- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Simple Protocol for Distinguishing Drug-induced Effects on Spatial Memory Acquisition, Consolidation and Retrieval in Mice Using the Morris Water Maze
Published: Vol 9, Iss 18, Sep 20, 2019 DOI: 10.21769/BioProtoc.3376 Views: 7826
Reviewed by: Lori NewmanEmma PuighermanalAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2606 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views
Abstract
The Morris water maze (MWM) is one of the most commonly used tests for assessing spatial learning and memory in mice. While the MWM is highly amenable to testing the effects of memory modifying drugs, most studies do not consider the timing or duration of drug exposure when conducting the MWM assay; factors that can strongly influence the effect of the drug on different stages of memory and interfere with data interpretation. Herein we describe a MWM protocol which offers the advantage of distinguishing the impact of a fast acting intraperitoneally (IP) injected drug on the different stages of spatial memory: acquisition, consolidation, and retrieval. Mice initially undergo habituation to both the MWM apparatus and IP injection procedure over the course of three days. For assessing the effect of a drug on memory acquisition, mice are injected with the drug prior to training sessions over four consecutive days, where mice learn to find an escape platform in a circular water tank using distal spatial cues. To determine the effect of the drug on memory consolidation, mice are injected with the drug immediately after each training session. For testing the effect of a drug on memory retrieval, mice receive mock IP injections on each training day and the drug is IP injected only once, prior to a probe trial, where mice attempt to locate the platform following its removal from the tank. This protocol provides a simple strategy for distinguishing the effect(s) of a CNS acting drug on the different stages of memory.
Keywords: Morris water mazeBackground
Numerous studies in rodents have demonstrated that memory is a process generally considered to occur during several stages: acquisition, consolidation and retrieval (Abel and Lattal, 2001). The acquisition phase is better known as learning, while consolidation is a labile phase during which the memory trace is stored. The standard model of memory consolidation posits that task engagement initially triggers intracellular signaling cascades and activation of immediate early gene (IEG) expression, within minutes to hours after learning, and ultimately elicits long-term synaptic changes primarily within the hippocampus (Dudai, 2004). Over time, hippocampal activity is theorized to promote extra-hippocampal consolidation, particularly in the neocortex, in a process termed “systems consolidation” that is largely independent of the hippocampus (Dudai, 2004). However, recent studies suggest that hippocampal and neocortical consolidation occur concurrently, and rapidly, after de novo learning (Runyan et al., 2019). Memory retrieval takes place during re-exposure to the learning context and is believed to involve reactivation of patterns of neural activity in hippocampal and neocortical networks associated with the original learning experience (Ben-Yakov et al., 2015). Surgical or chemical strategies that interfere with acquisition, consolidation and retrieval stages have shed light on the underlying processes of memory formation (Broadbent et al., 2004; Bast et al., 2005).
The Morris water maze (MWM) was originally developed by Richard Morris in order to test spatial learning and memory in rats (Morris, 1984). The MWM is a hippocampus dependent behavioral task in which rodents are placed in a circular pool filled with opaque water and learn to find (and climb up on) an escape platform hidden just below the water surface at an unchanging location within the pool. Initially, rodents placed in the pool find the escape platform by random navigation. However, over repeated training trials, mice slowly learn the escape platform location using objects or symbols placed outside the maze as cues, and progressively swim to the platform in a shorter time (decreased escape latency). This visuospatial task is a reliable test for rats and mice, and more recently in humans using an analogous virtual task (Kallai et al., 2005; Zhong et al., 2017). The popularity of the MWM task has resulted in the development of a plethora of different versions that vary in utility (D'Hooge and De Deyn, 2001). Generalized MWM protocols for mice have been described elsewhere ( Wenk, 2004; Choi et al., 2006; Vorhees and Williams, 2006; Bromley-Brits et al., 2011) and modified MWM protocols adapted for use with various strains, environments, genetics and ages have also been developed (Bromley-Brits et al., 2011; Barnhart et al., 2015; Weitzner et al., 2015; De Coninck et al., 2017).
Various pharmacological, genetic and lesion approaches have helped to define the brain regions, neural pathways and molecular processes involved in different stages of spatial memory using the MWM. Although lesion studies can be informative for discerning brain regions and neural circuits required for memory formation, the permanence of many types of lesions makes it difficult to precisely determine which stage(s) of memory the lesion affected. However, the MWM is highly amenable for testing the effects of injectable drugs on spatial memory. In many studies, memory modifying drugs are typically administered for a prolonged period of time or simply prior to the beginning of testing. The duration and timing of drug administration can have profound effects on the different stages of memory, thereby precluding proper determination of drug effects on learning or memory. Relatively few studies have attempted to systematically modify the timing of drug treatment during the MWM assay in order to tease apart the effect of the agent on learning/acquisition, consolidation and retrieval of spatial memory (Da and Takahashi, 2002; Florian and Roullet, 2004; Hou et al., 2006; Bonini et al., 2007). Herein we outline a protocol for distinguishing the influence of an intraperitoneally (IP) injected fast acting drug on different spatial learning and memory components.
Materials and Reagents
- Ballpoint pen (Paper Mate, catalog number: 89466)
- Paper towels (Cascades Pro, catalog number: CSDH170)
- 1 ml syringe (BD, catalog number: 309602)
- 0.2 µm filter unit (Thermo Scientific, catalog number 566002)
- 27 G 1¼ needle (BD, catalog number: 305136)
- Adult C57BL/6NCrl mice (Charles River, catalog number: 027)
Male or female mice can be utilized in this protocol. Aged mice can be used in this protocol as long as they are healthy enough to withstand the minor stress associated with swimming. - White tempera paint (Funstuff®, Reeves and Poole Group Inc., catalog number: 92080)
- NaCl (Sigma-Aldrich, catalog number: S7653)
- Fast acting drug of choice prepared in vehicle
Note: It is important that the drug of choice has well defined pharmacokinetic properties. In particular, the drug must be capable of crossing the blood brain barrier and have a half-life no longer than 1 h in order to discriminate effects on a certain stage of memory (i.e., acquisition) without influencing a subsequent stage (i.e., consolidation). We have recently evaluated the effect of the fast acting drug dichloroacetate (DCA, Sigma-Aldrich, catalog number: 347795) dissolved in sterile saline solution (0.9 % [w/v] NaCl) on memory acquisition and retrieval (Harris et al., 2019). - Vehicle solution without drug
Note: We typically use sterile saline solution (0.9% [w/v] NaCl) (see Recipes).
Equipment
- Thermometer (Fischer Scientific, S02674)
- Well-lit room (1,400 lux, room temperature 25 °C) to accommodate the MWM and a holding area outside of the testing area
- Morris Water Maze apparatus consisting of:
- White polyurethane water tank, 122 cm diameter, 81 cm height (San Diego Instruments, catalog number: 7000-0723)
- Adjustable height clear acrylic round water maze platform (10 cm diameter, 32-56 cm height), including removable flag pole (San Diego Instruments, catalog number: 7500-0310)
- Tank drain Kit (San Diego Instruments, catalog number: 7500-0217)
- Submersible aquarium heater (Aqueon, catalog number: TA300H)
- Four distal spatial cues of colored construction paper cut into different shapes e.g., Circle, triangle, plus sign, heart and diamond (~25 cm length/width) (Staples, catalog number: 25305)
Note: Ensure cues have a color maximally contrasted to the walls they are to be hung on. - Overhead digital camera (Logitech, catalog number: C920)
- PC computer running Windows operating system connected to camera (ASUS, catalog number: M51A)
- Heat mat (122 cm x 51 cm) with thermostat controller (VIVOSUN, catalog number: 4820)
- Transparent Tape (Staples, catalog number: 52384-PD)
Software
- ANY-Maze Behavioral Tracking Software (Stoelting, https://www.stoeltingco.com/any-maze-video-tracking-software-1224.html)
Note: This protocol outlines the use of ANY-Maze software for analysis. However, use of other software or manually scoring videos is also feasible with this protocol. - Prism (GraphPad, https://www.graphpad.com/scientific-software/prism/)
- RStudio (RStudio, https://www.rstudio.com/)
Procedure
- Room preparation
- Place the tank in the center of the sectioned/curtained off testing area.
- Hang different spatial cues on the north, east, south and west walls of the testing area around the tank with transparent tape, 1-2 m away from the tank.
Note: Ensure that the cues are large and contain contrasting colors to the walls/curtains of the testing area for better visibility and are at a distance and height where they are visible to mice from inside the tank (Figure 1).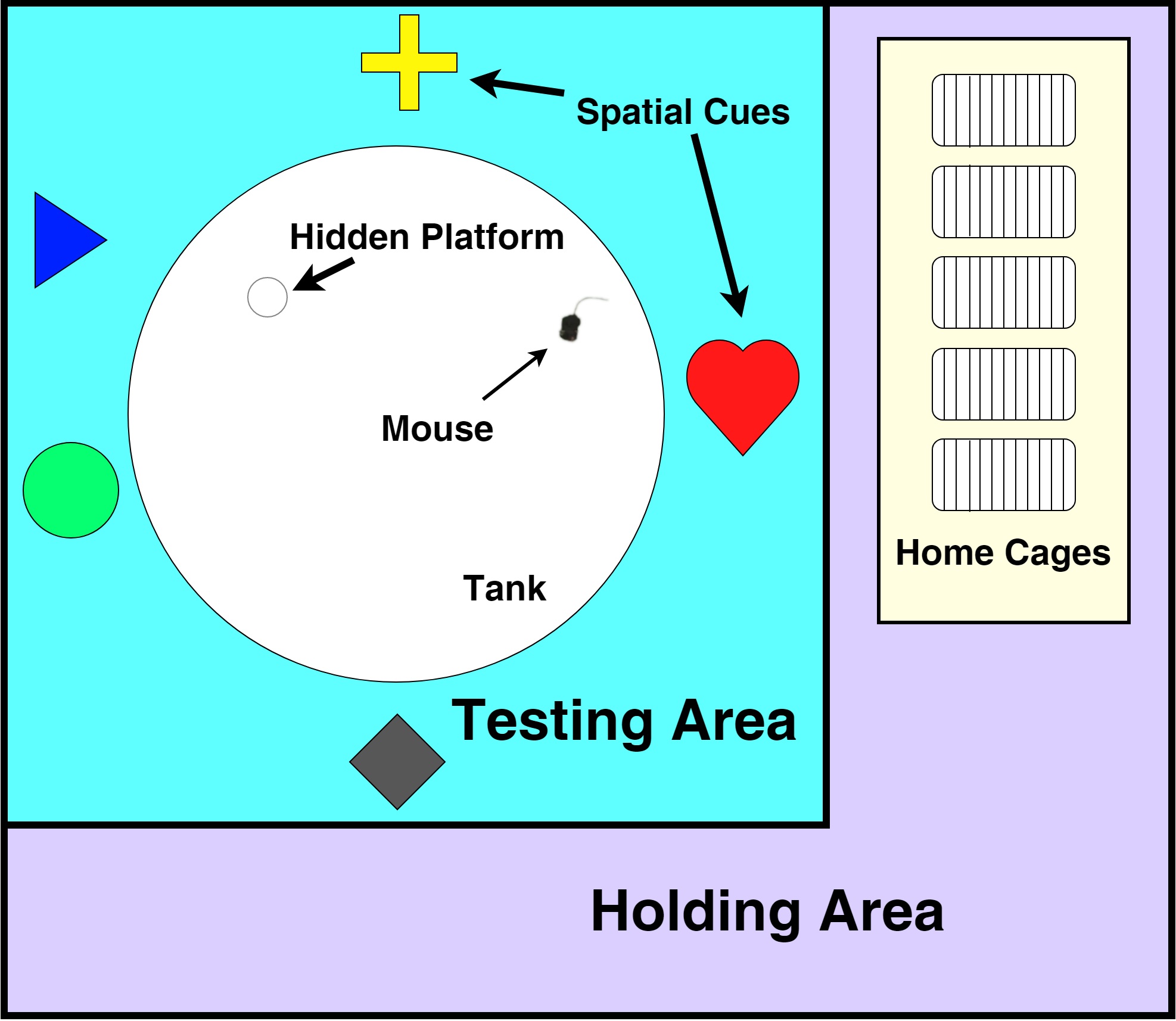
Figure 1. Holding and testing area setup. The tank must be curtained/walled off such that the holding area and experimenter are not visible to mice in the tank. Distinct spatial cues in the testing areas must be displayed at a height visible to the mice from within the tank. The digital camera is mounted above the center of the tank (not depicted). - Center the camera directly above the tank facing directly down and high enough above the tank to capture the entire diameter.
- Connect the webcam to the Windows PC situated outside of the testing area.
- Place the platform without flag in the center of the tank.
- Fill tank with water to within 20 cm of the top of the wall.
- Adjust the level of the platform so that it is submerged 1 cm below the water surface.
- Submerge the aquarium heater in the water in the tank and set it to 24 °C and allow at least 24 h to reach the set temperature. Remove heater immediately before behavioral testing and replace after each session.
- Add tempera paint to water until it becomes opaque. To reach opacity, approximately 0.34 g/L paint powder is needed. Opaque water prevents the mice from potentially using submerged objects, such as dropping from past trials or the base of the escape platform, as visual cues to navigate.
- Software setup
- Launch ANY-Maze and under “File” click “New Experiment”. ANY-Maze will then open a new window with the heading “Experiment details”. Under the “Experiment” tab enter experiment name under “Title”, then enter treatment names and the number of animals per treatment in the “Treatments” section.
- Assign animals to each treatment: Under the Tests tab, click on “Treatment codes report”, ANY-Maze assigns a letter to each treatment entered in the “Experiment details” section (e.g., control treatment is assigned letter code A and drug treatment is assigned letter code B). Click on “Animal treatments and data”, and under the “Treatment” column use the drop-down menu to assign a letter code to each animal to a treatment group (e.g., animal number 1 is assigned letter code A, animal number 2 is assigned letter code B etc.). This will ensure that all the animals being used in the experiment have been assigned a treatment group, and can be confirmed by clicking on the “Experiment” tab and looking at the “Treatments” window on the right.
- For protocol setup, click on the “Protocol” tab and following the following steps:
- Click on “Unnamed Protocol” and assign a name to your protocol.
- Click on the “Unnamed Protocol” folder icon to expand all options.
- Click on “Video Sources” and then on “New video source”.
- Select digital camera from the “Source of video images” drop-down menu. This video source can be renamed for use in future experiments by entering a name in the “Video source name” text box.
- After setting up the camera, proceed to configuring the MWM by the following steps:
- Click on “Apparatus” to expand all options, the water tank should be visible on the right side of the screen under the heading “New apparatus”.
- Rename the apparatus to Morris Water Maze in the “Apparatus name” text box.
- Select digital camera from the “Video Source” drop-down menu.
- Select the preferred key on the keyboard for starting and stopping the assay from the “Test control key” drop-down menu. External devices (such as a wireless mouse) can also be used, as long as they are connected to the computer and are visible under the “Test control key” drop-down menu.
- Select None from the “Test Control Switch” drop-down menu if using the keyboard.
- Select the option “If a user ends a test, save results without requesting confirmation”.
- Draw a virtual map onto the MWM tank by the following steps:
- In the window to the right, click the circle icon under the “Image” heading, and draw a circle on top of the MWM water tank such that the edges of the circle perfectly line up to the edge of the water in the tank. This will set the boundary of the MWM apparatus.
- Next, click the line icon under the “Image” heading, and draw a vertical and horizontal line cutting through the middle of the previously drawn circle, such that the circle is split into 4 equal quadrants.
- Place the water maze platform in the center of the target quadrant and draw a circle around the platform using the circle icon under the “Image” heading.
- In order to allow ANY-Maze to measure distances to scale within the MWM apparatus, physically measure any distance in the tank (e.g., diameter of tank, diameter of platform etc.) with a ruler. Record the measured distance in the text box in front of “The length of the ‘ruler line’ is (mm)”. Make sure to position the ‘ruler line’ along the distance that was measured in the tank, so that ANY-Maze can properly assess scale within the apparatus. (The ‘ruler line’ can be found in the bottom left corner of the window labeled “Image”)
- Once the virtual circle and quadrants have been drawn, each quadrant must be assigned to a specific zone as follows:
- Click on “Zones” and enter a name in the “Zone name” text box, then click on the quadrant in the “Image” window that you wish to assign to this zone.
- Repeat the above step for all 3 remaining quadrants and the platform.
- Once all 5 zones (4 quadrants and a platform) have been assigned, click on the first zone to verify the “Zone entry settings”.
- Make sure the check box in front of “Use the center of the animal is checked” is selected.
- Repeat this for all remaining zones.
- In order for ANY-Maze to compute the distance of the animal travels to the platform, a reference point needs to be assigned to the platform within the apparatus as follows:
- Click on “Points” to add a new point in the apparatus. Place point at the center of the platform in the “Image” window to the right. Rename this point to Platform in the “Point name” text box.
- Make sure that the check box in front of “Use the center of the animal when calculating the distance from this point” is selected.
- Adjust the test settings by clicking on the “Test settings and options” then:
- Under “Animal color”, select the “The animals are darker than the apparatus background” option.
- Under “Tracking the animal’s head”, select the “No, I don’t need to track the animal’s head” option.
- Under “Automatic starting of tests”, select the “No, I’ll start the tests manually” option.
- Under “What to record while testing”, select the “Record a maximum of 10 positions/second” option from the “Recording frequency” drop-down menu.
- Under “What to display while testing”, make sure the following options are selected:
- Show the time elapsed (i.e., count up).
- Mark the animal’s center point.
- List any active zones.
- Display the indicators in the top right corner of the image.
- Display the real the time in the bottom right corner of the image.
- When working from video don’t show the video file name.
- Under “Advanced tracking options”, select the “Suppress Water-maze reflections” option.
- To customize the settings for all the trials run during the MWM assay, click on “Stages”, select the “Manually schedule tests” option, then click on “Trial” under “Stages” to customize trial settings as follows:
- Rename “Stage name” to Trial.
- Set “Test Duration” to 90 s.
- Set “Maximum number of trials in this stage” to 4.
- Under “Automatic repetition” of trials, select the “Don’t automatically repeat the trials in this stage (default)” option.
- To configure automatic data analysis click on “Analysis” to expand all options and perform the following:
- Under “Analysis across time”, put 30s in the text box in front of “Specify the duration of the time segments”.
- Under “Heading errors”, select the “The following time after the start of test” option, and put 500 ms in the text box in front of it. Also check the “Calculate heading errors to the centre of zones” option.
- Under “Movement towards and away from the zones and points”, select the “Calculate time moving towards zones based on the entire zone area” option.
- Under “Additional scoring – overlapping key strokes”, select the “Merge overlapping key strokes” option.
- Click on “Results, reports and data” and adjust the following settings for the “Test Schedule report”:
- Under “Animal and test information” these options should be selected:
Test number
Animal number
Stage
Trial number
Apparatus
Test date - Under “Apparatus measures” these options should be selected:
Test duration
Total distance traveled - All “zone measures (excluding platform zone)” should have these options selected:
Time in the zone
Distance traveled in the zone - Under “Platform zone measures” these options should be selected:
Number of entries to the zone
Latency to the first entry to the zone
Initial heading error to the zone
Average heading error to the zone - Under “Field entries”, select the “Animal ID” option
- Under “Platform point measures” these options should be selected:
Average distance from the point
Maximum distance from the point
Minimum distance from the point
- Under “Animal and test information” these options should be selected:
- Adjust the following settings under “Animal and test details reports”:
Under “Apparatus measures” the options below should be selected:
Test duration
Total distance traveled
Average speed - Adjust the following settings under “Test data report”:
- Under “Animal position and activity” select the “The position of the animal” option.
- For all zones listed under “Test data report”, only the “Entries into the zone” option is selected.
- Adjust the following settings under “Data page”:
- Under “Animal and test information” these options should be selected:
Animal number
Treatment
Treatment code
Stage
Trial number
Apparatus
Test date - Under “Apparatus measures” these options should be selected:
Test duration
Total distance traveled
Average speed - All “zone measures” (excluding platform zone) should have these options selected:
Time in the zone
Distance traveled in the zone - Under “Platform point measures” these options should be selected:
Number of entries to the zone
Latency to first entry to the zone - Under “Field entries”, select the “Animal ID” option.
- Under “Platform point measures”, these options should be selected:
Average distance from the point
Maximum distance from the point
Minimum distance from the point
Time moving towards the point
Time moving away from the point
Initial heading error to the point
Average heading error to the point
- Under “Animal and test information” these options should be selected:
- To configure the results from each experiment click on the “Results” tab to perform the following:
- Adjust the following settings under “Text report”:
- Under “Apparatus measures”, select the “Test duration” option.
- Choose “Treatment” from the “On the x-axis show” drop-down menu.
- Choose the “Scatter graph” option from “Select the type of graph to display”.
- Choose Individual points and group means from “Select what the graph should show”.
- Adjust the following settings under “Graph report”:
- Under “Apparatus measures”, select the “Test duration” option.
- Choose “Treatment” from the “On the x-axis show” drop-down menu.
- Choose the “Scatter graph” option from “Select the type of graph to display”.
- Choose Individual points and group means from “Select what the graph should show”.
- Adjust the following settings under “Statistical analysis report”:
- Under “Apparatus measures”, select the “Test duration” option.
- Choose “Treatment” from the “Select the 1st independent variable” drop-down menu.
- Choose “Parametric” from the “Select the type of statistical tests to use” option.
- Choose the following options under “Select the control group to compare to”:1)Include a column for standard deviation.2)Show animal number next to data values.
- The following are settings under “Track plot report”:
- Select “Track of the animal’s center point” from the “Type of plot” drop-down menu.
- Select “Treatment” from the “Group plots by” drop-down menu.
- Adjust the following settings under “Text report”:
- Once all parameters and settings have been configured, save protocol as a file in desired folder. A duplicate of this protocol file can be made for each training day for each MWM experiment and named accordingly.
- Behavioral testing
- Days 1-3: Habituation session
- Day 1: Acclimatize mice to the holding area.
- Place mice in their home cages into the holding area for 30 min.
Mice may be housed in groups up to four per cage or in isolation. The number of mice per cage has little impact on MWM behavior in C57BL/6 mice (Võikar et al., 2005) unless elderly mice are to be used. With elderly mice social isolation may be a confound (Huang et al., 2018) and is not recommended. - Return to normal housing room.
- Place mice in their home cages into the holding area for 30 min.
- Day 2: Acclimatize mice to the intraperitoneal injection procedure and testing area.
- Place mice in their home cages in the holding area.
- Remove one mouse from cage and use one hand to restrain the mouse by firmly grasping the mouse by the scruff using the index finger and thumb while securing the tail with the 3rd and 4th finger.
Note: Always ensure that all procedures with mice are approved by your institutional animal care and use committee. - Mimic an intraperitoneal injection by poking the mouse with a ballpoint pen firmly. Do not depress the skin deeper than 0.5 cm.
- Return the mouse to home cage for 30 min.
- Repeat Steps C1b ii-C1b iv for each mouse.
- Place one mouse on the platform positioned in the center of the maze.1)Immediately after placing the mouse in the maze, vacate the testing area.2)Let the mouse sit on the platform for 15 s.
Note: During the training sessions, it is important for the mouse to take note of the extra-maze visual cues to help establish the spatial location of the platform. If the mouse fails to find the platform during a training trial, by guiding it to the platform and letting it sit there for 15 s will provide an opportunity for the mouse to see the visual cues and better establish its spatial location in the maze. The amount of time varies somewhat between various MWM protocols but 15 s is typically used and generally works well to generate learning and memory (Vorhees and Williams, 2006).3)If the mouse jumps off the platform, guide it back to the platform with your hands to ensure that it experiences sitting on the platform for an uninterrupted duration of 15 s. - Remove the mouse from the maze, dry it well with paper towels and return it to its home cage.
- Repeat Steps C1b vi-C1b vii for each mouse.
- Day 3: Acclimatize mice to the training procedure.
- Bring all mice in their home cages into the holding area and place on a mat that has been heated to 37 °C.
- Place the platform without the flag in the center of the maze in the testing area.
- Remove one mouse from its home cage and restrain by the scruff with one hand.
- Mimic an intraperitoneal injection by poking the mouse with a ballpoint pen firmly.
- Return the mouse to its home cage for 30 min.
- Repeat Steps C1c ii-C1c iv for each mouse.
- To begin the trial, place one mouse in the water on the perimeter of the maze at one randomly chosen cardinal direction (N, E, S, or W) and facing towards the wall of the maze (Figure 2).1)Immediately after placing the mouse in the maze, vacate the testing area.2)Allow the mouse 90 s to find the platform.3)After finding the platform let mouse reside there for 15 s.4)If the mouse does not find the platform after 90 s, or jumps off the platform, guide it back to the platform with your hands and ensure it experiences sitting on the platform for a total duration of 15 s.
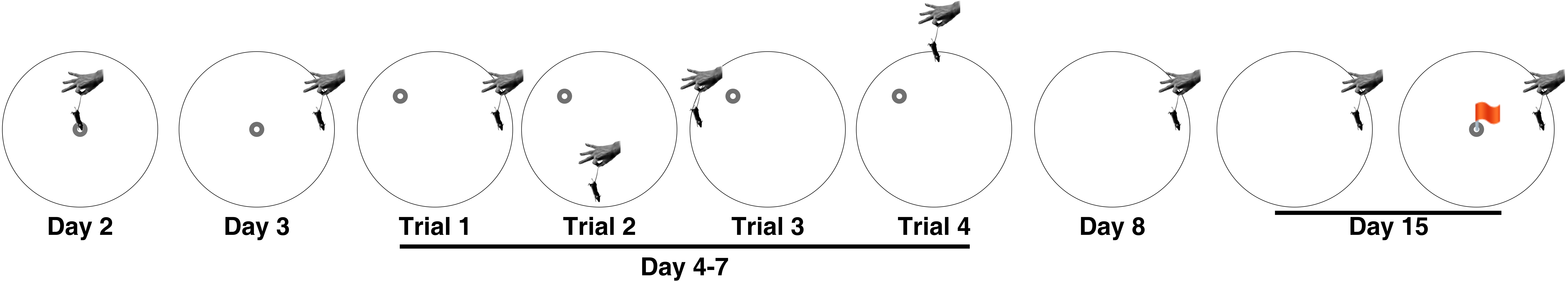
Figure 2. Platform and mouse placement. The starting location of mice beginning each trial is indicated by a hand holding a mouse. During all trials starting on day 3 and onward, mice are to begin facing the wall of the tank. The initial location chosen to start mice on day 3 determines the relative location mice are to begin each trial on subsequent days. The small grey circle indicates the location the submerged platform within the tank. - To end the trial, remove the mouse from the maze, dry it well with paper towels and return to home cage on the heated mat to prevent hypothermia.
- Repeat Steps C1c vii-C1c viii for each mouse. Starting in the same cardinal direction for all mice.
- Day 1: Acclimatize mice to the holding area.
- Days 4-7: Training sessions
- Bring all mice in their home cages into the holding area and place on a mat that has been heated to 37 °C.
- Place the platform without the flag in center of one of the quadrants of the maze, equidistant from the wall and the pool center.
Note: In this protocol, we place the platform in the same quadrant for every trial during the training session. Alternatively, one-quarter of the animals can be tested with the platform in each of the quadrants. The latter approach counterbalances for possible bias in finding the target quadrant. - Remove one mouse from home cage and restrain by the scruff with one hand.
- Spatial memory acquisition injection regime:
- For testing impact of drug treatment on spatial memory acquisition, inject drug or vehicle intraperitoneally into the mouse (Figure 3).
- For testing impact of drug treatment on spatial memory consolidation or retrieval, mimic an intraperitoneal injection by poking the mouse with a ballpoint pen firmly.
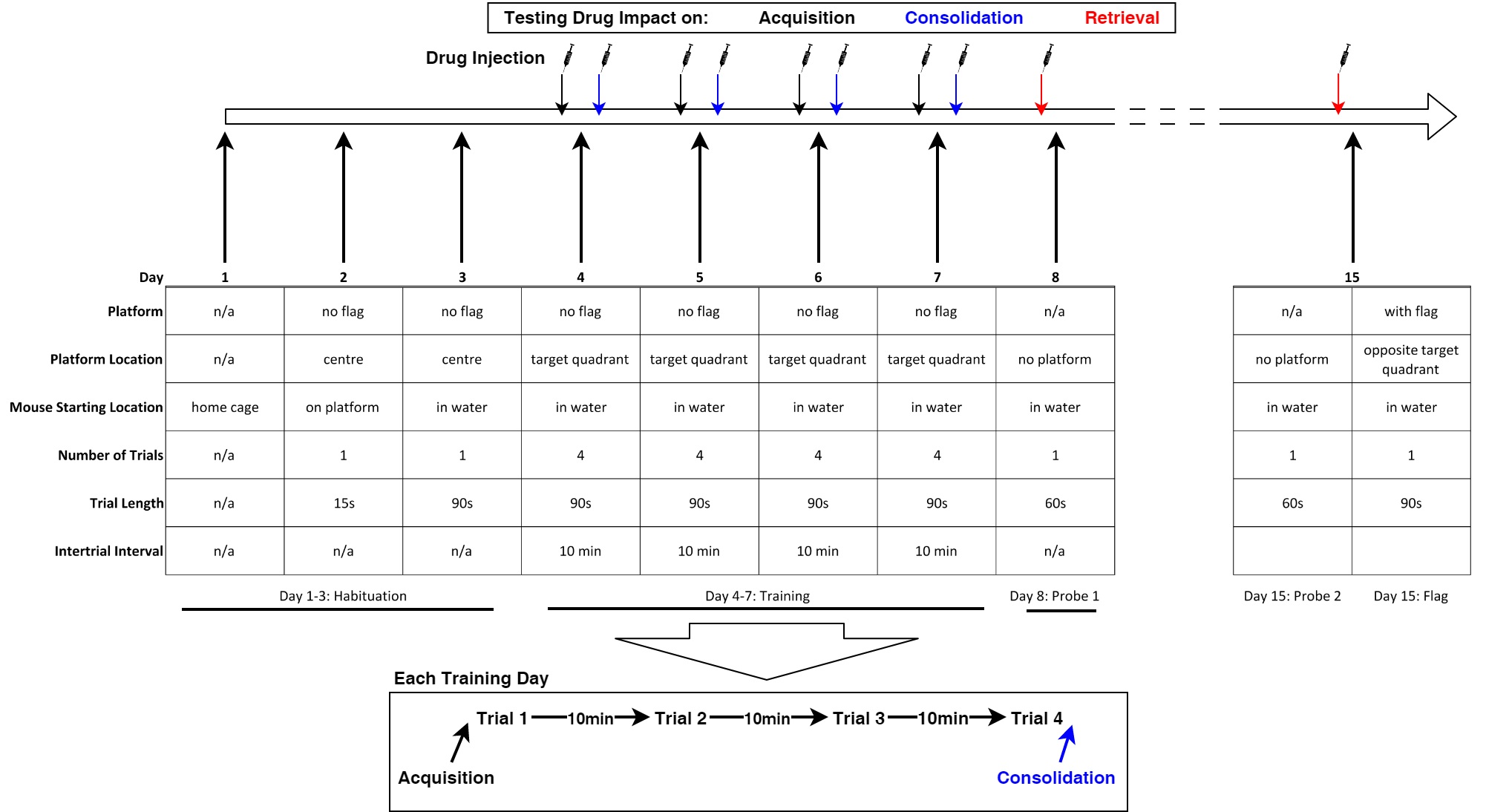
Figure 3. Behavioral testing timeline. Injection regime for testing the effect of drug on either memory acquisition, consolidation or retrieval separately. Arrows of indicated colors specify when drug injections are to occur. When testing drug impact on acquisition or consolidation, mock injections with a ballpoint pen poke mimicking an intraperitoneal injection occur prior to probe 1, probe 2 and the flag trials. When testing drug impact on retrieval, mock injections occur prior to each day of training. For all testing conditions, mock injections occur during habituation.
- Return the mouse to home cage for 30 min.
- Repeat Steps C2c-C2e for each mouse.
When performing an experiment, limit the number of mice used to an amount which can realistically be injected within a 30 min timespan. This prevents mice from having longer than 30 min between injection in Step C2d. and the beginning of training trials in Step C2h. - Launch ANY-Maze software and open previously configured protocol. Make sure that the video source (camera) is working. The MWM should appear in the window on the right.
- Click on the “Test” tab.
- Click on “Test Schedule Report”. All trial runs for each mouse should be visible in the MWM experiment in the left window.
- To begin the training trial, place mouse in the tank at the same cardinal direction used during the habituation session and facing the pool wall.
- Immediately after placing the mouse in the maze, press “Play” as soon as the mouse is placed into the water tank to start the timer and activate video recording in ANY-Maze. Vacate the testing area in order to ensure that the experimenter is not visible to the animal.
- Press “Stop” to stop the timer once the mouse has found the platform in the water tank. If the mouse cannot locate the platform during the allotted time of the trial, the software will automatically stop the timer once the allotted time has been reached.
- If the mouse has not found the platform after 90 s has elapsed, gently guide the mouse to the platform and ensure it experiences sitting on the platform for a total duration of 15 s.
- At the end of each trial, remove the mouse from the maze, dry it well with paper towels and return to home cage on the heated mat to prevent hypothermia.
- Repeat Steps C2h-C2i for each mouse four times.
- Ensure there is a 10 min inter-trial interval for each mouse.
- Start all mice in the same cardinal direction as the first training trial for that day.
- On subsequent trial days, start each mouse in the cardinal direction that is one quarter turn clockwise from the starting position of the previous trial day.
- Memory consolidation injection regime:
- For testing impact of drug treatment on spatial memory consolidation, intraperitoneally inject drug or vehicle immediately after the last training session for each day.
- For testing impact of drug treatment on spatial learning or memory retrieval, mimic an intraperitoneal injection by poking each mouse with a ballpoint pen firmly.
- Repeat Steps C2a-C2j on each subsequent day of training.
Note: For each training day, change the starting direction for the first trial to the cardinal direction that is one quarter turn clockwise from the previous day.
- Day 8: Probe trial for 24 h long-term memory
- Bring all mice in home cages into the holding area and place on a mat that has been heated to 37 °C.
- Remove the platform from the maze in the testing area.
- Remove one mouse from home cage and restrain by the scruff with one hand.
- Memory retrieval injection regime:
- For testing impact of drug treatment on memory retrieval, intraperitoneally inject drug or vehicle before the probe trial.
- For testing impact of drug treatment on spatial learning or memory consolidation, mimic an intraperitoneal injection by poking the mouse with a ballpoint pen firmly.
- Return the mouse to its home cage for 30 min.
- Repeat Steps C3c-C3e for each mouse.
- Launch ANY-Maze and open saved protocol.
- To begin the probe trial, place mouse in the tank at the same cardinal direction used during the habituation session and facing the pool wall.
- Immediately after placing the mouse in the maze, Press “Play” in ANY-Maze and vacate the testing area in order to ensure that the experimenter is not visible to the animal.
- Allow the mouse 60 s to explore the maze.
- ANY-Maze automatically stops recording after the mouse reaches the platform and records the time. If the mouse does not find the platform, the time is recorded as 60 s.
- To end the probe trial, remove the mouse from the maze, dry it well with paper towels and return to home cage on the heated mat to prevent hypothermia.
- Day 15: Probe trial for 7-day long-term memory and flag trial for testing visuospatial navigation
- Repeat Step C3 in an identical manner.
- After all mice have completed the second probe trial, place the platform with the flag in the maze in the center of the quadrant opposite the target quadrant used during the training and probe trials.
Note: Ensure the flag is visible above the surface of the water. - To begin the flag trial, place mouse in the tank at the same cardinal direction used during the habituation session, facing the pool wall and away from the flag.
- Immediately after placing the mouse in the maze, activate the video recording in ANY-maze and vacate the testing area.
- Allow the mouse 90 s to find the platform.
- Once 90 s has elapsed or the mouse has found the platform, end the trial.
- To end the trial, remove the mouse from the maze, dry it well with paper towels and return to the home cage on the heated mat to prevent hypothermia.
- Days 1-3: Habituation session
Data analysis
Visual data acquired by ANY-Maze during the training and probe trials are automatically processed and can provide information on a number of parameters. The averages +/- standard error for latency to find platform or path length for each trial should be plotted for each training day (Figure 4) and a two-way ANOVA with repeated measures can be used to detect the effect of the drug treatment, as well as the learning curve over the course of training days. A lack of decreased escape latency or path length to find the platform during training is indicative of compromised memory acquisition or consolidation. Average swimming speed should be measured over training as a control to ensure that the test drug has no adverse effects on locomotor ability.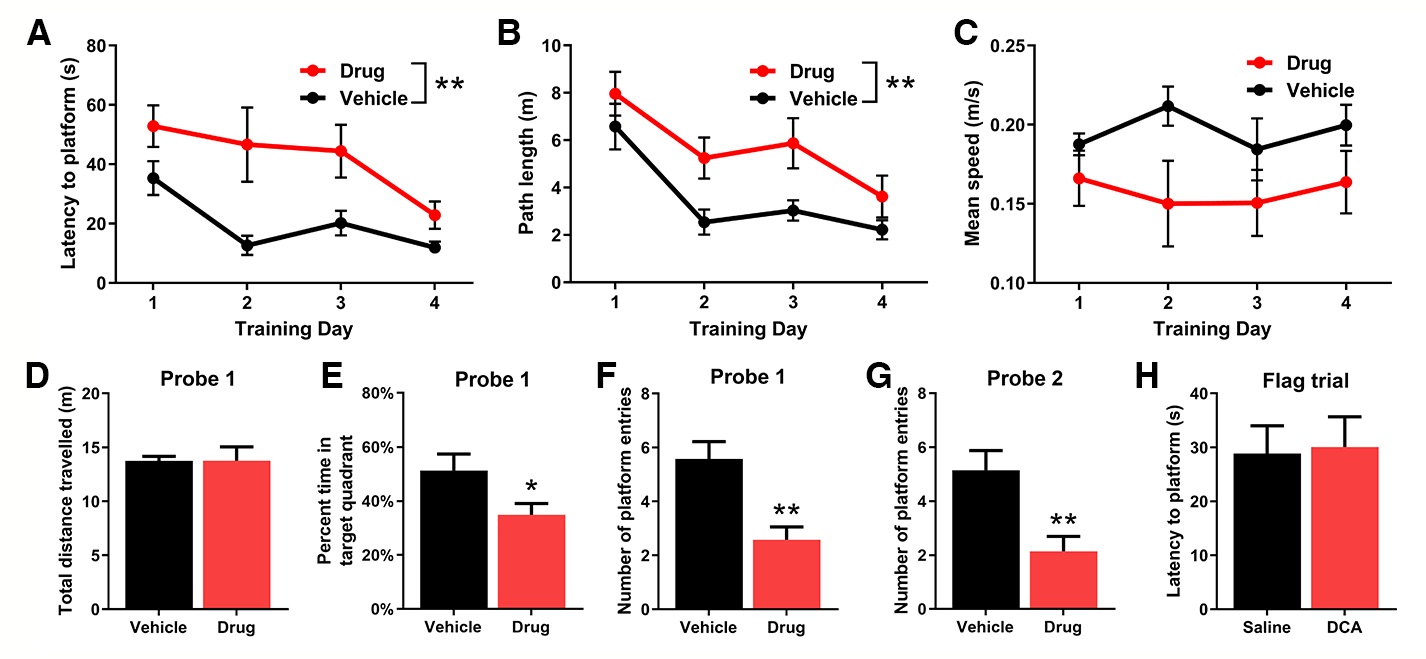
Figure 4. Representative Data for drug-mediated effects on memory acquisition. Average latency to find the platform (A), path length (B) and mean speed (C) for mice across all four trials for each training day. Total distance traveled (D) and percent time spent in target quadrant (E) for mice on Probe 1. The number of times mice enter the area where the platform was previously located during training for probe 1 (F) and probe 2 (G). Latency for mice to find the flagged platform on the final flag trial (H). Data is presented as mean and SEM (n = 7). No less than seven mice are recommended for each group. However, a larger number of mice may be required in order to detect statistically significant differences between drug and vehicle treated mice if the effect is small. Example data is provided demonstrating a drug showing impact on memory acquisition. Graphs of the same format are to be produced separately for tests of drug impact on memory consolidation and retrieval as well. Injection with drugs showing an impact on either memory consolidation or retrieval is not expected to result in significant differences in latency to find the platform and path length, as seen in (A) and (B) respectively, but is expected to result in reductions of percent time in target quadrant, as seen in (E), or the number of platform entries, as seen in (F) and (G) during probe trials.
For the probe trial the average percent time spent in the target quadrant or number of platform entries should be plotted and an unpaired t-test can be used to detect differences between drug and vehicle treatment. A decrease in time spent in the target quadrant or the number of platform entries is indicative of compromised memory retrieval. The total distance traveled or average swimming speed over the course of the probe trial can be measured as a control to ensure that the test drug has no effect on locomotor ability. Finally, the latency for mice to find the flagged platform on the final flag trial should also be plotted. For training phases and probe trials, a Bonferroni multiple comparisons correction for significance threshold should be implemented depending on how many measures are used to evaluate the effect of the drug on spatial memory. For example, if both the average percent time spent in the target quadrant and the number of platform entries are used to evaluate spatial memory during the probe trial (two independent hypotheses to be tested), then the significance threshold of α < 0.05 should be divided by 2 to yield a corrected threshold of α < 0.025. Statistical analysis can be performed using RStudio v0.97.551.
Notes
- Drugs that interfere with memory acquisition and consolidation will result in a longer latency to find the platform and a longer distance traveled compared to vehicle-injected mice. However, it is important that no differences in mean swimming speed during training trials and total distance traveled during the probe tests are observed between drug and vehicle treatments as this would indicate that the drug may affect swimming performance or motivation to find the platform, possibly confounding the analysis. In addition, to ensure that the drug has no adverse effect on visual acuity, no significant difference in the ability to find the flagged platform should be observed between vehicle and drug-treated mice.
- Occasionally some animals are unmotivated to escape and simply float. In this case, the mouse should remain in the MWM until the end of the trial. If it floats again on the second trial, then it should be returned to its cage and tested again the next day. If the animal now swims on the subsequent day of testing, then testing should be continued with the mouse but will be staggered one day behind the other animals in the testing schedule. If the animal fails to swim on the second day, then it should be excluded from further testing. In addition, some mice initially tend to swim along the wall of the pool (thigmotaxic or wall-hugging swimming). This behavior is generally reflective of anxiety and stress, although the extensive habituation in this protocol tends to reduce anxiety in mice. Animals that persist with a thigmotaxic strategy during the training trials and probe test cannot be excluded unless they fail to find the platform during the flag trial at the end of the assay. Anxiety effects can be measured explicitly using additional tests such as the elevated plus maze, elevated zero maze, light/dark test, or open field test.
- It has been suggested that the multiple training sessions used in the MWM over 4 days may result in memory traces that are reactivated and reconsolidated during each learning session, possibly obscuring events associated with the initial consolidation of a spatial memory (Florian and Roullet, 2004). An alternative one day massed-training protocol has been developed whereby mice are submitted to four consecutive trial sessions within a 70-90 min period followed by a probe trial 24 h later (Florian and Roullet, 2004; Stein et al., 2014; Villain et al., 2016). The one day protocol has the advantage that it can expose the effects of drug treatment on early consolidation after the training sessions without the complication of overlapping reconsolidation processes during multiday training protocols (Stein et al., 2014). However, potential problems with using the massed training include poorer establishment of a memory trace and the potential of hypothermic effects. Intrahippocampal injection of a protein kinase C (PKC) chemical inhibitor in rats revealed impaired spatial memory consolidation using either the one day massed or multi-day training protocols of the MWM (Bonini et al., 2007). Thus injection of drugs in the post-training phase using the multiday protocol can discern effects of the drug on the consolidation process, with the caveat that reconsolidation processes may also be affected.
- Some cellular aspects of memory consolidation may take place within 30 min of the initial exposure to training (Runyan et al., 2019). Since injection of a drug to assess for effects on memory consolidation occurs at the end of each training session (~40 min in duration), some of the early stages of consolidation may not be fully affected using this protocol. However, the effect of a drug on consolidation, using a massed training protocol for the MWM, has been noted 70 minutes after training (Villian et al., 2016).
- Mice are more susceptible to hyperthermia in the MWM than rats (Iivonen et al., 2003). The risk of hyperthermia is mitigated by ensuring the water temperature is maintained at 24 °C throughout the protocol, thoroughly drying mice after removal from the water, keeping home cages warm on heating mats, and ensuring inter-trial intervals are no less than 10 min. This is of particular importance if using aged or transgenic mice.
Recipes
- Sterile saline solution (0.9% w/v NaCl)
Dissolve 9 g of NaCl (Sigma-Aldrich) and filter sterilizing using a 0.2 µm filter unit (Thermo Scientific)
Acknowledgments
This research was supported by Natural Sciences and Engineering Research Council of Canada Grant 355803-2013 and Canadian Consortium on Neurodegeneration in Aging Grant 137794. This protocol was adapted from previous work (Harris et al., 2019).
Competing interests
The authors declare no competing interests.
Ethics
All animal procedures were performed in compliance with the Canadian Council on Animal Care guidelines under an animal protocol (2011-079) approved by the University of Western Ontario animal care committee.
References
- Abel, T. and Lattal, K. M. (2001). Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11(2): 180-187.
- Barnhart, C. D., Yang, D. and Lein, P. J. (2015). Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One 10(4): e0124521.
- Bast, T., da Silva, B. M. and Morris, R. G. (2005). Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci 25(25): 5845-5856.
- Ben-Yakov, A., Dudai, Y. and Mayford, M. R. (2015). Memory Retrieval in Mice and Men. Cold Spring Harb Perspect Biol 7(12).
- Bonini, J. S., Da Silva, W. C., Bevilaqua, L. R., Medina, J. H., Izquierdo, I. and Cammarota, M. (2007). On the participation of hippocampal PKC in acquisition, consolidation and reconsolidation of spatial memory. Neuroscience 147(1): 37-45.
- Broadbent, N. J., Squire, L. R. and Clark, R. E. (2004). Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101(40): 14515-14520.
- Bromley-Brits, K., Deng, Y. and Song, W. (2011). Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp (53). Doi: 10.3791/2920.
- Choi, S. H., Woodlee, M. T., Hong, J. J. and Schallert, T. (2006). A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods 156(1-2): 182-193.
- Da, S. and Takahashi, R. N. (2002). SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry 26(2): 321-325.
- De Coninck, M., Van Dam, D., Van Ginneken, C. and De Deyn, P. P. (2017). Adapted Morris Water Maze protocol to prevent interference from confounding motor deficits on cognitive functioning. Somatosens Mot Res 34(3): 172-178.
- D'Hooge, R. and De Deyn, P. P. (2001). Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36(1): 60-90.
- Dudai, Y. (2004). The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55: 51-86.
- Florian, C. and Roullet, P. (2004). Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behav Brain Res 154(2): 365-374.
- Harris, R. A., Lone, A., Lim, H., Martinez, F., Frame, A. K., Scholl, T. J. and Cumming, R. C. (2019). Aerobic glycolysis is required for spatial memory acquisition but not memory retrieval in mice. eNeuro 6(1). Doi: 10.1523/ENEURO.0389-18.2019.
- Hou, Y., Wu, C. F., Yang, J. Y. and Guo, T. (2006). Differential effects of haloperidol, clozapine and olanzapine on learning and memory functions in mice. Prog Neuropsychopharmacol Biol Psychiatry 30(8): 1486-1495.
- Huang H, Wang L, Cao M, Marshall C, Gao J, Xiao N, Hu G and Xiao M. (2015). Isolation Housing Exacerbates Alzheimer’s Disease-Like Pathophysiology in Aged APP/PS1 Mice. Int J Neuropsychopharmacol. 18(7): pyu116.
- Iivonen H, Nurminen L, Harri M, Tanila H and Puoliväli J. (2003). Hypothermia in mice tested in Morris water maze. Behav Brain Res. 141(2): 207-213.
- Kallai, J., Makany, T., Karadi, K. and Jacobs, W. J. (2005). Spatial orientation strategies in Morris-type virtual water task for humans. Behav Brain Res 159(2): 187-196.
- Morris, R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1): 47-60.
- Runyan, J. D, Moore, A. N and Dash, P. K. (2019). Coordinating what we’ve learned about memory consolidation: Revisiting a unified theory. Neurosci Biobehav Rev 100: 77-84.
- Stein, I. S., Donaldson, M. S. and Hell, J. W. (2014). CaMKII binding to GluN2B is important for massed spatial learning in the Morris water maze. F1000Res 3: 193.
- Villain, H., Florian, C. and Roullet, P. (2016). HDAC inhibition promotes both initial consolidation and reconsolidation of spatial memory in mice. Sci Rep 6: 27015.
- Võikar V, Polus A, Vasar E, Rauvala H. (2005). Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 4(4):240-252.
- Vorhees, C. V. and Williams, M. T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2): 848-858.
- Weitzner, D. S., Engler-Chiurazzi, E. B., Kotilinek, L. A., Ashe, K. H. and Reed, M. N. (2015). Morris water maze test: Optimization for mouse strain and testing environment. J Vis Exp (100): e52706.
- Wenk, G. L. (2004). Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci Chapter 8: Unit 8 5A.
- Zhong, J. Y., Magnusson, K. R., Swarts, M. E., Clendinen, C. A., Reynolds, N. C. and Moffat, S. D. (2017). The application of a rodent-based Morris water maze (MWM) protocol to an investigation of age-related differences in human spatial learning. Behav Neurosci 131(6): 470-482.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Frame, A. K., Lone, A., Harris, R. A. and Cumming, R. C. (2019). Simple Protocol for Distinguishing Drug-induced Effects on Spatial Memory Acquisition, Consolidation and Retrieval in Mice Using the Morris Water Maze. Bio-protocol 9(18): e3376. DOI: 10.21769/BioProtoc.3376.
- Harris, R. A., Lone, A., Lim, H., Martinez, F., Frame, A. K., Scholl, T. J. and Cumming, R. C. (2019). Aerobic glycolysis is required for spatial memory acquisition but not memory retrieval in mice. eNeuro 6(1). Doi: 10.1523/ENEURO.0389-18.2019.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










