- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Identification of Heteroreceptors Complexes and Signal Transduction Events Using Bioluminescence Resonance Energy Transfer (BRET)
(*contributed equally to this work) Published: Vol 9, Iss 19, Oct 5, 2019 DOI: 10.21769/BioProtoc.3385 Views: 4979
Reviewed by: Edgar Soria-GomezJoana Alexandra Costa ReisMaria Nguyen

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Detecting Native Protein–Protein Interactions by APEX2 Proximity Labeling in Drosophila Tissues
Jhen-Wei Wu [...] Yu-Chiuan Chang
Oct 20, 2024 2146 Views

Ub-POD: A Ubiquitin-Specific Proximity-Dependent Labeling Technique to Identify E3 Ubiquitin Ligase Substrates in Human Cells
Urbi Mukhopadhyay [...] Sagar Bhogaraju
Jun 20, 2025 2435 Views

Isolation of Antigen-Specific Nanobodies From Synthetic Libraries Using a Protein Selection Strategy That Combines MACS-Based Screening of YSD and FLI-TRAP
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
Jan 20, 2026 442 Views
Abstract
Detecting protein-protein interactions by co-immunoprecipitation provided a major advancement in the immunology research field. In the G-protein-coupled receptors (GPCRs) research field, colocalization and co-immunoprecipitation were used to detect interactions, but doubts arose due to specificity of the antibodies (monoclonal in the case of receptors related to immunology and polyclonal in the case of GPCRs) and due to the possibility of false positive due to the potential occurrence of bridging proteins. Accordingly, new methodological approaches were needed, and energy transfer techniques have been instrumental to detect direct protein-protein, protein-receptor or receptor-receptor interactions. Of the two most relevant methods (Förster, or fluorescence resonance energy transfer: FRET and Bioluminescence energy transfer: BRET), the protocol for BRET is here presented. BRET has been instrumental to detect direct interactions between GPCRs and has contributed to demonstrate that GPCR dimers/oligomer functionality is different from that exerted by individual receptors. Advantages outweigh those of FRET as no fluorescence source is needed. Interestingly, BRET is not only useful to validate interactions detected by other means or hypothesized in the basis of indirect evidence, but to measure signal transduction events. In fact, BRET may, for instance, be used to assess β-arrestin recruitment to activated GPCRs.
Keywords: G-protein-coupled receptorsBackground
Fluorimeters have been in research laboratories for decades and still are. Also, cell biology took advantage of fluorescent detection of molecules using antibodies conjugated to small fluorescent molecules. A next stage was reached upon the discovery and further development of big fluorescent molecules. A fluorescent protein may be fused to a protein of interest to detect it even in living cells; now it is even possible to follow proteins in tissues of transgenic animals engineered to express the fluorescent fusion protein. Here are now available a variety of proteins with different fluorometric properties, i.e., with different wavelengths of absorption and/or emission. The Nobel Prize in Chemistry in 2008 was awarded to O. Shimomura, M. Chalfie and R. Y. Tsien: “for the discovery and development of the green fluorescent protein, GFP”. The press note indicated: “By using DNA technology, researchers can now connect GFP to other interesting, but otherwise invisible, proteins. This glowing marker allows them to watch the movements, positions and interactions of the tagged proteins” (https://www.nobelprize.org/prizes/chemistry/2008/press-release/).
Energy transfer protocols are now instrumental to detect molecular interactions, which were previously suspected by colocalization and “confirmed” by co-immunoprecipitation, i.e. by bringing down one protein using a monoclonal antibody against the “interacting” partner (Burger et al., 1986; Springer et al., 1986; Dianzani et al., 1999; Serrador et al., 1999). Unfortunately, it was not (still is not) easy to develop monoclonal antibodies against G-protein-coupled receptors (GPCRs). The first one produced to detect a GPCR, the adenosine A2A receptor, is probably still the best one (Rosin et al., 1998). But the majority of “good” anti-GPCR antibodies are polyclonal and the first ones appearing in the literature for class A GPCRs recognized adenosine receptors (Ciruela et al., 1995; Jimenez et al., 2008). Many of the antibodies against GPCRs are polyclonal and were used for coimmunoprecipitation (Ginés et al., 2000). Critiques arrived soon from two points of view: doubts on the specificity of polyclonal antibodies and the possibility that in detergent-treated cells a (third) bridging protein could lead to artifacts.
Energy transfer between proteins can only occur if the donor and the acceptor are at a distance of < 10 nm. The first option is using two proteins of interest each fused to a different fluorescence protein. Such approach is known as Förster resonance energy transfer: FRET (Förster, 1948; Perkins et al., 1984). In parallel Bioluminescence resonance energy transfer: BRET, was developed in the basis of a bioluminescent donor and a fluorescent acceptor (Xu et al., 1999). This is the technique whose protocol will be detailed here. Conceptually, the technique requires one protein fused to an enzyme (Renilla reniformis luciferase; a protein of circa 36 kDa) that degrades a substrate and emits light that in turns excite another protein fused, for instance, to the yellow fluorescent protein (YFP) (Figure 1). Two possible BRETs exist but we will detail BRET1, as the protocol would be quite similar with BRET2 (the main two differences are the substrate of Rluc and the fluorescent acceptor). BRET can also be used for signal transduction measurements. Probably the best example in the field of GPCRs is the measurement of β-arrestin binding to activated GPCRs. In that case the β-arrestin is fused to Rluc and acts as donor of the fluorescent protein fused to the GPCR of interest. BRET is repeated at different times to get time-response curves of β-arrestin recruitment or at a given time but with different doses of the GPCR activator to get dose-response curves. Reviews on the BRET technique (including the so-called nano-BRET) and on different possibilities that it offers in different fields and/or to answer scientific questions can be elsewhere found (Pfleger and Eidne, 2005; Breton et al., 2010; Schann et al., 2013; Beautrait et al., 2014; Sun et al., 2016; Dale et al., 2019).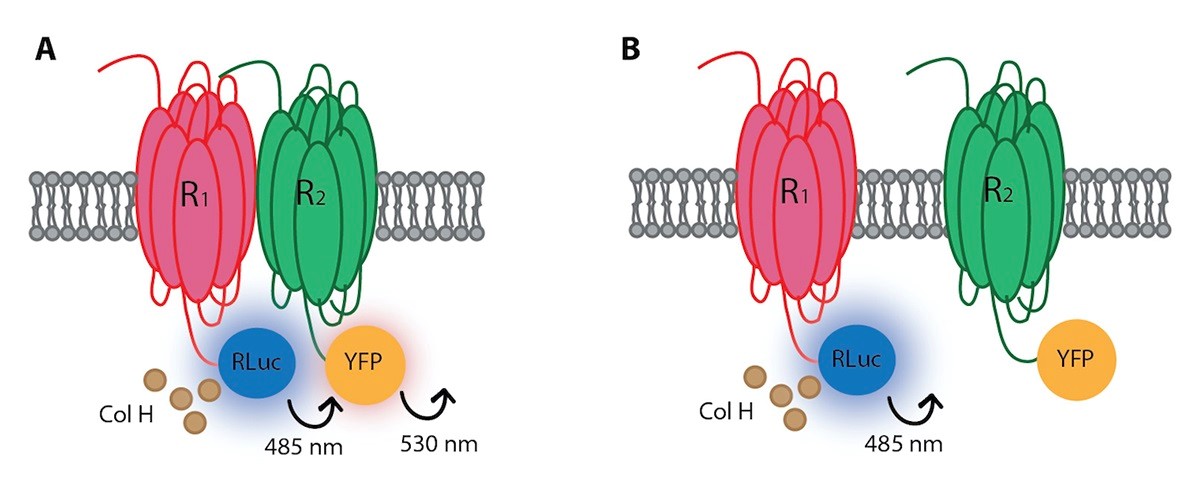
Figure 1. Cartoon representation of the BRET assay between a fusion protein constituted by R1 and Rluc and another fusion protein constituted by R2 and YFP. A. When both receptors (R1 and R2) are in close proximity (distance ≤ 100 A) there is energy transfer from Rluc-derived bioluminescence to YFP. B. When receptors are at a higher distance, no energy transfer can be detected. Rluc: Renilla luciferase. Col H: coelenterazine H, a substrate of Rluc. YFP: Yellow fluorescent protein. R1: cell surface receptor number 1. R1: cell surface receptor number 2.
Materials and Reagents
- Sterilization 0.22 µm pore size filter, 33 mm diameter (Millipore, catalog number: SLGP033RS)
- Sterile 6-well plates for cell culture (Cultek, catalog number: 153506)
- Transparent 96-well microplates (Sudelab, catalog number: 900011)
- 96-well black micro plates with a transparent bottom (Biogen, catalog number: PO-215003)
- 96-well white microplates with white bottom (Biogen, catalog number: PO-204003)
- Sterile 15 ml centrifuge falcon tubes (Cultek, catalog number: 45352096)
- Sterile 2 ml Eppendorf tubes
- Human embryonic kidney (HEK-293T) cells [293T (ATCC®, catalog number: CRL-3216TM)], store aliquots in liquid nitrogen
- Plasmids for cell transfection
Note: One containing the code for one protein fused to Rluc and another containing the code for the second protein fused to enhanced yellow fluorescent protein (EYFP). Plasmids including the sequences for Rluc or YFP are available from ADDGENE, store at -20 °C. - L-glutamine (Gibco, catalog number: 25030-024), store at -20 °C
- Penicillin/streptomycin (Gibco, catalog number: 15140-122), store at -20 °C
- Sodium chloride (NaCl) (PanReacApplichem, catalog number: 141659.1211), store solid at room temperature, or 150 mM aliquots at 4 °C
- NaCl (Panreac, catalog number: 131659.1211), store at room temperature
- Ethanol
- CaCl2·2H2O (Panreac, catalog number: 131232.1211), store at room temperature
- KCl (Panreac, catalog number: 131494.1211), store at room temperature
- KH2PO4 (Panreac, catalog number: 141509.1211), store at room temperature
- MgCl2, anhydrous (Sigma-Aldrich, catalog number: M8266), store at room temperature
- MgSO4·7H2O (Panreac, catalog number: 141404.1211), store at room temperature
- Na2HPO4·12H2O (Panreac, catalog number: 141678.1211), store at room temperature
- HEPES (Panreac, catalog number: A3724,0500), store at room temperature
- Glucose (PanReac, catalog number: A1422,1000), store at room temperature
- Bradford reagent (BioRad, catalog number: 500-0006), store at 4 °C
- Bovine serum albumin (Sigma-Aldrich, catalog number: A6003-25g) store either solid at 4 °C, or in solution at -20 °C
- Coelenterazine H (Attendbio, catalog number: E-102181), store at -20 °C, protected from light
- Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, catalog number: 21969-035), store at 4 °C
- MEM Non-Essential Amino Acids Solution (Gibco, catalog number: 11140-035), store at 4 °C
- Fetal Bovine Serum (FBS) (Gibco, catalog number: 10270106), store at -20 °C
- Polyethylenimine (PEI) (Sigma-Aldrich, catalog number: 408727), store either solid at room temperature, or in 40 µM aliquots at -20 °C
- Sterile 40 µM PEI (see Recipes)
- Sterile 150 mM NaCl (see Recipes)
- Serial BSA dilutions (0-1 mg/ml) (see Recipes)
- Supplemented DMEM (see Recipes)
- HBSS (see Recipes)
- Coelenterazine H solution (see Recipes)
Equipment
- Water bath (Kotterman, model: D-3162)
- Centrifuge (Eppendorf, model: 5084R)
- Centrifuge (Eppendorf, model: 5415R)
- Cell incubator (ThermoFisher, model: 3308)
- Laminar flow hood (Faster, model: Bio60)
- Spectrophotometer (ThermoLabsystems, multiskan Ascent; 354)
- Fluostar Optima Fluorimeter (BMG Labtech, FLUOstar OPTIMA) equipped with a high-energy xenon flash lamp, a 10 nm bandwidth excitation filter at 485 nm and a 530 nm emission filter
- Mithras LB 940 plate reader (Berthold Technologies) equipped with 485 nm and 530 nm filters
Software
- GraphPad Prism software (GraphPad, https://www.graphpad.com/scientific-software/prism/)
- Microsoft Excel (Microsoft, https://products.office.com/es/excel)
- Multiskan software, provided by manufacturer of the Plate reader for measuring protein concentration
- OPTIMA software, provided by manufacturer of the fluorescence plate reader
- Mikrowin 2000 software, provided by manufacturer of the BRET reader
Procedure
- Fusion proteins
Description of cloning for preparation of the fusion proteins is out of the scope of the current protocol.
Note: In the case of GPCRs, added (Rluc or EYFP) proteins must be at the C terminal end, i.e., cytoplasmic (Figure 2). Exception may occur in special cases (for instance if one wants to look for interactions between the huge extracellular domains of class C GPCRs. In brief, place the cDNAs coding for the proteins of interest without their stop codon inside the pRLuc-N1 or the pEYFP-N1 vectors to obtain the fusion proteins that contain RLuc or EYFP. One of the receptors (R1) should be fused to Rluc and the other one (R2) to EYFP. We recommend trying both pair combinations as, often, one combination works better than the other. For negative control, a non-interacting receptor (R3) should be fused to EYFP to perform BRET using R1-Rluc.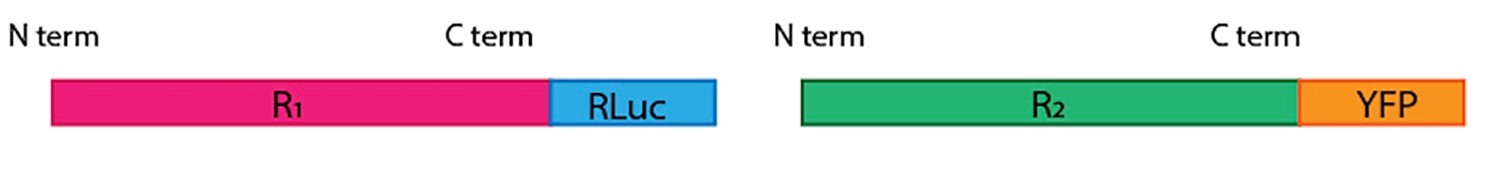
Figure 2. Cartoon representation of the fusion proteins containing Rluc or YFP at the C terminus of each receptor - Cell culture and transient transfection (any current cell transfection reagent suitable for eukaryotic cells may be used; we routinely use PEI)
- Grow HEK-293T cells in DMEM supplemented with 2 mM L-glutamine, 100 U/ml penicillin/streptomycin, 1% (v/v) MEM Non-Essential Amino Acid Solution and 5% (v/v) heat inactivated Fetal Bovine Serum (FBS) at 37 °C and 5% CO2 humid atmosphere. Cell growth is optimal without need to use higher FBS concentrations. Plate cells in 6-well plates at a density of 250,000 cells/well. A single BRET assay requires 9 transfection conditions, and each will require 2 wells. In total 9 x 2 = 18 wells = three 6-well plates. Discard every batch of cells after 10 passages and thaw a new liquid nitrogen-stored vial.
- Twenty-four hours after plating, transfect HEK cells (they should be ~50% confluent). Increase the amount of the EYFP plasmid every two wells (as above indicated, same transfection conditions are performed in 2 wells). Rluc must be kept relatively constant and this requires preliminary assays to optimize the EYFP/Rluc ratios. An example of transfection conditions is given in Table 1. The first condition, that contains only RLuc, is used as a blank for cell fluorescence. In further experiments, DNA amounts must be adjusted to obtain the desired protein-RLuc and protein-EYFP expression values (see below).
Table 1. Example of DNA amounts used for transfection (total DNA required for two wells needed for every condition is shown)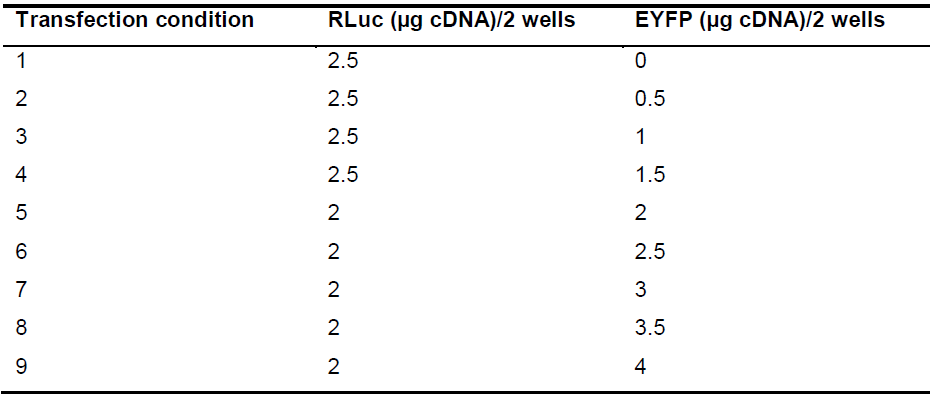
- Add 250 µl 150 mM NaCl (per tube) to each of 9 sterile 15 ml centrifuge tubes.
- Add the desired amount of DNA and mix using vortex.
- In parallel, prepare a mixture of 40 µM PEI and 150 mM NaCl (1:20), vortex vigorously.
- Add 250 µl of the PEI:NaCl mixture to each tube, mix using vortex.
- Incubate for 10 min at room temperature.
- Add 1.5 ml/tube of non-supplemented DMEM, mix (now each tube should contain 2 ml of transfection mixture).
- Aspirate the culture medium from the 6-well plates and add carefully 1 ml of the transfection mixture to each well.
- Place cells in the incubator for 4 h at 37 °C, in 5% CO2 humid atmosphere.
- Replace the transfection mixture with 2 ml of supplemented DMEM and place again cells in the incubator for 48 h.
- Cell preparation for BRET assay
- Prepare HBSS (see Recipes) supplemented with 0.1% (w/v) glucose, prewarm the mixture in the water bath at 37 °C.
- Aspirate culture medium from transfected cells.
- Wash cells with the prewarmed solution of HBSS-glucose.
- Add 1 ml HBSS-glucose per well.
- Detach cells, pool cells from the 2 wells with the same transfection condition in a 2 ml Eppendorf tube.
- Centrifuge for 5 min at 1,000 x g (room temperature).
- Resuspend each pellet in 200 µl of HBSS-glucose.
- To adjust the protein amount, proceed to Bradford protein assay.
- In a 96-well transparent microplate, add 10 µl/well of each bovine serum albumin (BSA) dilution (0-1 mg/ml; in duplicates).
- Add 10 µl cell sample/well.
- Add Bradford reagent (200 µl/well; previously diluted 1:5 in MilliQ water).
- Shake the plate at room temperature for 2 min.
- Read the absorbance at 595 nm.
- Use GraphPad software to calculate the standard parameters and use them to calculate protein concentration in each cell suspension. Calculate the amount of HBSS-glucose that must be added to each cell suspension to obtain a protein concentration equivalent to 0.2 mg/ml.
- Assessing the amount of EYFP expression
- Load 100 µl/well (20 µg protein) of each sample (in duplicates) on 96-well black microplates with transparent bottom.
- Using a Fluostar Optima Fluorimeter plate reader, excite samples with a high-energy xenon flash lamp using a 10 nm bandwidth excitation filter at 485 nm, and read fluorescence at 530 nm. See example fluorescence measurements in Table 2.
Table 2. Example of fluorescence measurements from protein-EYFP expression for each of the conditions indicated in Table 1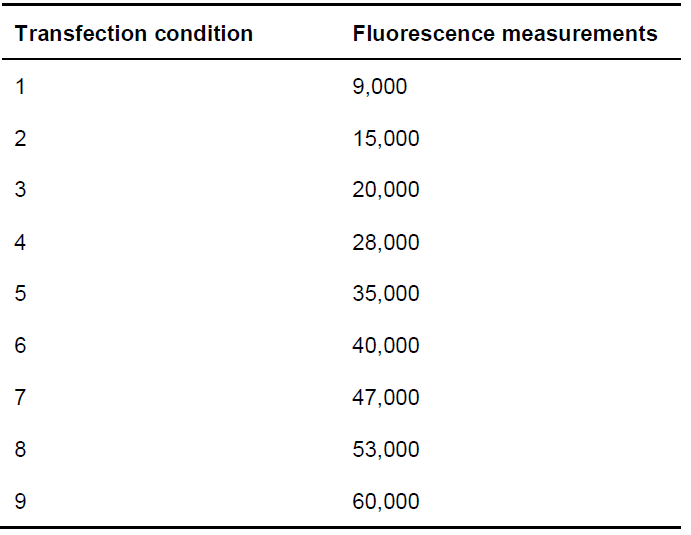
- BRET measurements
- Prepare a Coelenterazine H: HBSS-glucose dilution from the Coelenterazine H stock (see Recipes) (1:40), keep on ice and protected from light.
- Load 90 µl/well of each cell suspension (in duplicates) on 96-well white microplates with opaque bottom.
- Add 10 µl/well of the Coelenterazine H solution (final concentration of coelenterazine is 5 µM); gently shake the plate.
- Read the energy transfer (e.g., on a Mithras LB 940 plate reader) 1 min after the addition of coelenterazine H, using a 485 nm filter (short wavelength) to detect the bioluminescence emitted by protein-Rluc, and a 530 nm filter (long-wavelength) to detect the fluorescence emitted by protein-EYFP upon energy transfer from protein-RLuc. The Mithras reader is able to integrate both wavelengths and provide the BRET values, that is, the ratio between the two readings (see Table 3 for examples of BRET readings).
Table 3. Example of BRET readings for each of the conditions indicated in Table 1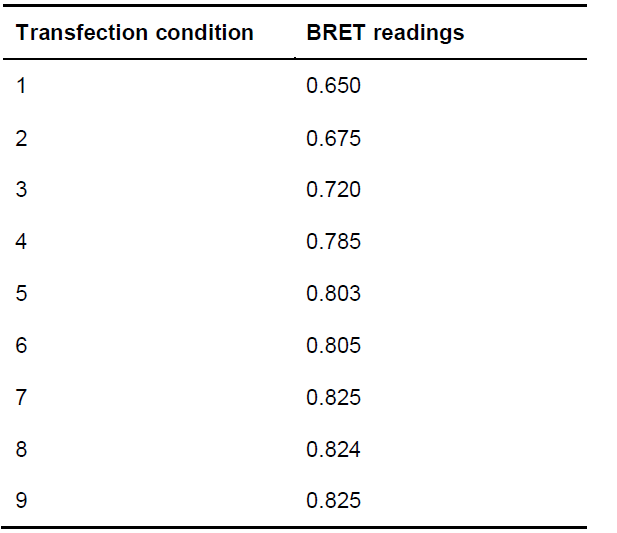
- Assessing the amount of Rluc expression
To determine protein-Rluc expression, read the bioluminescence of the sample 10 min after the addition of coelenterazine H on the Mithras LB 940 plate reader using the 485 nm filter (use the same samples used for BRET readings). See Table 4 for examples of protein-RLuc readings.
Table 4. Example of bioluminescence readings from protein-RLuc expression for each of the conditions indicated in Table 1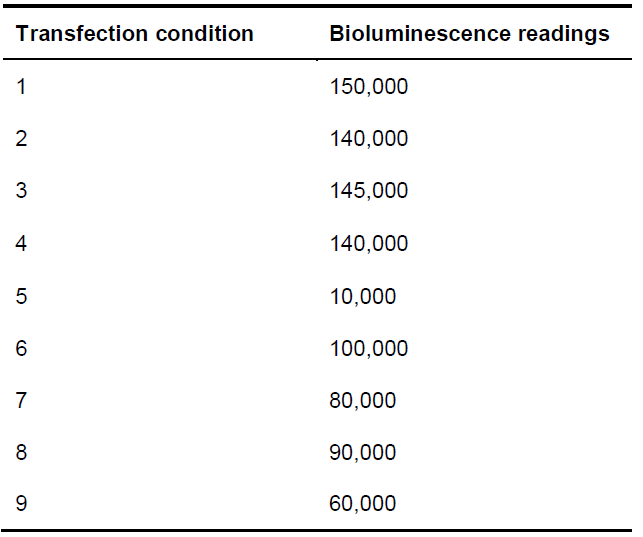
Data analysis
Notes:
- When reading protein-YFP fluorescence, the value of the first transfection condition (i.e., the one lacking protein-EYFP) should be around 9,000 fluorescence units, and those of the rest of the conditions should increase up to 60,000 fluorescence units (see Table 2).
- When reading protein-Rluc expression, values should be around 50,000-150,000 units (see Table 4).
- If expression values are not in this range, discard the data. Readjust transfection amounts until obtaining expression values in the indicated range.
- Calculations
- The X axis of the BRET graph (Figure 3) shows the ratio between the expression of both fusion proteins in the assay. Subtract the value of protein-YFP emission of the first transfection condition (blank) from all conditions. Then, divide this fluorescence value by protein-RLuc expression of the corresponding transfection condition. Finally, multiply this ratio by 1,000 for easier data handling (Table 5).
Table 5. Example of calculations of the independent variable (X axis values)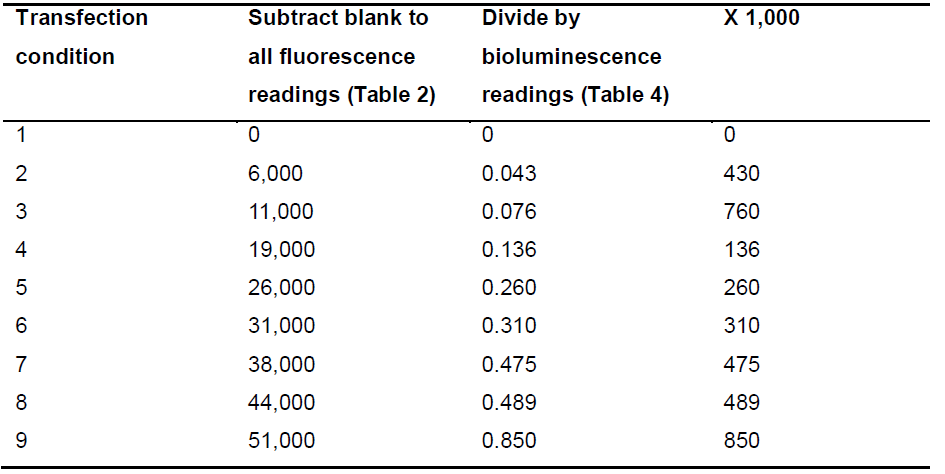
- The Y axis of the graph shows net BRET (Figure 3). Net BRET is defined as [530 nm emission/485 nm emission] minus the [530 nm emission/485 nm emission] value obtained in the first condition (blank). When measuring BRET, Mithras software already gives for each well plate the ratio between protein-EYFP emission (due to energy transfer) and protein-Rluc emission (see Table 3). To all these values subtract the value of the ratio of the first condition (blank). The netBRET value is usually expressed in milliBRET units (mBu) = net BRET x 1,000. Negative netBRET values should be discarded. netBRET values under 20 mBu indicate lack of energy transfer (in the conditions of the assay) (Table 6).
Table 6. Example of calculations of the dependent variable (Y axis values)Table 6. Example of calculations of the dependent variable (Y axis values)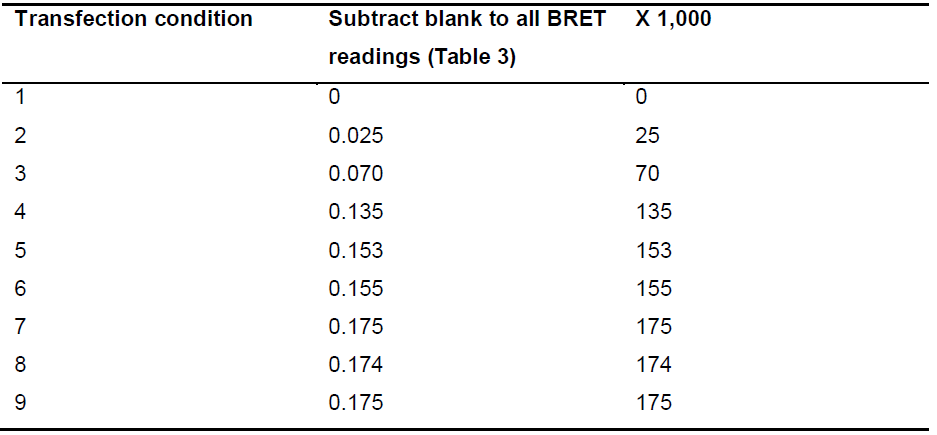
- The X axis of the BRET graph (Figure 3) shows the ratio between the expression of both fusion proteins in the assay. Subtract the value of protein-YFP emission of the first transfection condition (blank) from all conditions. Then, divide this fluorescence value by protein-RLuc expression of the corresponding transfection condition. Finally, multiply this ratio by 1,000 for easier data handling (Table 5).
- Data plotting and calculation of parameters: BRETmax and BRET50
Around 5 replicates (all showing good expression values) of each condition are needed to build an appropriate BRET curve. When enough data is collected, enter X/Y pair of values for each condition into GraphPad software and adjust values by non-linear regression to the “One-site specific binding equation” (provided by the software: Y = Bmax·X/(KD + X)). GraphPad will show parameter values for Bmax and KD; Bmax is equivalent to the maximal BRET (BRETmax) and KD is equivalent to the ratio (in X axis) providing a BRET reading (Y axis) equal to BRETmax/2. These parameters are useful to compare data from different BRET experiments/conditions.
If data result in a saturable curve, which at least reaches a BRETmax of 20 mBu, the BRET is considered as positive, that is, there is an interaction between the two receptors (Figure 3). If necessary, in order to obtain readings at higher X-axis values (to reach saturation) do further transfection conditions with higher protein-EYFP amount. Any linear relationship is considered a negative result, i.e., a lack of interaction. In this case, e.g., when plotting the negative control, data must be adjusted by linear regression (x = 0, y = 0, Figure 4). - Advantages, pitfalls and trouble solving
The advantage of BRET over FRET is the lack of fluorescence emission source to excite the fluorescence donor. In addition, fluorescent illumination may lead to high background signals and, eventually, to acceptor excitation and/or signal quenching. As an exception, FRET is the choice when interactions are investigated in sections of tissue by means of a confocal-FRET approach. Lack of specific signal may be not related with lack of interactions. As already mentioned, the two options may be tried: R1-Rluc and R2-YFP or R2-Rluc and R1-YFP. Eventually BRET2 (using a receptor fused to Rluc and another receptor fused to GFP2) may solve the issue as the intensity of the signals is higher than in BRET1.
Notes:- Five different BRET experimental sessions must, at least, be performed.
- Each reading is considered a value for making calculations using the GraphPad software. It is not correct to make the mean of results of “same” condition in different days. Reason: transfection efficiency varies from day to day. Accordingly, the ratio in the X-axis changes from experiment to experiment and, therefore, the X-value mean of replicates of each point in a given experiment is considered an individual value. The SD is obtained after nonlinear regression including all “individual” data points obtained in all experimental sessions. During several years, researchers used to put mean X values from different experiments and “horizontal” bar errors, but this procedure is incorrect.
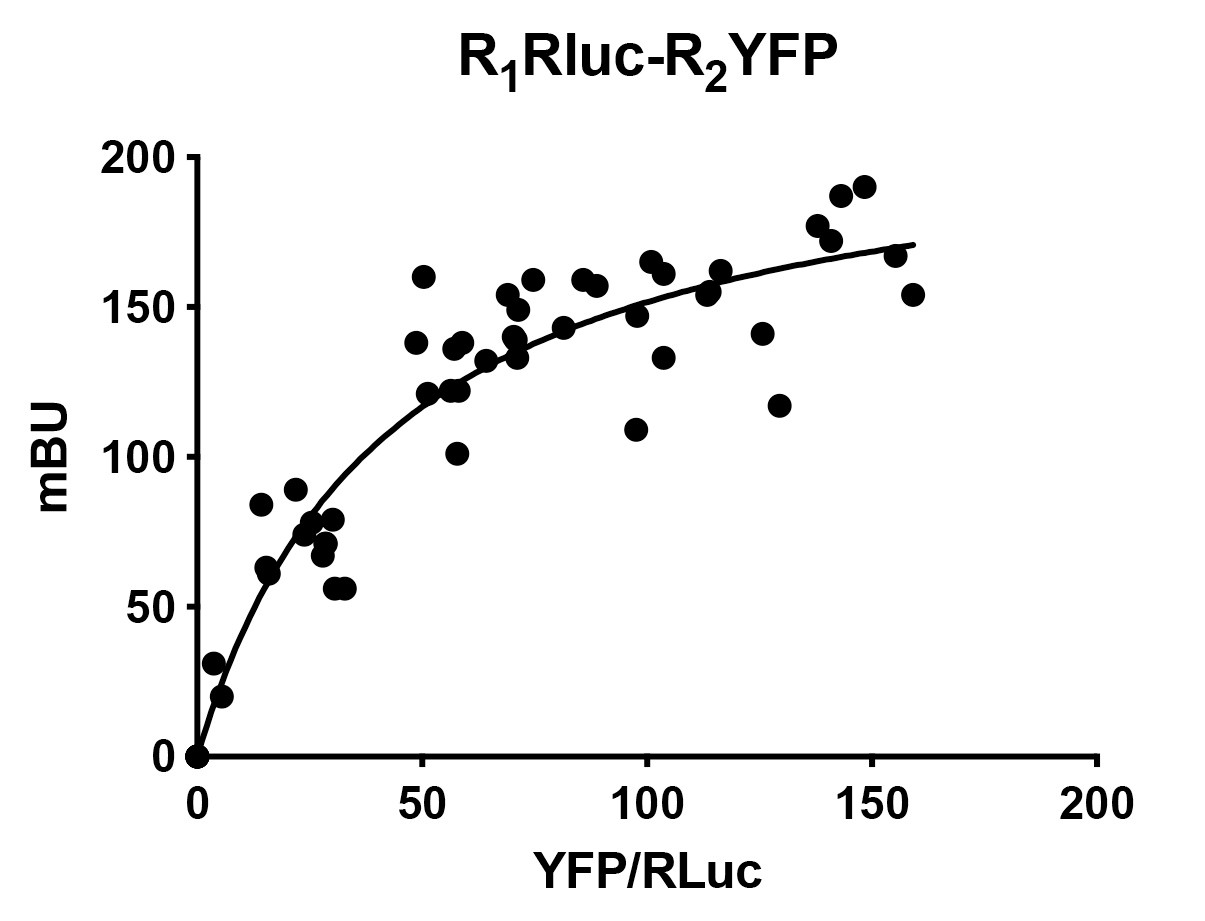
Figure 3. Example of positive BRET. When two receptors form heteromers, BRET assay results in a saturable curve. Bmax and KD for the curve are provided by non-linear-regression. For this curve, BRETmax= 220 ± 10 mBU and BRET50= 43 ± 7 (data are mean ± SEM).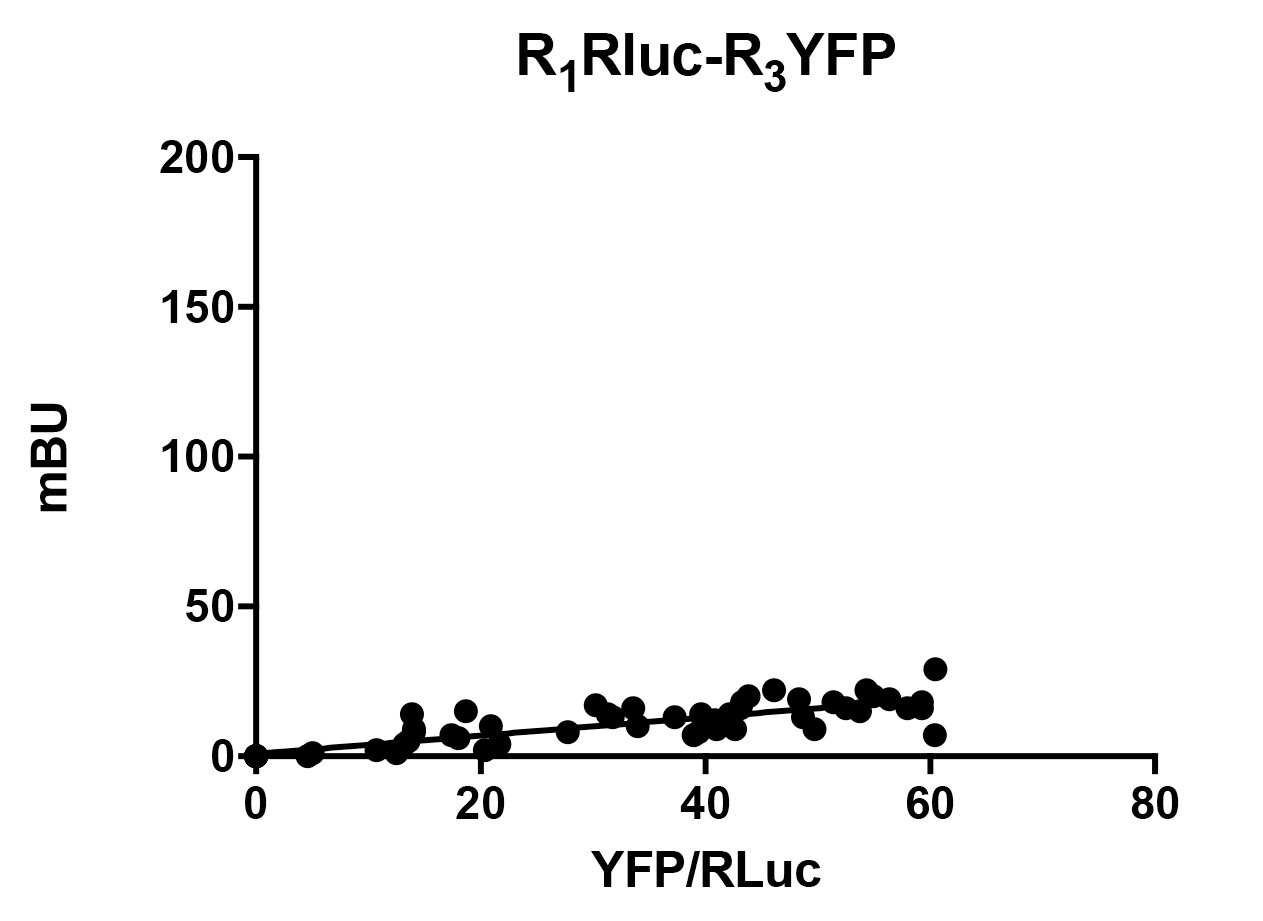
Figure 4. Example of negative BRET. In the case of two receptors that do not form heteromers, data fit to a linear regression.
Recipes
- Sterile 40 µM PEI
- Dilute 1 g PEI in 1 L MilliQ water, adjust to pH= 5.5 with 1 M HCl
- Filter through a 0.22 µm filter
- Store in small volume aliquots to avoid continuous freeze/thaw cycles (e.g., 5 ml)
- Sterile 150 mM NaCl
Dilute 8.7 g NaCl in 1 L MilliQ water, filter through a 0.22 µm filter - Serial BSA dilutions (0-1 mg/ml)
Resuspend 1 mg BSA in 1 ml of MilliQ water, and do serial dilutions in MilliQ water to obtain 0.5, 0.4, 0.3, 0.2, 0.1 and 0 mg/ml solutions - Supplemented DMEM
Add 25 ml of heat-inactivated FBS (incubate FBS for 30 min at 56 °C), 5 ml of L-glutamine, 5 ml of penicillin/streptomycin and 5 ml of MEM Non-Essential Amino Acids Solution in a 500 ml DMEM bottle - HBSS
1.26 mM CaCl2
5 mM KCl
0.44 mM KH2PO4
0.5 mM MgCl2
0.4 mM MgSO4
137 mM NaCl
0.34 mM Na2HPO4
10 mM HEPES
For 5 L:
0.92 g CaCl2·2H2O
1.85 g KCl
1.85 g KH2PO4
0.235 g MgCl2, anhydrous
0.5 g MgSO4·7H2O
40 g NaCl
0.605 g Na2HPO4·12H2O
11.925 g HEPES
Dissolved in 5 L MilliQ water, pH = 7.4
Store solution at 4 °C
For HBSS-glucose 0.1% (w/v):
Dilute 100 mg glucose in 100 ml HBSS (prepare fresh) - Coelenterazine H solution
Resuspend 1 mg stock in 1,227 µl ethanol
Acknowledgments
This work was supported by the Spanish Ministry of Innovation and Competitiveness (MINECO). Grant Ref No. SAF2009-07276, SAF2012-39875-C02-01 and BFU2015-64405-R (they may include EU FEDER funds).
This protocol was commissioned upon publication of Navarro et al., 2018. A selection of articles from our laboratory in which the protocol has been used are: Canals et al. (2003 and 2004), Carriba et al. (2008), Martínez-Pinilla et al. (2014 and 2015), Navarro et al. (2016 and 2018a and 2018b), Rodríguez-Ruiz et al. (2017) and Hinz et al. (2018).
Competing interests
Authors declare no competing of interests.
References
- Beautrait, A., Michalski, K. R., Lopez, T. S., Mannix, K. M., McDonald, D. J., Cutter, A. R., Medina, C. B., Hebert, A. M., Francis, C. J., Bouvier, M., Tesmer, J. J. and Sterne-Marr, R. (2014). Mapping the putative G protein-coupled receptor (GPCR) docking site on GPCR kinase 2: insights from intact cell phosphorylation and recruitment assays. J Biol Chem 289(36): 25262-25275.
- Breton, B., Sauvageau, E., Zhou, J., Bonin, H., Le Gouill, C. and Bouvier, M. (2010). Multiplexing of multicolor bioluminescence resonance energy transfer. Biophys J 99(12): 4037-4046.
- Burger, R., Schrod, L. and Schaefer, H. (1986). Functionally relevant membrane proteins of human and guinea-pig T lymphocytes. Mol Immunol 23(11): 1149-1156.
- Canals, M., Burgueno, J., Marcellino, D., Cabello, N., Canela, E. I., Mallol, J., Agnati, L., Ferre, S., Bouvier, M., Fuxe, K., Ciruela, F., Lluis, C. and Franco, R. (2004). Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Neurochem 88(3): 726-734.
- Canals, M., Marcellino, D., Fanelli, F., Ciruela, F., de Benedetti, P., Goldberg, S. R., Neve, K., Fuxe, K., Agnati, L. F., Woods, A. S., Ferre, S., Lluis, C., Bouvier, M. and Franco, R. (2003). Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278(47): 46741-46749.
- Carriba, P., Navarro, G., Ciruela, F., Ferre, S., Casado, V., Agnati, L., Cortes, A., Mallol, J., Fuxe, K., Canela, E. I., Lluis, C. and Franco, R. (2008). Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods 5(8): 727-733.
- Ciruela, F., Casado, V., Mallol, J., Canela, E. I., Lluis, C. and Franco, R. (1995). Immunological identification of A1 adenosine receptors in brain cortex. J Neurosci Res 42(6): 818-828.
- Dale, N. C., Johnstone, E. K. M., White, C. W. and Pfleger, K. D. G. (2019). NanoBRET: The bright future of proximity-based assays. Front Bioeng Biotechnol 7: 56.
- Dianzani, U., Bragardo, M., Tosti, A., Ruggeri, L., Volpi, I., Casucci, M., Bottarel, F., Feito, M. J., Bonissoni, S. and Velardi, A. (1999). CD44 signaling through p56lck involves lateral association with CD4 in human CD4+ T cells. Int Immunol 11(7): 1085-1092.
- Förster, T. (1948). Intermolecular energy transfer and fluorescence. Ann Phys Leipz 2: 55-75.
- Hinz, S., Navarro, G., Borroto-Escuela, D., Seibt, B. F., Ammon, Y. C., de Filippo, E., Danish, A., Lacher, S. K., Cervinkova, B., Rafehi, M., Fuxe, K., Schiedel, A. C., Franco, R. and Muller, C. E. (2018). Adenosine A2A receptor ligand recognition and signaling is blocked by A2B receptors. Oncotarget 9(17): 13593-13611.
- Jimenez, S., Baglietto-Vargas, D., Caballero, C., Moreno-Gonzalez, I., Torres, M., Sanchez-Varo, R., Ruano, D., Vizuete, M., Gutierrez, A. and Vitorica, J. (2008). Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer's disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci 28(45): 11650-11661.
- Navarro, G., Borroto-Escuela, D., Angelats, E., Etayo, I., Reyes-Resina, I., Pulido-Salgado, M., Rodriguez-Perez, A. I., Canela, E. I., Saura, J., Lanciego, J. L., Labandeira-Garcia, J. L., Saura, C. A., Fuxe, K. and Franco, R. (2018). Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer's disease and levodopa-induced dyskinesia. Brain Behav Immun 67: 139-151.
- Navarro, G., Cordomí, A., Brugarolas, M., Moreno, E., Aguinaga, D., Pérez-Benito, L., Ferre, S., Cortés, A., Casadó, V., Mallol, J., Canela, E. I., Lluis, C., Pardo, L., McCormick, P. J. and Franco, R. (2018a) Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain. BMC Biol 16(1):24.
- Navarro, G., Cordomi, A., Zelman-Femiak, M., Brugarolas, M., Moreno, E., Aguinaga, D., Perez-Benito, L., Cortes, A., Casado, V., Mallol, J., Canela, E. I., Lluis, C., Pardo, L., Garcia-Saez, A. J., McCormick, P. J. and Franco, R. (2016). Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with Gi and Gs. BMC Biol 14: 26.
- Navarro, G., Reyes-Resina, I., Rivas-Santisteban, R., Sanchez de Medina, V., Morales, P., Casano, S., Ferreiro-Vera, C., Lillo, A., Aguinaga, D., Jagerovic, N., Nadal, X. and Franco, R. (2018b). Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem Pharmacol 157: 148-158.
- Perkins, W. J., Weiel, J., Grammer, J. and Yount, R. G. (1984). Introduction of a donor-acceptor pair by a single protein modification. Förster energy transfer distance measurements from trapped 1,N6-ethenoadenosine diphosphate to chromophoric cross-linking reagents on the critical thiols of myosin subfragment. J Biol Chem 259(14): 8786-8793.
- Pfleger, K. D. and Eidne, K. A. (2005). Monitoring the formation of dynamic G-protein-coupled receptor-protein complexes in living cells. Biochem J 385(Pt 3): 625-637.
- Rosin, D. L., Robeva, A., Woodard, R. L., Guyenet, P. G. and Linden, J. (1998). Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol 401(2): 163-186.
- Schann, S., Bouvier, M. and Neuville, P. (2013). Technology combination to address GPCR allosteric modulator drug-discovery pitfalls. Drug Discov Today Technol 10(2): e261-267.
- Serrador, J. M., Nieto, M. and Sanchez-Madrid, F. (1999). Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol 9(6): 228-233.
- Springer, T. A., Miller, L. J. and Anderson, D. C. (1986). p150,95, the third member of the Mac-1, LFA-1 human leukocyte adhesion glycoprotein family. J Immunol 136(1): 240-245.
- Sun, S., Yang, X., Wang, Y. and Shen, X. (2016). In vivo analysis of protein-protein Interactions with bioluminescence resonance energy transfer (BRET): progress and prospects. Int J Mol Sci 17(10).
- Xu, Y., Piston, D. W. and Johnson, C. H. (1999). A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc Natl Acad Sci U S A 96(1): 151-156.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Reyes-Resina, I., Jiménez, J., Navarro, G. and Franco, R. (2019). Identification of Heteroreceptors Complexes and Signal Transduction Events Using Bioluminescence Resonance Energy Transfer (BRET). Bio-protocol 9(19): e3385. DOI: 10.21769/BioProtoc.3385.
Category
Neuroscience > Basic technology > Receptor-receptor interactions
Molecular Biology > Protein > Protein-protein interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









