- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Protocol to Generate Decellularized Bovine Spinal Cord Extracellular Matrix-based Scaffolds (3D-dCBS)
Published: Vol 9, Iss 19, Oct 5, 2019 DOI: 10.21769/BioProtoc.3380 Views: 4867
Reviewed by: Alessandro DidonnaSarah Daniela DiermeierAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Accurate Identification of Cell Cycle Stages in RPE1 Cells Using the ImmunoCellCycle-ID Method
Syon Reddy [...] Aussie Suzuki
Aug 5, 2025 1870 Views

Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
Quang Bach Le [...] Deepak Choudhury
Nov 20, 2025 1536 Views

Vascularization of Human Pancreatic Islets With Adaptive Endothelial Cells for In Vitro Analysis and In Vivo Transplantation
Ge Li [...] Shahin Rafii
Dec 20, 2025 750 Views
Abstract
Extracellular matrix (ECM)-based tissue engineering scaffolds have an essential role in promoting tissue regeneration. Nerve tissue engineering aims at facilitating the repair of permanent damage to the peripheral and central nervous systems, which are difficult to heal. For this purpose, a variety of biomaterials are being developed consisting of numerous synthetic and/or natural polymers to provide axonal reinnervation and to direct the growth of axons. Here, we present a novel protocol that enables to fabricate a 3-dimensional (3D) decellularized scaffold derived from the bovine spinal cord (BSC) ECM (3D-dCBS) for neural tissue engineering applications. In this protocol, a viscous ECM-derived gel from BSC is prepared, molded, and chemically crosslinked with EDC/NHS (3D-CBS) before decellularization process. Decellularization of 3D-CBS is performed with 1% SDS to attain 3D-dCBS. As compared with other available methods, our protocol is a novel decellularization method that preserves a more significant part of the ECM. We believe that the mentioned protocol has the potential to produce a bioengineered scaffold from spinal cord tissue with desired geometry for regenerative medicine applications related to neural tissue engineering.
Keywords: Bovine spinal cordBackground
Recently, nerve injuries have become a widespread issue that affects an approximate number of 20 million people in the United States alone and remain a significant burden on society (Du et al., 2018). According to data from the World Health Organization (2013), 250,000 to 500,000 people suffer a spinal cord injury due to traffic injuries, falls, and violence. When investigated the National Spinal Cord Injury Database, it is seen that spinal cord injury (SCI) caused by falls has dramatically increased. For instance, while that kind of injuries had occurred 17% in the 1970s, the value reached 31% during 2010-2013. In addition to this, the rate of fall-related SCI is indicated as 75% among persons 76 years of age and over (Chen et al., 2015). Following the SCI, mechanical stress and secondary injuries cause axonal degeneration. Following the SCI, mechanical stress and secondary injuries cause axonal degeneration. Although there are a few treatment approaches for axonal regeneration, it is still impossible to completely cure the damaged axons (Ban et al., 2017). Central nervous system (CNS) axons, unfortunately, have limited regeneration capacity after injury in contrast to axons of the peripheral nervous system (PNS). Therefore, the physiological response is considerably different in the repair of CNS and PNS. Because the macrophages assigned to repair of CNS injury cannot entry to the damaged zone due to blood-spine barrier, and thus tissue repair takes place slightly below of the desired level (Schmidt and Leach, 2003). Many strategies have been developed to provide axonal reinnervation and direct axonal growth, but recent approaches aim at preventing secondary injury, regeneration, and replacement of damaged spinal cord tissues.
Surgical operations and pharmacological methods have been preferred to minimize secondary damage, only treatment for SCI patients. However, there is currently no treatment available to repair nerve tissue function completely (Cristante et al., 2012; Varma et al., 2013; Schmidt and Leach, 2003). Cell-based regenerative therapy and tissue engineering strategies have a promising potential for recovering severe SCI (Assunção-Silva et al., 2015). More recently, neural tissue engineering has focused on natural or synthetic biopolymers that are combined to create a tissue engineering product (Boni et al., 2018). Natural and synthetic derived materials have some advantages and disadvantages, including mechanical, chemical, and biological features (O’ Brien, 2011). The most important properties of natural biopolymers such as gelatin, elastin, fibrinogen, silk, and collagen are a biocompatible, biodegradable and inherent structural similarity to mimic natural ECM. Despite these unique advantages, they have weak mechanical strength and inconsistency, which limit to develop ideal scaffolds for regenerative medicine applications (Haraguchi et al., 2012; Ulery et al., 2011; Sekula and Zuba‐Surma, 2018).
On the other hand, synthetic biopolymers such as poly (ε-caprolactone), poly (lactic acid) and their copolymers, poly (p-dioxanone), copolymers of trimethylene carbonate and glycolide are easily fabricated on a large scale by controlling their degradation rates (Gunatillake and Adhikari, 2003; Abbasian et al., 2019). However, various disadvantages such as toxic by-products resulting from biodegradation of the synthetic polymers restrict their applications in the field of tissue engineering (Tabata, 2009; Arslan et al., 2017). Due to limitations of synthetic and/or natural biopolymers and shortage of donors, acellular biologic scaffolds have become a new option for the treatment of missing or damaged tissues (Yu et al., 2016). Decellularization technique is a promising technology for tissue engineering and regenerative medicine applications because the technique aims at removing host cells from ultrastructure of native ECM by protecting unique ECM molecules (Kelleher and Vacanti, 2010). Many different methods including chemical, enzymatic, physical and/or their combinations, are used for the decellularization of native ECM. Preservation of structural integrity and mechanical properties of ECM in the decellularization process, which allows removing cellular components such as cells, cell debris, chromosome fragments, and xenogeneic antigens, is a crucial fact for achieving effective healing of the tissues (Crapo et al., 2011; Patnaik et al., 2014). Furthermore, acellular biologic scaffolds regulate the homeostasis and regeneration of tissues and organs by mimicking the ECM that acts as a niche for the cells (Tapias and Ott, 2014; Dzobo et al., 2018).
The ultimate goal of the proposed decellularization protocol is to effectively remove the nuclear contents (i.e., dsDNA) of native tissues and organs while maintaining the critical structural, biochemical, and biomechanical cues present in the ECM. This study has demonstrated a novel decellularization protocol, which enables an optimized strategy to fabricate a 3D bioscaffold for neural tissue engineering applications (Arslan et al., 2019). By using 1% sodium dodecyl sulfate (SDS), 50 mM Trizma® hydrochloride and 5 mM EDTA, we have executed the decellularization process at room temperature (RT) for 72 h. The results showed that the amounts of dsDNA in the native BSC and 3D-dCBS were found to be 520.76 ± 18.11 and 28.80 ± 0.20 ng/mg dry weight, respectively (n = 3; P < 0.001; ANOVA). The proposed method removed approximately 94.47% of nuclear material from the native BSC. It has been reported that the amount of dsDNA should be < 50 ng per mg dry weight and < 200 bp residual of the DNA fragment for the optimal decellularization (Pati et al., 2014).
Consequently, the main benefit of the presented method is constructing a 3D biomatrix from decellularized BSC with desired geometry. We believe that the recellularization of 3D-dCBS with neural stem and/or progenitor cells could have a positive effect on the supporting of deformed nerve tissue. In conclusion, we believe that the proposed approach to decellularization of BSC will open new horizons for the experts working in the field of neural tissue engineering.
Materials and Reagents
- 48-well Clear TC-treated Multiple Well Plates, Individually Wrapped, Sterile (Corning Incorporated, Costar®, catalog number: 3548)
- Falcon, 50 mI conical centrifuge tubes (Isolab, sterile, catalog number: 078.02.008)
- Falcon, 15 mI conical centrifuge tubes (Isolab, sterile, catalog number: 078.02.007)
- Borosilicate laboratory bottle, 250 mI, GL-45 (Interlab, catalog number: 061.01.250)
- Borosilicate laboratory bottle, 500 mI, GL-45 (Interlab, catalog number: 061.01.500)
- Metal pot, stainless steel, 250 mm diameter and 85 mm depth
- Scalpel, length 25 mm (Interlab, catalog number: 048.51.010)
- Scalpel blade holder, length 130 mm (Interlab, catalog number: 048.50.001)
- Glass petri dish, diameter 60 mm; height 15 mm (Interlab, catalog number: 081.01.060)
- Parafilm M (Sigma-Aldrich, catalog number: P7543)
- Magnetic stirring bar; length 10 mm; diameter 6 mm (Interlab, catalog number: 057.01.010)
- FinnpipetteTM F2 Fixed Volume Single-Channel Pipettes; 100 to 1,000 μl (Thermo-Scientific, catalog number: 4642090)
- FinnpipetteTM F2 Fixed Volume Single-Channel Pipettes; 10 to 100 μl (Thermo-Scientific, catalog number: 4642070)
- Glass Erlenmeyer flask; volume 50 mI (Interlab, catalog number: 027.01.050)
- Erlenmeyer flask cap, NS 12/21 (Interlab, catalog number: 051.08.012)
- Screwcap GL-45, blue (Interlab, catalog number: 051.09.45B)
- Laboratory forceps, length 115 mm, 304 stainless steel (Interlab, catalog number: 048.08.115)
- Spoon spatula, length 150 mm, 304 stainless steel (Interlab, catalog number: 047.01.150)
- Borosilicate graduated cylinder, 250 mI (Interlab, catalog number: 015.01.250)
- Borosilicate graduated cylinder, 500 mI (Interlab, catalog number: 015.01.500)
- μDropTM Plate (Thermo-Scientific, MultiskanTM with mdropTM plate)
- BSC, the bovine spinal cord (BSC) from Holstein-Friesian cattle [thoracic region (T2–T11)] was obtained within 4-6 h following the slaughtering of healthy animals (16-month-old) at a local slaughterhouse in Balikesir, Turkey
- Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA-Na2) (Sigma-Aldrich, catalog number: E5134)
- Trizma® hydrochloride (Sigma-Aldrich, catalog number: T5941)
- Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L4509)
- Peracetic acid solution (Sigma-Aldrich, catalog number: 433241)
- Ethanol 99% (Sigma-Aldrich, catalog number: 32221)
- Magnesium chloride (Sigma-Aldrich, catalog number: M8266)
- Deoxyribonuclease I from bovine pancreas, DNase I (Sigma-Aldrich, catalog number: DN25)
- Ribonuclease A from bovine pancreas, RNase A (Sigma-Aldrich, catalog number: R5503)
- Sodium hydroxide (Sigma-Aldrich, catalog number: 06203)
- Hydrochloric acid (Sigma-Aldrich, catalog number: 07102)
- 2-(N-Morpholino) ethanesulfonic acid (MES) (Sigma-Aldrich, catalog number: M3885)
- N-Hydroxysuccinimide (NHS) (Sigma-Aldrich, catalog number: 8.04518)
- N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (Sigma-Aldrich, catalog number: 8.00907)
- Milli-Q water (Merck-Millipore, Type I-III, catalog number: ZRQSVP3WW)
- 0.1 N NaOH solution (see Recipes)
- 1 M NaOH solution (see Recipes)
- 1 M HCl solution (see Recipes)
- 70% ethanol solution (see Recipes)
- 0.05 M 2-(N-Morpholino) ethanesulfonic acid (MES) buffer (pH 5.5) (see Recipes)
- Crosslinking (EDC/NHS) solution (see Recipes)
- Hypertonic solution (see Recipes)
- RNase A and DNase I enzyme solutions (see Recipes)
- Decontamination solution (4% ethanol/0.1% peracetic acid) (see Recipes)
Equipment
- Ultrasonic bath (Elma, Elmasonic S30H)
- Lyophilizer -55 °C (Telstar, LyoQuest)
- Milli-Q® Integral water purification system for ultrapure water (Merck-Millipore, Type I, Direct-Q® 3 UV)
- Freeze dryer (Telstar, LyoQuest -55)
- Vortex (IKA Genious 4, V4B)
- Analytical balance (Shimadzu, model: ATX224)
- pH Meter (Mettler Toledo, model: FiveEasyTMFE20)
- Magnetic stirrer (Multi Position) (Jeiotech, MS-12BB, catalog number: AAH330115BK)
- Mechanical homogenizer (IKA, T18 Basic Ultra TURRAX)
- Automatic single-channel pipettes, 10-100 and 100-1,000 µl (Gilson-compatible, Diamond® Eco-PackTM)
- Refrigerator (Siemens, model: KG57NPW24N)
- Ultra-low temperature freezer (Panasonic, model: MDF-U5386S-PE)
- Orbital shaker incubator-temperature controlled (Benchmark, model: Incu-ShakerTM Mini)
Procedure
- Dissection of BSC
Note: Carry out the dissection process in a fume hood safety cabinet under sanitary conditions to minimize cross-contaminations.- Harvest BSC from the thoracic region (T2-T11) of Holstein-Friesian cattle.
- Cut muscle, fat, and other meningeal tissues from the spinal cord using a scalpel.
- Rinse the tissue 3 times each with 200 ml of Milli-Q water in order to decrease connective tissue and blood contamination.
- After removing soft tissues, cut the spinal cord into several pieces on a glass petri dish (see Figure 1, Step 1).
- Producing strategy of viscous gel from BSC
Notes:- Carry out the tissue homogenization process at room temperature (RT) in an ice bath (prepared in a metal pot) to prevent over-heating of the mixture and the degradation of biomolecules.
- Homogenization step (Step B4) should be repeated at least three times.
- Mince the BSC tissue (purged from external contaminants) in small pieces of approximately 2 x 2 cm dimensions.
- Place BSC tissues into a glass Erlenmeyer flask using surgical equipment such as laboratory forceps made of nickel-plated steel, and stainless-steel micro spoon spatula.
- Weigh small pieces of BSC (approx. 5 g and in the wet form) using an analytical balance, and then place into 20 ml of 0.1 M NaOH solution.
- Homogenize BSC tissue pieces in 20 ml of 0.1 M NaOH solution using a homogenizer for 5 min at 10,000 rpm.
- Adjust the final volume of the gel solution to 30 ml with 0.1 M NaOH solution using a glass graduated measuring cylinder (see Figure 1, Step 2).
- Fabrication of 3D-CBS (Figure 1)
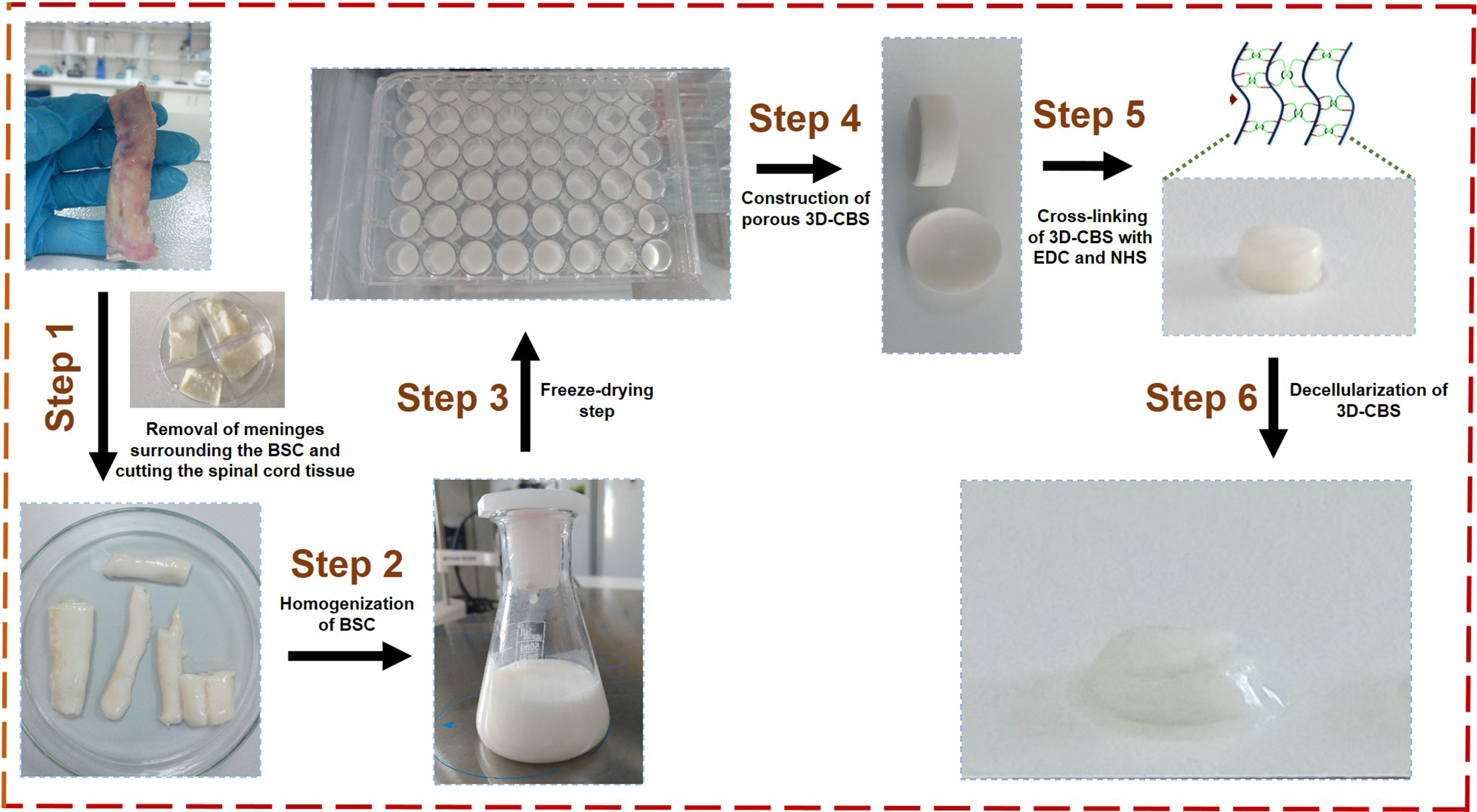
Figure 1. 3D-dCBS fabrication process- Pipette 200 μl of the gel solution into each well of a 48-well plate to prepare 3-dimensional (3D) bovine spinal cord extracellular matrix-based scaffolds (referred to as 3D-CBS).
- Incubate the plate at 4 °C for 4 h for the maturation of the gel solution.
- Then, freeze the 48-well plate in an ultra-low temperature freezer at -80 °C overnight.
- Finally, freeze-dry homogenized BSC matrix overnight using a -55 °C lyophilizer (see Figure 1, Steps 3 and 4).
- Crosslinking process of 3D-CBS (Figure 1)
- Weigh 25 mg of lyophilized 3D-CBS to prepare crosslinked scaffolds.
- Place each 3D-CBS into a 15 ml falcon tube including 6 ml of ethanol 99.9% at RT for 15 min while gently shaking at 120 rpm using orbital shaker incubator.
- Carefully decant the ethanol solution and crosslink each 25 mg of 3D-CBS with 5.37 ml of crosslinking (EDC/NHS) solution at RT for 10 min using the orbital shaker incubator.
- Afterward, rinse the scaffolds with 200 ml of Milli-Q water at RT for 2 h in a 250 ml borosilicate laboratory bottle with screw cap.
- Finally, freeze crosslinked 3D-CBS in a 48-well plate at -80 °C and freeze-dry overnight using a -55 °C lyophilizer (see Figure 1, Step 5).
Notes:- Whereas the dimensions of non-crosslinked 3D-CBS are h=3 mm and Ø=8, the crosslinked ones are h=1.2 mm and Ø=6.
- Whereas mass of non-crosslinked 3D-CBS is approx. 25 mg, the crosslinked one is approx. 15 mg.
- Decellularization process of 3D-CBS (Figure 1)
Note: Carry out the process in the orbital shaker incubator at RT.- Soak crosslinked 3D-CBS in 200 ml of hypertonic solution [50 mM Trizma® hydrochloride, 5 mM EDTA-Na2 (pH 8.0)] containing 1% SDS at RT for 72 h.
- After being treated with the hypertonic solution, rinse the scaffolds 8 times each with 250 ml of Milli-Q water for 24 h in a 250 ml borosilicate laboratory bottle with screw cap.
- Following the washing step, treat 3D-CBS with 50 ml of an enzyme solution containing deoxyribonuclease I (DNase I) and ribonuclease A (RNase A) at 37.5 °C for 24 h in the shaker at 120 rpm.
- Rinse 3D-CBS 3 times each with 250 ml of Milli-Q water and decontaminate with 200 ml of 4% ethanol and 0.1% peracetic (decontamination solution) acid for 5 h at RT.
- Repeat the washing step (Step E4).
- Finally, freeze the scaffolds at -80 °C ultra-low temperature freezer and lyophilize overnight again to achieve 3D decellularized BSC ECM-based scaffolds (3D-dCBS) (see Figure 1, Step 6).
Note: Mass of 3D-dCBS is approx. 2.5 mg.
Notes
- Carefully weigh all of the materials (i.e., chemicals, reagents, tissue samples, etc.) using an analytical balance.
- Check the pH using a benchtop pH meter that has been calibrated at the desired pH range. Use 1 M HCl or 1 M NaOH to adjust the pH of prepared solutions.
- The storage temperature of the prepared solutions varies between 4 °C and RT.
- Coat falcon tubes or glass bottles with Parafilm M laboratory film to prevent drying.
- Carry out the decellularization and washing processes in 250 mI borosilicate laboratory bottles with screw cap.
- Figure 1 demonstrates the fabrication and decellularization processes of the 3D-CBS at a glance.
Recipes
- 0.1 N NaOH solution
- Dissolve 0.4 g NaOH pellets in Milli-Q water so that the final volume is 100 mI
- Completely dissolve the pellets in the ultrasonic bath
- Storage: RT; Shelf life: 3 months
- 1 M NaOH solution
- Dissolve 2 g NaOH pellets in Milli-Q water so that the final volume is 50 mI
- Completely dissolve the pellets in the ultrasonic bath
- Storage: RT; Shelf life: 3 months
- 1 M HCl solution
- Add carefully 4.10 ml concentrated HCl (12.18 M) to 45.9 mI Milli-Q water in a fume hood
- Gently vortex
- Storage: RT; Shelf life: 3 months
- 70% ethanol solution
- Mix 350 mI of ethanol (99.9%) with 150 mI of Milli-Q water
- Be sure to obtain a homogeneous mixture
- Storage: RT, Shelf life: 3 months
- 0.05 M 2-(N-Morpholino) ethanesulfonic acid (MES) buffer (pH 5.5)
- Dissolve 0.54 g MES in 70% ethanol (v/v) so that the final volume is 50 mI
- Gently vortex for 1 min and sonicate at 280 W for 10 min
- Adjust the pH to 5.5 using 1 M HCl solution
- Storage: 4 °C; Shelf life: 1 month.
- Crosslinking (EDC/NHS) solution
- For each 25 mg of 3D-CBS, dissolve 57.5 mg of N-(3-dimethylaminopropyl) N'ethylcarbodiimide hydrochloride (EDC) and 14 mg of N-hydroxysuccinimide (NHS) in 5.37 mI of MES buffer
- Gently sonicate at 280 W for 2 min
- Storage: 4 °C; Shelf life: prepared fresh
Notes:- Should mix EDC (2.30 g/g of sample) and NHS (0.56 g/g of sample) (molar ratio of EDC/NHS = 2.5) in MES buffer at RT.
- The crosslinking process previously described by Buttafoco et al. (2006) was modified and applied (215 mI of MES buffer containing EDC/NHS for each 1 g of 3D-CBS).
- Hypertonic solution
- Dissolve 1.58 g of Trizma® hydrochloride, 3.72 g of ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA-Na2) and 2 g of sodium dodecyl sulfate (SDS) in Milli-Q water
- Incubate at 40 °C for 10 min and then sonicate until a homogeneous solution is obtained (approx. 15 min)
- After being adjusted the pH 8.0 using 1 M NaOH, complete the final volume to 200 mI (referred to as the hypertonic solution)
- Storage: RT; Shelf life: 1 week
- RNase A and DNase I enzyme solution
- Dissolve 0.19 mg of magnesium chloride (MgCl2) and 1.58 g of Trizma® hydrochloride in Milli-Q water using vortex
- After being adjusted the pH 7.5 using 1 M NaOH, complete the final volume to 200 mI (referred to as buffer solution)
- Add 40 mg of DNase I and 10 mg of RNase A into the buffer solution then dissolve by gentle vortexing (referred to as enzyme solution)
Note: Should prepare as fresh and use immediately.
- Decontamination solution (4% ethanol/0.1% peracetic acid)
- Mix 200 µl of peracetic acid solution (36-40%), 8 mI of ethanol (99.9%) and 191.8 mI of Milli-Q water to obtain 200 mI of decontamination solution
- Be sure to obtain a homogeneous mixture
- Storage: RT, Shelf life: 1 month
Acknowledgments
This work was financially supported by the Ministry of Science, Industry, and Technology, Republic of Turkey (Project ID. 0089.TGSD.2013).
Competing interests
The authors have declared that no competing interests exist.
References
- Abbasian M., Massoumi B., Mohammad-Rezaei R., Samadian H. and Jaymand M. (2019). Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Inter J Biol Macromol 134: 673-694.
- Arslan, Y. E., Efe, B. and Sezgin Arslan, T. (2019). A novel method for constructing an acellular 3D biomatrix from bovine spinal cord for neural tissue engineering applications. Biotechnology Progress 35(4):e2814. doi: 10.1002/btpr.2814.
- Arslan, Y. E., Sezgin Arslan, T., Derkus, B., Emregul, E. and Emregul, K. C. (2017). Fabrication of human hair keratin/jellyfish collagen/eggshell-derived hydroxyapatite osteoinductive biocomposite scaffolds for bone tissue engineering: from waste to regenerative medicine products. Colloids Surf B Biointerfaces 154:160-170.
- Assunção-Silva, R. C., Gomes, E. D., Sousa, N., Silva, N. A. and Salgado, A. J. (2015). Hydrogels and cell based therapies in spinal cord injury regeneration. Stem Cells Int 2015: 948040.
- Ban, D. X., Liu, Y., Cao, T. W., Gao, S. J. and Feng, S. Q. (2017). The preparation of rat’s acellular spinal cord scaffold and co-culture with rat’s spinal cord neuronin vitro. Spinal Cord 55(4):411-418.
- Boni, R., Ali, A., Shavandi, A. and Clarkson, A. N. (2018). Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci 25: 90.
- Buttafoco, L., Kolkman, N. G., Engbers-Buijtenhuijs, P., Poot, A. A., Dijkstra, P. J., Vermes, I. and Feijen, J. (2006). Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 27(5): 724-734.
- Chen Y., Tang Y., Allen V. and De Vivo J.M. (2016). Fall-induced spinal cord injury: External causes and implications for prevention. J Spinal Cord Med 39(1): 24-31.
- Crapo, P. M., Gilbert, T. W. and Badylak, S. F. (2011). An overview of tissue and whole organ decellularization processes. Biomaterials 32(12):3233-3243.
- Cristante, A. F., Barros Filho, T. E., Marcon, R. M., Letaif, O. B. and Rocha, I. D. (2012). Therapeutic approaches for spinal cord injury. Clinics (Sao Paulo) 67(10): 1219-1224.
- Du, J., Chen, H., Qing, L., Yang, X. and Jia, X. (2018). Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration. Biomater Sci 6(6): 1299-1311.
- Dzobo K., Thomford N. E., Senthebane D. A., Shipanga H., Rowe A., Dandara C., Pillay M. and Motaung K. S. C. M. (2018). Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells International 2018:2495848.
- Gunatillake P. A. and Adhikari R. (2003). Biodegradable synthetic polymers for tissue engineering. Eur Cells Mater 5: 1-16.
- Haraguchi Y., Shimizu T., Yamato M. and Okano T. (2012). Concise review: cell therapy and tissue engineering for cardiovascular disease. Stem Cells Transl Med 1(2):136-141.
- Kelleher C. M. and Vacanti J. P. (2010). Engineering extracellular matrix through nanotechnology. J R Soc Interface 7: S717-S729.
- O’Brien F. J. (2011). Biomaterials & scaffolds for tissue engineering. Mater Today 14 (3): 88-95.
- Pati, F., Jang, J., Ha, D. H., Won Kim, S., Rhie, J. W., Shim, J. H., Kim, D. H. and Cho, D. W. (2014). Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5: 3935.
- Patnaik, S. S., Wang, B., Weed, B., Wertheim, J. A. and Liao, J. (2014). Decellularized scaffolds: concepts, methodologies, and applications in cardiac tissue engineering and whole-organ regeneration. In: Tissue Regeneration Where Nano-Structure Meets Biology. World Scientific, 77-124.
- Schmidt C. E. and Leach J. B. (2003). Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng 5: 293-347.
- Sekuła, M. and Zuba‐Surma, E. K. (2018). Biomaterials and stem cells: promising tools in tissue engineering and biomedical applications. In: Biomaterials in Regenerative Medicine. Bentham Science Publishers, 361-378.
- Tabata, Y. (2009). Biomaterial technology for tissue engineering applications. J R Soc Interface 6 Suppl 3: S311-324.
- Tapias, L. F. and Ott, H. C. (2014). Decellularized scaffolds as a platform for bioengineered organs. Curr Opin Organ Transplant 19(2):145-152.
- Ulery, B. D., Nair, L. M. and Laurencin, C. T. (2011). Biomedical Applications of Biodegradable Polymers. J Polym Sci B Polym Phys 49(12): 832-864.
- Varma, A. K., Das, A., Wallace, G. 4th., Barry, J., Vertegel, A. A., Ray, S. K. and Banik, N. L. (2013). Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res 38(5): 895-905.
- Yu, Y., Alkhawaji, A., Ding, Y. and Mei, J. (2016). Decellularized scaffolds in regenerative medicine. Oncotarget 7(36): 58671–58683.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Arslan, Y. E., Efe, B. and Sezgin Arslan, T. (2019). A Novel Protocol to Generate Decellularized Bovine Spinal Cord Extracellular Matrix-based Scaffolds (3D-dCBS). Bio-protocol 9(19): e3380. DOI: 10.21769/BioProtoc.3380.
Category
Cell Biology > Cell viability > Cell proliferation
Cell Biology > Cell engineering > Tissue engineering
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












