 20 μm. B. Output image. A part of the original image is enlargedly shown.
- Calculate the adhesion ratio, i.e., the proportion of adhesive zoospores to whole zoospores: C3/C2. Correction values per unit area should be used for the calculation.
Notes
In the adhesion test, zoospores must be freshly prepared. Use zoospores within 1 h after collection. The collected zoospores should not be diluted because the dilution with a buffer may affect the motility of zoospores.
Recipes
- YBNM agar medium
0.1% yeast extract
0.2% meat extract
0.2% N-Z-Amine®
1% D (+)-maltose monohydrate
Adjust pH to 7.0
Add agar to 2% prior to autoclaving - PYM broth
0.5% peptone
0.3% yeast extract
0.1% MgSO4·7H2O
Adjust pH to 7.0
Autoclave the broth - HAT agar medium
0.1% saccharose
0.01% casamino acids, technical
0.05% K2HPO4
2% nitrohumic acid solution
1% trace element solution
Adjust pH to 7.5
Add agar to 2% prior to autoclaving - Nitrohumic acid solution
- Grind 10 g of nitrohumic acid with a mortar and pestle
- Add 100 ml of 0.8% NaOH solution little by little and suspend the nitrohumic acid powder
- Autoclave the suspension at 105 °C for 15 min
- Stir the suspension until cooling down to RT
- Autoclave again the suspension at 105 °C for 15 min
- Stir the suspension until cooling down to RT
- Centrifuge the suspension at 1,500 x g for 10 min at 4 °C
- Transfer the supernatant to a sterilized glass bottle and store at 4 °C
- Trace element solution
0.004% ZnCl2
0.02% FeCl3·6H2O
0.001% CuCl2·2H2O
0.001% MnCl2·4H2O
0.001% Na2B4O7·10H2O
0.001% (NH4)6Mo7O24·4H2O
Autoclave the solution
Acknowledgments
This protocol is adapted from Kimura et al. (2019). The above work was supported in part by Grants-in-Aid for Scientific Research no. 19H05685 (to Y.O.), 18H02122 (to Y.O.), 26252010 (to Y.O.), and 17K07711 (to T.T.), Grants-in-Aid for Young Scientists no. 16H06230 (to D.N.) and 15K18669 (to T.T.), and a Grant-in-Aid for JSPS Research Fellow no. 15J07768 (to T.K.) from the Japan Society for the Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT).
Competing interests
The authors declare no conflicts of interest associated with this manuscript.
References
- Hayakawa, M., Tamura, T. and Nonomura, H. (1991). Selective isolation of Actinoplanes and Dactylosporangium from soil by using γ-collidine as the chemoattractant. J Ferment Bioeng 72(6): 426-432.
- Hiratsuka, Y., Miyata, M., Tada, T. and Uyeda TQ. (2006). A microrotary motor powered by bacteria. Proc Natl Acad Sci USA 103(37): 13618-13623.
- Kasai, T. and Miyata, M. (2013). Analyzing inhibitory effects of reagents on Mycoplasma gliding and adhesion. Bio-protocol 3(14): e829.
- Kimura, T., Tezuka, T., Nakane, D., Nishizaka, T., Aizawa, S. and Ohnishi, Y. (2019). Characterization of zoospore type IV pili in Actinoplanes missouriensis. J Bacteriol 201(14): e00746-18.
- Letcher, P. M. and Powell, M. J. (2017). Hypothesized evolutionary trends in zoospore ultrastructural characters in Chytridiales (Chytridiomycota). Mycologia 106(3): 379-396.
- Sharma, M., Ghosh, R., Tarafdar, A. and Telangre, R. (2015). An efficient method for zoospore production, infection and real-time quantification of Phytophthora cajani causing Phytophthora blight disease in pigeonpea under elevated atmospheric CO2. BMC Plant Biol 15: 90.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tezuka, T., Nakane, D., Kimura, T. and Ohnishi, Y. (2019). Preparation of Actinoplanes missouriensis Zoospores and Assay for Their Adherence to Solid Surfaces. Bio-protocol 9(24): e3458. DOI: 10.21769/BioProtoc.3458.
Category
Microbiology > Microbial physiology
Cell Biology > Cell movement > Cell adhesion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









