- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Technique for Imaging and Analysis of Hair Cells in the Organ of Corti Using Modified Sca/eS and Machine Learning
Published: Vol 9, Iss 16, Aug 20, 2019 DOI: 10.21769/BioProtoc.3342 Views: 6658
Reviewed by: Zinan ZhouSvetlana KurilovaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
Catalina Matias [...] Jeffrey J. Brault
Apr 5, 2025 2900 Views

A Novel Optimized Silver Nitrate Staining Method for Visualizing and Quantifying the Osteocyte Lacuno-Canalicular System (LCS)
Jinlian Wu [...] Libo Wang
Apr 20, 2025 1518 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 483 Views
Abstract
Here, we describe a sorbitol-based optical clearing method, called modified Sca/eS that can be used to image all hair cells (HCs) in the mouse cochlea. This modification of Sca/eS is defined by three steps: decalcification, de-lipidation, and refractive index matching, which can all be completed within 72 h. Furthermore, we established automated analysis programs that perform machine learning-based pattern recognition. These programs generate 1) a linearized image of HCs, 2) the coordinates of HCs, 3) a holocochleogram, and 4) clusters of HC loss. In summary, a novel approach that integrates modified Sca/eS and programs based on machine learning facilitates quantitative and comprehensive analysis of the physiological and pathological properties of all HCs.
Keywords: CochleaBackground
Sound waves reach the inner ear via conduction mechanisms in the external and middle ear. The cochlea is an organ in the inner ear that transduces mechanical stimulation into electronic signals. As a result, generated auditory information is transferred to the brain. Hair cells (HCs) in the organ of Corti, an auditory sensory epithelium, are protected by the temporal bone and are precisely disposed to detect every frequency band from the base (high pitch) to the apex (low pitch) (von Békésy, 1990). Functional analysis of the cochlea is limited due to its anatomical specificity. Surface preparation and sectioning (paraffin or frozen sections) are the most popular methods in histological analysis; however, these techniques do not preserve the three-dimensional structure (Fujimoto et al., 2017; Mizushima et al., 2017). To better understand the processing of auditory information, the location and diversity of HCs must be accurately determined. Consequently, a novel technique to identify intact HCs has long been sought. Established optical clearing methods such as CLARITY, 3DISCO, Sca/eS, iDISCO, and CUBIC (Dodt et al., 2007; Chung et al., 2013; Renier et al., 2014; Susaki et al., 2014; Hama et al., 2015) enable imaging of intact brains and other organs, but are not optimized for imaging of all HCs (Urata et al., 2019). Based on recently developed optical clearing methods for hard tissues such as vDISCO, Bone CLARITY, PEGASOS, CUBIC-X, PACT, and PARS (Calve et al., 2015; Treweek et al., 2015; Berke et al., 2016; Greenbaum et al., 2017; Cai et al., 2018; Jing et al., 2018; Tainaka et al., 2018), we present a sorbitol-based optical clearing method (modified Sca/eS) that is optimized for intact imaging of all mouse HCs in the cochlea (Urata et al., 2019).
By acquiring successive images of HCs using modified Sca/eS, we established a novel algorithm for comprehensive analysis of HCs. These programs are available on GitHub (https://github.com/okabe-lab/cochlea-analyzer.git). Use of modified Sca/eS in combination with these programs facilitates quantitative and comprehensive analysis of the physiological and pathological properties of all HCs.
Materials and Reagents
- Blu Tack (Bostik, catalog number: 371351)
- Glass microscope slide (Matsunami, catalog number: S024410)
- Glass microscope coverslip (Matsunami, catalog number: C018181)
- Basukoku (Cemedine; silicone-based adhesive, catalog number: HJ-148)
- Alexa Fluor 488-conjugated goat anti-rabbit IgG (H+L) (Life Technologies, catalog number: 1937195)
- 4% paraformaldehyde diluted in phosphate-buffered saline (PBS) (Wako, catalog number: 163-20145)
- Triton X-100 (Nacalai-Tesque, catalog number: 12967-45)
- Urea (SIGMA, catalog number: U0631-1KG)
- Guanidine hydrochloride (Nacalai-Tesque, catalog number: 17318-82)
- D-sorbitol (SIGMA, catalog number: S1816-1KG)
- D-glucose (SIGMA, catalog number: G8270-100G)
- Rabbit polyclonal anti-myosin VIIa antibody (Proteus Biosciences, catalog number: 25-6790)
- 10% EDTA-2Na (Muto Pure Chemicals, catalog number: 85653)
- NaCl
- KCl
- Na2HPO4·12H2O
- KH2PO4
- Solution 1 (see Recipes)
- Solution 2 (see Recipes)
- PBS (see Recipes)
Equipment
- Dumont tweezers, straight (World Precision Instruments, catalog number: 500233)
- Dumont tweezers, straight (World Precision Instruments, catalog number: 14098)
- Stereoscopic microscope (for microdissection), e.g., SMZ-2B (Nikon)
- Fluorescence microscope
Confocal microscope (for imaging at a depth of up to 300-500 μm), e.g., A1MP confocal microscope (Nikon)
Multiphoton microscope (recommended for deeper imaging), e.g., A1MP two-photon microscope (Nikon) - Objective lenses (long working distance objectives), e.g., N25X-APO-MP (NA1.10, WD2.00) for multiphoton imaging and VC 20x (NA0.75, WD1.00) for confocal imaging
- Water bath at 37 °C
- Fridge at 4 °C
Software
- Microsoft Excel (Microsoft)
- MATLAB (MathWorks, version R2017b)
Image Processing Toolbox
Statistics and Machine Learning Toolbox
Neural Network Toolbox
Procedure
- Modified Sca/eS protocol (see Figure 1)
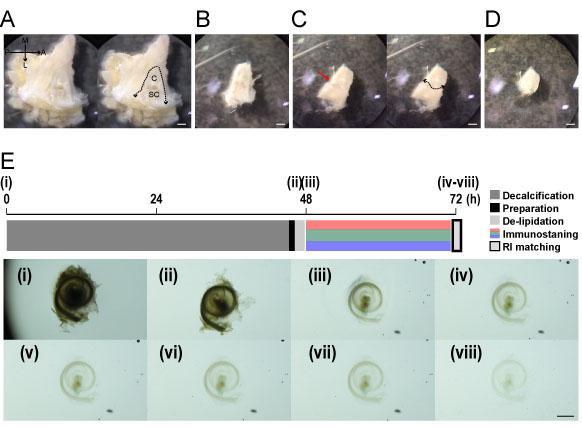
Figure 1. Time course of modified Sca/eS. The inner ear embedded in the temporal bone was removed (panel A) and dissected along the fistula (B) (dotted lines in the left of panel A). The cochlea was separated (dotted line in panel C) from the semicircular canals along the fissure (red arrow in panel C). The cochlea was removed [panel D and (i)] and decalcified (ii). The sample was cleared immediately after being submerged in solution 1 (iii). The sample was then submerged in solution 2 and its transparency gradually increased (iv-viii). A: anterior, P: posterior, M: medial, L: lateral, C: cochlea, SC: semicircular canal, RI: refractive index. Scale bar: 1 mm.- Extraction of the cochlea from the temporal bone
- Fix the cochlea in 4% paraformaldehyde prepared in PBS overnight at 4 °C with gentle shaking.
- Wash the cochlea three times (15 min each) with PBS.
- Incubate the samples in 500 mM EDTA prepared in PBS for 48-120 h at 37 °C.
Note: The incubation duration depends on the sample size. For example, 48 h for pups, 72 h for young (2-week-old) mice, and 120 h for middle-age (6-month-old) mice. - Wash the cochlea three times (15 min each) with PBS.
- Remove excess bone tissue surrounding the cochlea.
- Remove the vestibule and semicircular canal.
- Tissue de-lipidation (see Figure 1 and Solution 1 in Recipes)
Incubate the samples in Solution 1 for 2 h at 37 °C. - Antibody staining (see Figure 1)
- Wash the samples with PBS containing 0.1% Triton X-100 for 30 min with continuous rocking at 40 rpm.
- Incubate the samples in a solution containing the appropriate dilutions of primary antibodies for 2-48 h at 37 °C.
- Wash the samples three times (30 min each) with PBS containing 0.1% Triton X-100 with continuous rocking at 40 rpm.
- Detect primary antibodies by incubating the samples with a solution containing secondary antibodies for 12-48 h at 37 °C followed by three washes with PBS containing 0.1% Triton X-100.
- Sample preparation for imaging (see Figures 2 and 3)
- Roll up a piece of Blu Tack into a cylindrical shape that is slightly thicker than the cochlea [Figure 2 (i)].
- Align Basukoku in a horseshoe shape on the glass slide [Figure 2 (ii)].
- Place the Blu Tack on the Basukoku [Figure 2 (iii, iv)].
- Place a drop of Basukoku inside the Blu Tack horseshoe to make a pedestal for the cochlea [Figure 2 (v)].
- Carefully place the cochlea on the pedestal [Figure 2 (vi)].
Note: Position the cochlea so that the axis of the center of the cochlea is aligned perpendicular to the glass slide (Figure 2). - Place the cover glass on the surface of the Blu Tack and gently push it toward the surface of the cochlea [Figure 2 (vii)].
Note: Keep pushing the cover glass until it reaches the surface of the cochlea. This process is critical to image the whole cochlea [Figure 2 (vii-a)]. - With the horseshoe opening facing upward, add 100 μl of solution 2 (see Recipes) to the gap within the horseshoe until the imaging chamber is filled [Figure 2 (viii)].
Note: Images iv-viii in Figure 1 show how transparent the cochlea becomes after 20 min (images were acquired at increments of 5 min). - Fill the gap between the Blu Tack with Basukoku to seal the horseshoe opening [Figure 2 (ix)].
Note: Air bubbles should not remain in the chamber; this would indicate potential leaking of solution 2 and cause the sample to dry out.
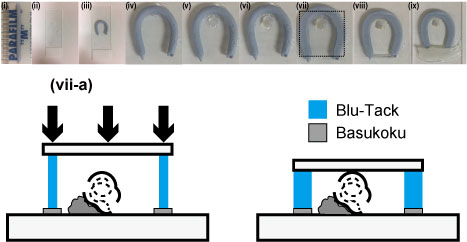
Figure 2. Sample preparation for the imaging step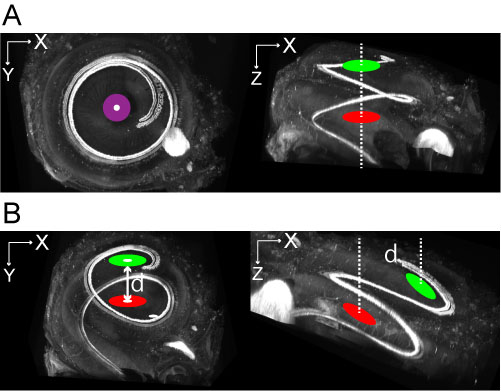
Figure 3. Cochlea position. The center of the first (green) and second (red) turns of the cochlea. Images on the left are the ideal position for imaging of HCs in the whole cochlea, where d represents the distance between the centers of the green and red areas. Upper panels (A) show all HCs imaged using a two-photon microscope in a successful case. Lower panels (B) are representative cases where the approach failed. Images on the left are from the top view, while images on the right are from the side view. The modiolus of the cochlea should be perpendicular to the slide glass [i.e., centers should overlap (purple)]. - Imaging of the sample
- Place one drop of distilled water on the cover glass and then place the objective lens against the cover glass.
Note: Use an appropriate immersion media for high-resolution imaging. Water-immersion lenses perform much better than air-immersion objective lenses. - To image the whole cochlea, images should be successively acquired with 10-40% overlap [Figure 2 (i)].
- See example images of sample processed with previous well known clearing methods (Figure 4).
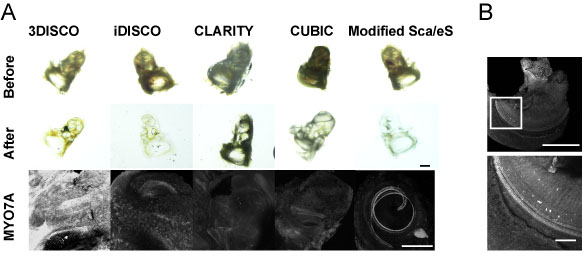
Figure 4. Comparison with other clearing methods. MYO7A-immunopositive HCs in samples were not detected by 3DISCO, iDISCO, CLARITY, or CUBIC (A). Microdissection of the membrane labyrinth of the iDISCO-processed samples in panel A confirmed the presence of MYO7A-immunopositive HCs, suggesting that the surrounding bone tissue prevented the detection of fluorescence (B). Scale bars: 500 µm in panel A and upper image of panel B, 100 µm in lower image of panel B. - Place one drop of distilled water on the cover glass and then place the objective lens against the cover glass.
- Extraction of the cochlea from the temporal bone
- Automated HC analysis protocol based on machine learning
HCs were automatically detected and analyzed using custom MATLAB scripts (R2017b, MathWorks), the details of which were provided in a previous study (Urata et al., 2019). The MATLAB source code is available on GitHub (https://github.com/okabe-lab/cochlea-analyzer.git). Data analysis procedures including statistical analysis were described in the original publication of the protocol (Urata et al., 2019). The actual running of the program was shown in this protocol paper.- The main0.m program (1 min) checks the integrity of the software so that automated analysis can be flawlessly performed (see Video 1).
Note: If an error occurs during the process, double check to see if any required files are absent under “Function Files” on GitHub (https://github.com/okabe-lab/cochlea-analyzer.git).Video 1. Checking the integrity of the software before performing automated analysis using main0.m - The main1.m program merges all the files together to create a linearized image of HCs, which takes about 15 min in total (Step B2 in Figure 5 and see Video 2).
Note: If the main0.m program completes without an error, it is guaranteed to function properly. A TIFF image is produced in this step.Video 2. Merging of all the acquired files together to create a linearized image of HCs using main1.m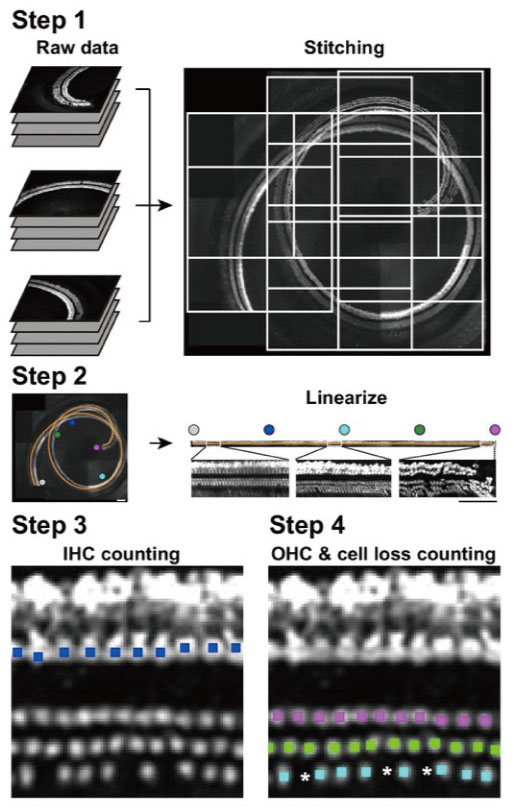
Figure 5. Image generation by the programs. Raw images are stitched (Step 1), and the lines between inner and outer HCs are illustrated (orange line in Step 2). Finally, HCs are linearized and representative images of the apical, middle, and basal portions are enlarged. Colored dots in Step 3 and Step 4 indicate the presence of HCs, and asterisks represent loss of HCs. - The main2.m (inner HCs, duration 5 min, see Video 3) and main3.m (outer HCs, duration 3 min, see Video 4) programs detect 169 HCs.
Notes:- An EXCEL file containing the coordinates of recorded (or imaged) HCs is generated in this step.
- Advanced analysis is available using the main4-6.m programs. The main4.m program analyzes (< 1 min) the spatial distribution of outer HCs and the main5.m program generates (< 1 min) a holocochleogram (see Video 5). The main6.m program analyzes (10 min) clusters of HC loss (see Video 6).
Video 3. Imaging of inner HCs using the main2.m programVideo 4. Imaging of outer HCs using the main3.m programVideo 5. Analysis of the spatial distribution of outer HCs using the main4.m program and generation of a holocochleogram using the main5.m programVideo 6. Analysis of clustered HC loss using the main6.m program
- The main0.m program (1 min) checks the integrity of the software so that automated analysis can be flawlessly performed (see Video 1).
Recipes
- Solution 1 (20 ml)
5.7 g of guanidinium chloride
7 g of D-sorbitol
3 g of D-glucose
800 µl (w/v) of Triton X-100 in PBS (pH 6.0-8.0) - Solution 2 (20 ml)
5.7 g of guanidinium chloride (or 4.8 g of urea)
12 g of D-sorbitol
20 µl of Triton X-100 in PBS (pH 7.1)
Note: The solution can be kept at room temperature for up to 2 months for good clearing. - PBS
80 g of NaCl
2 g of KCl
29 g of Na2HPO4·12H2O
2 g of KH2PO4
Add distilled H2O to prepare 1 L of 10x PBS
Dilute 10-fold with distilled H2O to prepare 1x PBS
Acknowledgments
We thank Dr. Shigeo Okabe for helpful suggestions and supervision. This method was adapted from our original publication of the protocol (Urata et al., 2019). This work was supported by grants from the Japan Society for the Promotion of Science KAKENHI (15K10743, 2653081, and 16K15717), the Japan Science and Technology Agency (JPMJCR14W2), the Japan Agency for Medical Research and Development (17gm5010003), the Japan Society for the Promotion of Science (17H01387), the UTokyo Center for Integrative Science of Human Behavior, and the Ministry of Education, Culture, Sports, Science, and Technology (18H04727 and 26111506).
Competing interests
The authors have no conflicts of interest to declare.
References
- Berke, I. M., Miola, J. P., David, M. A., Smith, M. K. and Price, C. (2016). Seeing through musculoskeletal tissues: improving in situ imaging of bone and the lacunar canalicular system through optical clearing. PloS One 11(3): e0150268.
- Cai, R., Pan, C., Ghasemigharagoz. A., Todorov, M. I., Foerstera, B., Zhao, S., Bhatia, H. S., Mrowka, L., Theodorou, D., Rempfler, M., Xavier, A., Kress, B. T., Benakis, C., Liesz, A., Menze, B., Kerschensteiner, M., Nedergaard, M. and Erturk, A. (2018). Panoptic vDISCO imaging reveals neuronal connectivity, remote trauma effects and meningeal vessels in intact transparent mice. BioRxiv. doi:10.1101/374785.
- Calve, S., Ready, A., Huppenbauer, C., Main, R. and Neu, C. P. (2015). Optical clearing in dense connective tissues to visualize cellular connectivity in situ. PLoS One 10(1): e0116662.
- Chung, K., Wallace, J., Kim, S. Y., Kalyanasundaram, S., Andalman, A. S., Davidson, T. J., Mirzabekov, J. J., Zalocusky, K. A., Mattis, J., Denisin, A. K., Pak, S., Bernstein, H., Ramakrishnan, C., Grosenick, L., Gradinaru, V. and Deisseroth, K. (2013). Structural and molecular interrogation of intact biological systems. Nature 497(7449): 332-337.
- Dodt, H. U., Leischner, U., Schierloh, A., Jahrling, N., Mauch, C. P., Deininger, K., Deussing, J. M., Eder, M., Zieglgansberger, W. and Becker, K. (2007). Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods 4(4): 331-336.
- Fujimoto, C., Iwasaki, S., Urata, S., Morishita, H., Sakamaki, Y., Fujioka, M., Kondo, K., Mizushima, N. and Yamasoba, T. (2017). Autophagy is essential for hearing in mice. Cell Death Dis 8(5): e2780.
- Greenbaum, A., Chan, K. Y., Dobreva, T., Brown, D., Balani, D. H., Boyce, R., Kronenberg, H. M., McBride, H. J. and Gradinaru, V. (2017). Bone CLARITY: Clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Sci Transl Med 9(387). doi: 10.1126/scitranslmed.aah6518.
- Hama, H., Hioki, H., Namiki, K., Hoshida, T., Kurokawa, H., Ishidate, F., Kaneko, T., Akagi, T., Saito, T., Saido, T. and Miyawaki, A. (2015). ScaleS: an optical clearing palette for biological imaging. Nat Neurosci 18(10): 1518-1529.
- Jing, D., Zhang, S., Luo, W., Gao, X., Men, Y., Ma, C., Liu, X., Yi, Y., Bugde, A., Zhou, B. O., Zhao, Z., Yuan, Q., Feng, J. Q., Gao, L., Ge, W. P. and Zhao, H. (2018). Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res 28(8): 803-818.
- Mizushima, Y., Fujimoto, C., Kashio, A., Kondo, K. and Yamasoba, T. (2017). Macrophage recruitment, but not interleukin 1 beta activation, enhances noise-induced hearing damage. Biochem Biophys Res Commun 493(2): 894-900.
- Renier, N., Wu, Z., Simon, D. J., Yang, J., Ariel, P. and Tessier-Lavigne, M. (2014). iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159(4): 896-910.
- Susaki, E. A., Tainaka, K., Perrin, D., Kishino, F., Tawara, T., Watanabe, T. M., Yokoyama, C., Onoe, H., Eguchi, M., Yamaguchi, S., Abe, T., Kiyonari, H., Shimizu, Y., Miyawaki, A., Yokota, H. and Ueda, H. R. (2014). Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157(3): 726-739.
- Tainaka, K., Murakami, T. C., Susaki, E. A., Shimizu, C., Saito, R., Takahashi, K., Hayashi-Takagi, A., Sekiya, H., Arima, Y., Nojima, S., Ikemura, M., Ushiku, T., Shimizu, Y., Murakami, M., Tanaka, K. F., Iino, M., Kasai, H., Sasaoka, T., Kobayashi, K., Miyazono, K., Morii, E., Isa, T., Fukayama, M., Kakita, A. and Ueda, H. R. (2018). Chemical landscape for tissue clearing based on hydrophilic reagents. Cell Rep 24(8): 2196-2210 e2199.
- Treweek, J. B., Chan, K. Y., Flytzanis, N. C., Yang, B., Deverman, B. E., Greenbaum, A., Lignell, A., Xiao, C., Cai, L., Ladinsky, M. S., Bjorkman, P. J., Fowlkes, C. C. and Gradinaru, V. (2015). Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat Protoc 10(11): 1860-1896.
- Urata, S., Iida, T., Yamamoto, M., Mizushima, Y., Fujimoto, C., Matsumoto, Y., Yamasoba, T. and Okabe, S. (2019). Cellular cartography of the organ of Corti based on optical tissue clearing and machine learning. Elife 8: e40946.
- von Békésy, G. (1990). Experiments in hearing. J Acoust Soc Am 88: 2905.
Article Information
Copyright
Urata et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Urata, S., Iida, T., Suzuki, Y., Lin, S., Mizushima, Y., Fujimoto, C., Matsumoto, Y. and Yamasoba, T. (2019). A Novel Technique for Imaging and Analysis of Hair Cells in the Organ of Corti Using Modified Sca/eS and Machine Learning. Bio-protocol 9(16): e3342. DOI: 10.21769/BioProtoc.3342.
- Urata, S., Iida, T., Yamamoto, M., Mizushima, Y., Fujimoto, C., Matsumoto, Y., Yamasoba, T. and Okabe, S. (2019). Cellular cartography of the organ of Corti based on optical tissue clearing and machine learning. Elife 8: e40946.
Category
Cell Biology > Cell imaging > Confocal microscopy
Cell Biology > Tissue analysis > Tissue imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










