- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Imaging Cryptococcus spp. Capsule by Differential Interference Contrast Microscopy Using Percoll®
Published: Vol 9, Iss 22, Nov 20, 2019 DOI: 10.21769/BioProtoc.3423 Views: 4151
Reviewed by: Samantha E. R. DundonJulie WeidnerAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live-cell Imaging by Super-resolution Confocal Live Imaging Microscopy (SCLIM): Simultaneous Three-color and Four-dimensional Live Cell Imaging with High Space and Time Resolution
Kazuo Kurokawa and Akihiko Nakano
Sep 5, 2020 5998 Views

Fabrication of Microfluidic Devices for Continuously Monitoring Yeast Aging
Richard O’Laughlin [...] Nan Hao
Aug 5, 2023 1464 Views

Imaging the Entire Sexual Life Cycle of the Budding Yeast Saccharomyces cerevisiae Using a Microfluidic Platform
Taylor Kennedy [...] Orlando Argüello-Miranda
Dec 5, 2025 1496 Views
Abstract
The most important virulence factor in the Cryptococcus genus is the polysaccharide capsule. This genus includes several species that cause life-threatening invasive disease. An increase in capsule thickness is important during fungal infection. The capsule is usually imaged using India ink, and crucial insights on the dynamics of its growth have been obtained using capsule-binding proteins such as specific antibodies or complement. We have developed an alternative method that allows both static and time-lapse imaging of the capsule using Percoll®, a suspension of nanometric spheres that do not penetrate the capsule. Given that these particles have a higher refractive index than the capsule, the latter can be imaged by differential interference contrast (DIC) microscopy. Static observation of the capsule with DIC and Percoll® results in capsule thickness measurements that match those made with India ink. Using capsule-inducing media, a glass-bottom incubation chamber and a live-imaging system equipped for DIC microscopy, this method allows time-lapse imaging of capsule growth. In contrast with India ink staining, DIC imaging of Percoll® exclusion halos result in crisp images. The greatest advantage of this method, though, is that unlike India ink, the Percoll® particles are non-toxic and unlike opsonins they do not bind the capsule, resulting in observations of capsule growth that are free from interference of bound proteins on capsule physiology.
Keywords: Cryptococcus neoformansBackground
Cryptococcosis affects more than two hundred thousand people each year, of which approximately 180 thousand die (Rajasingham et al., 2017). It presents most commonly as a severe meningoencephalitis in patients with AIDS, but the fungi can affect other immunocompromised and immunocompetent people and cause damage in other organs such as lungs and skin (Perfect et al., 2010). A hallmark characteristic of the Cryptococcus yeast cell is a polysaccharide capsule that surrounds the cell, which helps in evasion from the host’s immune responses (Agustinho et al., 2018).
The transparent capsule is most frequently observed using India ink, a suspension of dark particles that get excluded from the capsule and generate negative staining. It can also be observed using the Quellung reaction, in which the capsule becomes visible by differential interference contrast microscopy due to the increase in refractive index that results from antibody binding to it (Mukherjee et al., 1995; MacGill et al., 2000). In addition to observing the capsule statically, the binding of antibodies-as well as complement-has also been exploited to understand the mechanism used by C. neoformans to increase capsule thickness (Cordero et al., 2013a; García-Rodas et al., 2014). These methods brought a lot of information about the structure and formation of the most important Cryptococcus virulence factor, but they have intrinsic limitations. For one, we found India ink to be toxic to cryptococcal cells and thus not useful for time-lapse imaging of capsule growth. Binding of antibodies to the C. neoformans capsule was also found to change the capsular rigidity (Cordero et al., 2013b) and cellular metabolism (McClelland et al., 2010), so methods that rely on binding of an antibody or complement to observe capsule growth can give misleading results.
Here we provide additional details for a new method to image the capsule that we developed recently (Paes et al., 2018), which is able to overcome these limitations and allows imaging of the capsule in the absence of any protein binding to it. It relies on the same principle as India ink, that of negative staining using particles that are large enough to be excluded from the capsule. Instead of dark particles that absorb light, however, we used nanoparticles with high refractive index, which generate contrast in DIC microscopy (Figure 1). We have successfully used it to do one-time observations of the capsule and also in time-lapse experiments showing how the capsule thickness increased during growth and reproduction.
Figure 1. Example of capsule imaging by DIC using Percoll®. C. neoformans strain H99 was incubated for five days in the capsule-inducing medium Sab-MOPS. The cells were then imaged as described below. Brightness and contrast were adjusted in the image to improve clarity.
Materials and Reagents
- Tweezers, autoclave-sterilized
We prefer smaller tweezers (approximately 10 cm length) with a fine point that is either flat or serrated - P200 micropipette
- Microscope slides
We prefer pre-cleaned frosted slides (e.g., Fisher Scientific, catalog number: 12-550-143) - Coverslips, #1.5 or preferentially 1.5H thickness
We use 18 x 18 mm square coverslips (Zeiss, 474030-9000-000) - Sterile 1 ml syringe and two compatible 30-gauge sterile needles (e.g., BD, catalog numbers 309628 and 305128, respectively)
- Polypropylene tubes in 1.5 ml and 2.0 ml volumes (USA Scientific, catalog number: 1615-5500 and 1620-2700, respectively)
- Wooden toothpicks, autoclave-sterilized
- C. neoformans and C. gattii species complex strains
- NaCl (Fisher Scientific, catalog number: S271-3)
- KCl (Fisher Scientific, catalog number: P217-3)
- Na2HPO4 (Fisher Scientific, catalog number: S374-500)
- KH2PO4 (Fisher Scientific, catalog number: P-285)
- Hydrochloric acid (Fisher Scientific, catalog number: A144-212)
- Percoll® (GE Life Sciences, catalog number: 17089102), stored at 2-8 °C, shelf-life of two years
- 3-(N-Morpholino) propanesulfonic acid-MOPS (Sigma-Aldrich, catalog number: M3183)
- Peptone (BD, catalog number: 211677)
- Dextrose (BD, catalog number: 238230) or Dextrose (Sigma, catalog number: G7021)
- Yeast extract
- Agar (BD, catalog number: 242720)
- Ampicillin (Sigma-Aldrich, catalog number: A5354)
- Percoll®/Sab-MOPS suspensions (see Recipes)
- YPD broth and agar (see Recipes)
- Sabouraud dextrose broth (see Recipes)
- Phosphate buffer saline (see Recipes)
- Ampicillin solution (100 mg/ml) (see Recipes)
Equipment
- Microscope equipped for DIC microscopy
We use a Zeiss AxioObserver Z1 temperature-controlled inverted microscope (Carl Zeiss GmbH), fitted with a 63x NA 1.4 oil immersion objective, DIC polarizers and prisms, motorized stage and focus and a cooled monochromatic CCD camera - Incubation chamber for live-cell imaging
We use the POCmini-2 Cell Cultivation System (PeCon GmbH) in its closed cultivation mode (Figure 2) - Microcentrifuge with fixed-angle rotor to fit 1.5-2.0 ml tubes
- Orbital shaker incubator
We generally use heating incubated shakers with orbits between 25 and 28 mm, but most shakers used for microbiological cultures should work - Hemocytometer
- Biosafety cabinet
- Autoclave
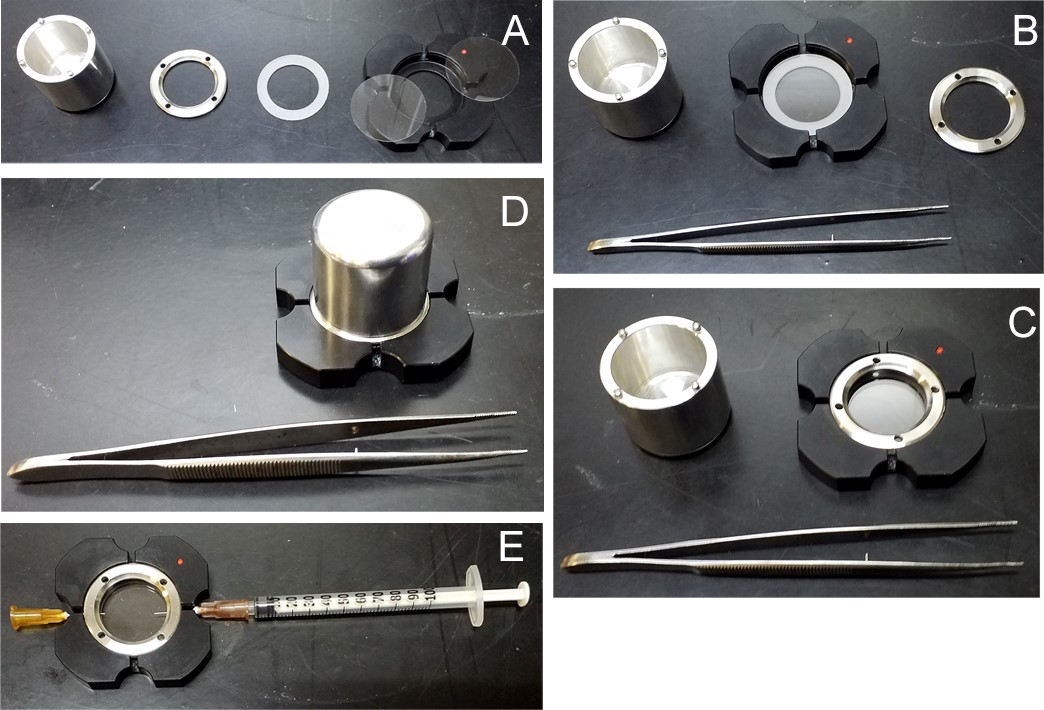
Figure 2. Assembly of the POCmini-2 chamber. A. The separate components from left to right: POCmini2 system specific screwdriver; screw-on gasket; spacer; metal frame with 170 µm glass coverslips on top of it. B. Glass coverslips mounted into the frame with the spacer between them, done with the tweezers shown. C. Screw-on gasket partially fitted into the frame. The system can be autoclaved like this. D. Screwdriver in position for tightening the screw-on gasket onto the coverslips. E. mounted system with the syringe and needle on opposite orifices, for injecting the cell suspension while expelling the air between the spacers.
Software
- Image collection: Zen 2 (Zeiss GmbH)
https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html - Image processing: ImageJ (NIH) (Schindelin et al., 2012; Schneider et al., 2012)
https://imagej.nih.gov/ij/
Procedure
General guidelines:
- Perform all steps in a biosafety cabinet. Pathogenic cryptococci are biosafety level 2 organisms.
- Proper Köhler illumination and perfect alignment of the polarizers and prisms are crucial for DIC microscopy. Make sure to check these settings prior to performing the experiments.
- Before doing live-imaging experiments, run tests with a suspension of capsule-induced cells in a 50% (v/v) mixture of Percoll® and Sab-MOPS to ensure that the system is properly set up for capsule imaging.
- In order to make precise measurements of capsule size, make sure the microscope is properly calibrated with a micrometer scale. If your microscope does not automate scaling, you need to collect an image of a micrometer scale prior to imaging the Cryptococcus cells.
- The cells are incubated for imaging on a closed chamber. The most common mistake during assembly of the incubation system is to screw on the metal parts too loose, causing the chamber to leak when you try to fill it with the cell suspension, or causing the spacers to bend instead of being pierced by the syringe needle; or too much, causing the glass plates to crack. Practice mounting and filling it with water before you do the experiment the first time.
- Live-imaging experiments are very challenging and can require several repetitions for success. Here is a list of problems that happen very often and suggestions to minimize them:
- As the cells are not attached to the coverslip, they frequently move sideways out of the field of view. This problem can be minimized to a certain extent in microscopes with a motorized stage. We usually set experiments up collecting a panel consisting of 3 x 3 fields, with the cell of interest in the center.
- The cells can also drift on the Z-axis, leading to loss of focus. We minimize this by collecting Z-stacks with at least 11 slices spaced 1 μm apart, with the best focus being in the middle.
- Some cells just do not multiply or significantly induce their capsules during the time of the experiment. Again, using a motorized stage, we collect images from several regions in each experiment to increase the chance that in at least some of them we can observe the capsule in a single cell for several hours.
- Static imaging of the capsule
- Obtain the Cryptococcus spp. cells for capsule imaging according to your experimental design. We have successfully imaged capsules from cultures grown in solid and liquid media, from both C. neoformans and C. gattii species complex strains.
- Dilute the cells in PBS and mix in a 1:1 proportion with Percoll®. We have imaged capsules in cell suspensions in a wide range of concentrations, from around 103 to over 106 cells per ml.
- Add 10 μl of the mixture to a slide and cover with a coverslip.
- Observe and image the cells on a DIC microscope.
- Time-lapse imaging of capsule growth–preparing the cells
- In the afternoon, with a sterile toothpick, collect a single Cryptococcus colony from a fresh (younger than two weeks) YPD agar plate and seed it into a culture tube containing 5 ml of YPD broth.
- Grow the yeast culture overnight in an orbital shaker at 30 °C and 200 rpm.
- On the same day, sterilize the POCmini-2 Cell Cultivation System components (use the 1-mm spacers) according to manufacturer’s instructions by autoclaving. Also autoclave small tweezers to maintain sterility later while mounting the cultivation chamber.
Note: It is easier to close the chamber after sterilization if it is autoclaved already partially assembled, with the gasket screwed in just a little. Do not screw it completely, though, because it will not be effectively sterilized. - In the morning of the next day, before you begin processing the yeast culture, switch on the microscope and set the heating system to 37 °C, to prewarm the whole machine in preparation for the experiment.
Note: A microscope such as the Axio Z1 takes at least one hour to reach 37 °C. If you forget this step, this will cause a delay later. The microscope must be at the incubation temperature before the time-lapse microscopy begins because thermal expansion of its parts will change the focal plane otherwise. - Collect 2 ml of the culture into a sterile 2 ml polypropylene tube and pellet the cells at 1,000 x g for 5 min at room temperature in a microcentrifuge.
- Remove the supernatant without disturbing the pellet, resuspend the cells into 1 ml of sterile PBS, saline or water, transfer the suspension to a 1.5 ml polypropylene tube and repeat the centrifugation at 1,000 x g for 5 min at room temperature. Repeat this wash two more times to wash off remaining material from the culture broth.
- After the last centrifugation, resuspend cells in 500 µl of a 60% dilution of Percoll® in Sab-MOPS.
- Centrifuge cells at 2,000 x g for 5 min at room temperature.
- Carefully remove 400 µl of the supernatant without disturbing the pellet. Resuspend the cells in the remaining supernatant by pipetting up and down.
Notes:- Use a P200 micropipette for this step. Do not try to remove all the supernatant: the tiny pellet that forms at the previous step is quite loose and will be aspirated if you try.
- Steps B7 to B9 are optional, and we have successfully made experiments without them. They can be skipped altogether, with the washed cells from Step B6 resuspended in PBS and counted as in Step B10. We do recommend making the centrifugation in Percoll®, though, to enrich the suspension for cells denser than the 50% Percoll®/Sab-MOPS mixture that is used for time-lapse microscopy (see below). In several experiments we found that most cryptococcal cells were less dense than the 50% Percoll® suspension, so they floated off the focal plane of the microscope during incubation. A 60% mixture of Percoll® in medium is slightly denser than the 50% mixture that is used in the actual experiment, which ensures that any cells that are dense enough to pellet in this step will not fail to settle down in the incubation chamber later. The final pellet on 60% Percoll® is usually very small, which is why we start from at least 2 ml of the culture.
- Collect a 10 µl aliquot of the suspension from Step B9 (or Step B7 if you skip the centrifugation in Percoll®) to estimate cell density in a hemocytometer. In a separate tube, prepare 1 ml of a 50% mixture of Percoll® in Sab-MOPS (include an antibiotic, for example ampicillin at 50 µg/ml) and transfer a volume of the cell suspension from Step B9 (or Step B7 if you skip the centrifugation in Percoll®) equivalent to 1,000-2,000 cells. Mix well by pipetting up and down.
Notes:- The reason we use so few cells are that a higher cell density in the incubation chamber will cause neighboring cell clusters to coalesce during incubation, rendering documentation of capsule growth impossible.
- Of all documented capsule-inducing media, we have tested this protocol with three. CO2-independent medium (Ost et al., 2015) produced less capsule growth than the SAB-MOPS medium we describe in this protocol. Minimal medium, however, formed an opaque gel with Percoll® at 37 °C after a few hours.
- The addition of ampicillin is not rigorously necessary, but we found it helped avoid bacterial contamination that happened sometimes, probably during manipulation of the incubation chamber.
- Time-lapse imaging of capsule growth-Collecting images
- Mount the POCmini-2 Cell Cultivation System as instructed by the manufacturer. Use a 1-mm spacer and manipulate the glass plates with a sterile tweezer.
Note: As explained above, it is a good idea to practice mounting and filling the cultivation chamber with water before you do the experiment the first time. - Introduce a sterile 30-gauge needle through one of the orifices, carefully piercing the spacer.
- Fill a 1-ml insulin syringe fitted with a 30-gauge needle with the cell suspension from Step B10. Make sure to expel all the air in the syringe.
- Carefully pierce the spacer with the syringe needle through the orifice opposite to the one you put the needle through in Step C2.
- Slowly inject the cell suspension into the POCmini-2, expelling the air through the needle opposite. When the chamber is nearly full, carefully remove first the opposite needle when the air is fully expelled, then the syringe and needle with the rest of the cell suspension. The POCmini-2 holds about 800 µl with a 1-mm spacer.
- Put the POCmini-2 in the appropriate sample holder in the microscope and close the incubation chamber.
- Scan the POCmini-2 visible area manually to pinpoint isolated, single, non-moving cells. Adjust Köhler illumination and the DIC slider for optimal contrast.
Note: Because so few cells were introduced into the POCmini-2, it takes time and patience to find them. It can be helpful to roughly find the appropriate focus by switching to a 10x objective, but this should only be done with long working distance 10x objectives to avoid getting them soiled with immersion oil. - Using the microscope firmware (Zen 2 in our case), set up the equipment to collect images every 5-15 min during 24-72 h.
- Wait about 20 min and confirm that the cells you selected are still visible and in position before starting the experiment.
- Mount the POCmini-2 Cell Cultivation System as instructed by the manufacturer. Use a 1-mm spacer and manipulate the glass plates with a sterile tweezer.
Data analysis
Measurement of the capsule thickness in DIC images
- Collect the images according to the protocols above. Depending on which software you use to operate your microscope, back up the original data in the proprietary format to ensure that all metadata is preserved. For Zeiss ZEN software, this format is .czi.
- Zeiss ZEN software allows automated scaling and has a measurement tool. To do so, select the “Circle (Diameter)” (Figure 3) or “Length” tool and first measure the total diameter of the cell, including capsule. Next measure the cell body diameter. Repeat this for all cells in each image until you measure 50 cells.
- Transfer the data to your favorite software for statistical analysis and graphing.
- To obtain capsule thickness, subtract the cell body diameter from the whole cell diameter and divide the value by two. To obtain capsule volume, subtract the cell body volume from the whole cell volume.
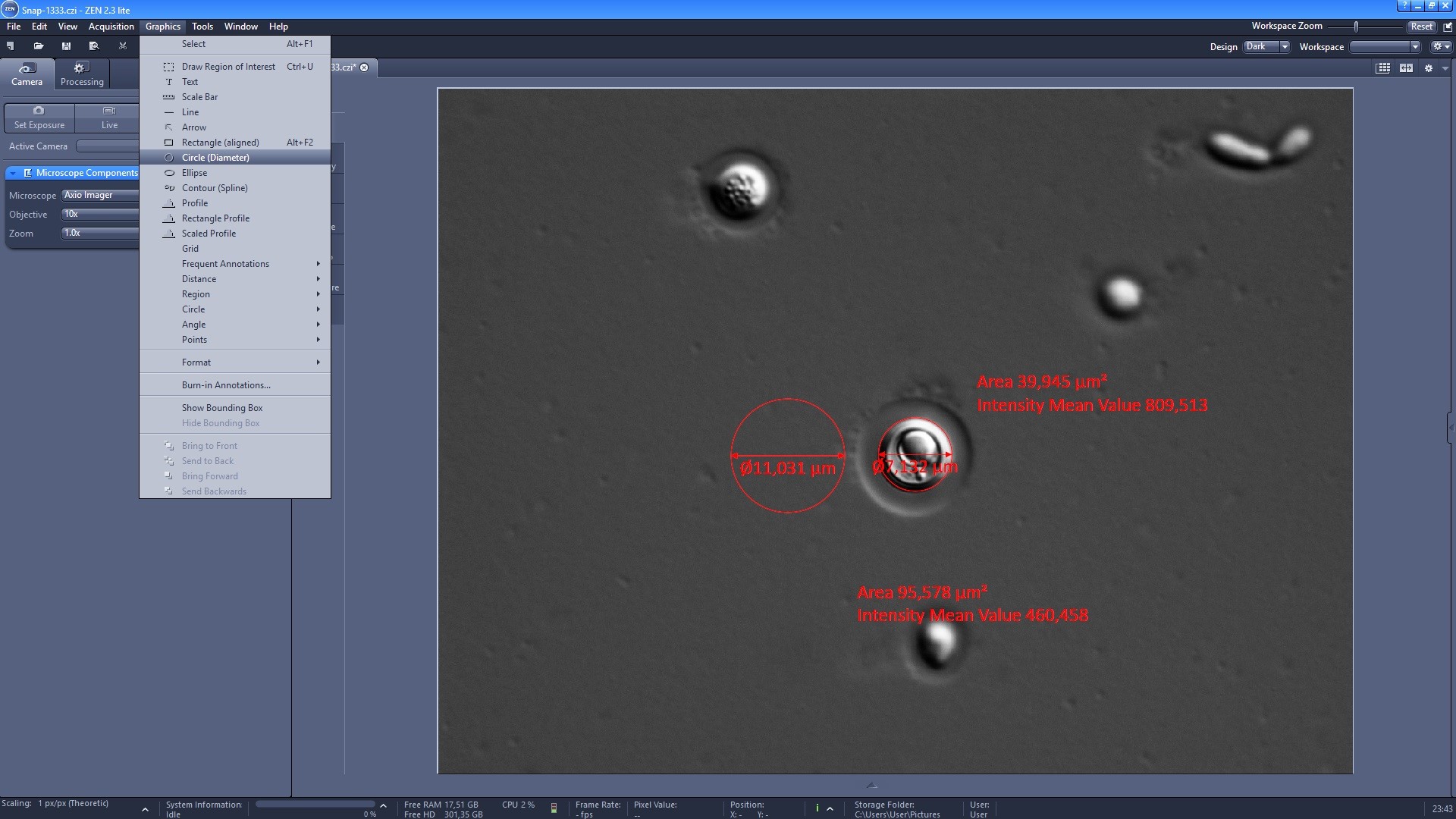
Figure 3. Measuring cell and capsule diameter on Zen 2. Select Graphics > Circle (Diameter). That enables a click and drag function whereby you can draw a circle lining the cell or the capsule and once you release the cursor, you will see its diameter and area. The values are then transcribed into a spreadsheet to calculate capsule width and volume. Brightness and contrast were adjusted in the image to improve clarity.
Notes
- Static observations of the capsule can be easily accomplished in simple upright or inverted microscopes, as long as they are equipped with high numeric aperture objectives and DIC prisms and polarizers. Live imaging of capsule growth, however, requires specialized inverted microscopes fitted with temperature controls and incubation chambers and software-controlled image acquisition.
- High-quality optics make a great difference in the ability to image the capsule using this protocol. The best images were obtained using high numeric aperture, plan-apochromatic objectives.
- Most microscopes are optimized for imaging samples beneath a coverslip that is 170 μm thick. The most commonly available coverslips have thicknesses that deviate significantly from this optimal value. Despite being more expensive and harder to find, #1.5H (170 μm thick) coverslips do make a difference in image quality at the high numerical apertures necessary for good capsule images, especially for live imaging.
- Other commercially available or house-made incubation chambers might work for live imaging. The most common ones (glass-bottom plates and multi-well chambers), however, gave poor results because evaporation transformed the suspension of fungi in Percoll® into an opaque gel. It is important to use a 170 µm thick glass-bottom chamber that can be closed to avoid evaporation.
Recipes
- Percoll®/Sab-MOPS suspensions
- To ensure that the final concentration of Sabouraud medium and MOPS are equal to that of the original description of Zaragoza and Casadevall (2004) (respectively 10% and 50 mM), prepare a stock solution four times as concentrated:
MOPS 200 mM, pH 7.5
Sabouraud dextrose broth (see below) diluted 2.5 times (v/v) - To prepare a 50% or 60% dilution of Percoll®, mix the following proportions per unit of volume:
50% or 60% of Percoll®
25% of the solution prepared in step a
Suspension of yeasts (Step B9 or Step B7 if you skip the centrifugation in Percoll®) as needed
Sterile deionized water to complete the volume, plus antibiotic
- To ensure that the final concentration of Sabouraud medium and MOPS are equal to that of the original description of Zaragoza and Casadevall (2004) (respectively 10% and 50 mM), prepare a stock solution four times as concentrated:
- YPD broth and agar
- Dissolve in deionized water (m/v):
Yeast extract at 1%
Peptone at 2%
Dextrose at 2% - Adjust pH to 5.3-5.6 with hydrochloric acid
- To make solid medium, add agar at 1.5% (m/v)
- Sterilize by autoclaving
- Dissolve in deionized water (m/v):
- Sabouraud dextrose broth
- Dissolve in deionized water (m/v):
Dextrose at 4%
Peptone at 1% - Adjust pH to 5.3-5.6 with hydrochloric acid
- Dissolve in deionized water (m/v):
- Phosphate buffered saline
- Dissolve in deionized water (m/v):
137 mM NaCl
2.7 mM KCl
10 mM Na2HPO4
1.8 mM KH2PO4 - Adjust the pH to 7.2-7.4 with hydrochloric acid, and sterilize by autoclaving or filtering
- Dissolve in deionized water (m/v):
- Ampicillin solution
Dissolve ampicillin at 100 mg/ml in deionized water and sterilize by filtration
Acknowledgments
AC is supported by National Institutes of Health Grants 5R01A1033774, 5R37AI033142, and 5T32A107506, and CTSA Grants 1 ULI TR001073-01, 1 TLI 1 TR001072-01, and 1 KL2 TR001071 from the National Center for Advancing Translational Sciences. HP, MF and AN are currently supported by grants from the Brazilian funding agencies CNPq, FAP-DF and Capes. This protocol was adapted from a work we have previously published (Paes et al., 2018).
Competing interests
The authors report no competing interests.
References
- Agustinho, D. P., Miller, L. C., Li, L. X. and Doering, T. L. (2018). Peeling the onion: the outer layers of Cryptococcus neoformans. Mem Inst Oswaldo Cruz 113(7): e180040.
- Cordero, R. J., Bergman, A. and Casadevall, A. (2013a). Temporal behavior of capsule enlargement by Cryptococcus neoformans. Eukaryot Cell 12(10): 1383-1388.
- Cordero, R. J., Pontes, B., Frases, S., Nakouzi, A. S., Nimrichter, L., Rodrigues, M. L., Viana, N. B. and Casadevall, A. (2013b). Antibody binding to Cryptococcus neoformans impairs budding by altering capsular mechanical properties. J Immunol 190(1): 317-323.
- Garcia-Rodas, R., Cordero, R. J., Trevijano-Contador, N., Janbon, G., Moyrand, F., Casadevall, A. and Zaragoza, O. (2014). Capsule growth in Cryptococcus neoformans is coordinated with cell cycle progression. MBio 5(3): e00945-14.
- MacGill, T. C., MacGill, R. S., Casadevall, A. and Kozel, T. R. (2000). Biological correlates of capsular (quellung) reactions of Cryptococcus neoformans. J Immunol 164(9): 4835-4842.
- McClelland, E. E., Nicola, A. M., Prados-Rosales, R. and Casadevall, A. (2010). Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest 120(4): 1355-1361.
- Mukherjee, J., Cleare, W. and Casadevall, A. (1995). Monoclonal antibody mediated capsular reactions (Quellung) in Cryptococcus neoformans. J Immunol Methods 184(1): 139-143.
- Ost, K. S., O'Meara, T. R., Huda, N., Esher, S. K. and Alspaugh, J. A. (2015). The Cryptococcus neoformans alkaline response pathway: identification of a novel rim pathway activator. PLoS Genet 11(4): e1005159.
- Paes, H. C., Frazao, S. O., Rosa, C. P., Albuquerque, P., Casadevall, A., Felipe, M. S. S. and Nicola, A. M. (2018). Opsonin-free, real-time imaging of Cryptococcus neoformans capsule during budding. Virulence 9(1): 1483-1488.
- Perfect, J. R., Dismukes, W. E., Dromer, F., Goldman, D. L., Graybill, J. R., Hamill, R. J., Harrison, T. S., Larsen, R. A., Lortholary, O., Nguyen, M. H., Pappas, P. G., Powderly, W. G., Singh, N., Sobel, J. D. and Sorrell, T. C. (2010). Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis 50(3): 291-322.
- Rajasingham, R., Smith, R. M., Park, B. J., Jarvis, J. N., Govender, N. P., Chiller, T. M., Denning, D. W., Loyse, A. and Boulware, D. R. (2017). Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17(8): 873-881.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J. Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P. and Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7): 676-682.
- Schneider, C. A., Rasband, W. S. and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7): 671-675.
- Zaragoza O and Casadevall A. (2004) Experimental modulation of capsule size in Cryptococcus neoformans. Biol Proced Online 6:10-15.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Paes, H. C., Frazão, S. D. O., Felipe, M. S. S., Casadevall, A. and Nicola, A. M. (2019). Imaging Cryptococcus spp. Capsule by Differential Interference Contrast Microscopy Using Percoll®. Bio-protocol 9(22): e3423. DOI: 10.21769/BioProtoc.3423.
Category
Microbiology > Microbial cell biology > Cell imaging
Microbiology > Microbial physiology > Cell wall
Cell Biology > Cell imaging > Live-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










