- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantification of Prostaglandin E2 Concentration in Interstitial Fluid from the Hypothalamic Region of Free-moving Mice
Published: Vol 9, Iss 15, Aug 5, 2019 DOI: 10.21769/BioProtoc.3324 Views: 5569
Reviewed by: Changyi ZhangEhsan KheradpezhouhAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1655 Views

In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2691 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1704 Views
Abstract
Prostaglandin E2 (PGE2) is a well-established chemical mediator for the generation of the fever at the hypothalamus of the brain. PGE2 mediates fever generation via PGE receptor 3 (i.e., EP3) on neurons in the preoptic area. The role of PGE2 has been analyzed by measuring PGE2 concentration in cerebrospinal fluid (Ccsf); however, local PGE2 concentration at the hypothalamus may not necessarily be consistent with Ccsf. In this protocol, we introduce our method to measure directly the alteration in PGE2 concentration in interstitial fluid in the hypothalamus (Cisf) of awake (free-moving) mice using a microdialysis technique. Male mice (c57BL/6J) were anesthetized and fixed in the stereotaxic instrument, and a microdialysis probe was inserted into the hypothalamus through a guide cannula. On the fifth postoperative day, Cisf was monitored in free-moving mice that were intraperitoneally (i.p.) injected with lipopolysaccharide (LPS). PGE2 and other eicosanoids recovered in Krebs-Ringer phosphate buffer and defused through a microdialysis probe were extracted into ethyl acetate/formic acid and then quantified with LC-MS/MS. Our method is useful to understand the role of key regulators of prostaglandin concentration such as those of transporters, which have been unappreciated in inflammation-based brain diseases.
Keywords: Prostaglandin E2 (PGE2)Background
PGE2 is intracellularly synthesized by cyclooxygenase pathways and released into extracellular spaces, where it binds to PGE receptors (i.e., EP1-4) to exhibit its activity. Prostanoids such as PGE2 are present in an anionic form under physiological conditions, and membrane transport has been suggested to be important for the local distribution and physiological action of PGE2 (Schuster, 2012; Nakanishi and Tamai, 2018). To date, several carrier proteins that are involved in PGE2 membrane transport have been identified and characterized; however, their roles in PGE2 distribution are not fully understood, particularly in the brain. Among these, organic anion transporting polypeptide 2A1 (OATP2A1) encoded by SLCO2A1 is a membrane transporter responsible for cellular uptake of prostanoids including PGE2, PGD2, and PGF2α, which are important for inflammatory reactions and homeostasis (Kanai et al., 1995). The roles of OATP2A1 in PGE2 disposition have been analyzed in SLCO2A1-deficient (SLCO2A1-/-) mice (Chang et al., 2010; Nakanishi et al., 2015). More recently, we reported that OATP2A1 located in intracellular organelles was involved in exocytotic PGE2 secretion from certain types of cells including macrophages (Shimada et al., 2015). Some previous articles described a potential role of OATP2A1 in the febrile response (Ivanov et al., 2003; Ivanov and Romanovsky, 2004). Indeed, OATP2A1 is expressed at the apical membranes of the choroid plexus (Tachikawa et al., 2012) and subarachnoidal blood vessels in rodents (Hosotani et al., 2015) and in primary cultured cerebral endothelial cells prepared from rats (Kis et al., 2006). Furthermore, in patients with Alzheimer’s disease, immunoreactivity for anti-OATP2A1 antibody has been reported in some glial cells including microglia and astrocytes (Choi et al., 2008). Therefore, our laboratory performed microdialysis to measure alteration in Cisf (concentration in interstitial fluid) of PGE2 at the hypothalamus of the brain to understand the physiological significance of OATP2A1 in LPS-induced fever generation. Our recent study determined Cisf of PGE2 at the hypothalamus in LPS-treated SLCO2A1+/+ and SLCO2A1-/- mice and clearly demonstrated that it was neither identical to Ccsf nor its concentration in hypothalamic tissues (Chyp) and was maintained by OATP2A1 independently (Nakamura et al., 2018). As far as we know, there is no report showing Cisf of PGE2 measured in free-moving small mammals like mice, although it has been analyzed by a microdialysis technique in rats (Gerozissis et al., 1995), rabbits (Kao, T. Y. et al., 2007; Kao, C. H. et al., 2007), and guinea pigs (Sehic et al., 1996). In the case of small animals such as mice, Cisf of PGE2 is expected to be lower than the detection limit of enzyme immunoassays, although an immunoassay is the most convenient and prevalent way to determine prostanoid concentration (Pradelles et al., 1985). However, protanoids can be easily detected using LC-MS/MS when extracted from biological fluids and concentrated using a solid phase column because accurate and sensitive quantification methods for prostanoids are established with LC-MS/MS (Schmidt et al., 2005; Cao et al., 2008). Fine microdialysis techniques to measure alteration in prostanoid concentration in the extracellular spaces of the brain in mouse models have been used widely in the field of neuroscience to promote an understanding of the mechanisms underlying neuroinflammation. Therefore, in this article, we present our protocols for analysis of PGE2 in microdialysis samples collected from the hypothalamus of mice, where PGE2 functions as a chemical mediator for fever generation.
Materials and Reagents
- 1.5-ml microtube (BIO-BIK, catalog number: CF-0150)
- 0.6-ml microtube (Thermo Fisher Scientific, catalog number: 502-GRD-Q)
- 2.0-ml microtube (Watson, catalog number: 132-620C)
- 10-ml glass conical tube (EMKS-16.5, Nichiden-Rika Glass, catalog number: 113111)
- Capillary glass tube (0.69 mm inner diameter, 1.19 mm outer diameter, 90 mm length, Drummond Scientific Company, catalog number: 3-000-210-G)
- 0.2-µm filter (Kanto Chemical, catalog number: 96923-00)
- Glass slide
- BD Lo-Dose U-100 insulin syringe (29 gauge of needle, Nippon Becton Dickinson, catalog number: 326666)
- Cotton wool (Osaki Medical, catalog number: 100006)
- Glass micropipette
a.Attach a capillary glass tube to the glass micropipette puller, and heat it with the instrument.
b.Pull a capillary glass tube according to the product protocol, and prepare the glass micropipette.
c.Brake the pipette at approximately 1 mm from the tip with tweezers before use. - Male c57BL/6J mice (10-25 weeks old)
- Pentobarbital sodium salt (Nacalai Tesque, catalog number: 26427-14)
- Paraffin (Leica, catalog number: 39601095)
- Liquid nitrogen
- Instant adhesive (Aron Alpha A, Daiichi-Sankyo)
- LPS O111:B4 (LPS, Sigma-Aldrich, catalog number: L4130)
- Formic acid (Wako Pure Chemical Industries, catalog number: 063-05895)
- Acetonitrile (Wako Pure Chemical Industries, catalog number: 015-08633)
- Ethyl acetate (Wako Pure Chemical Industries, catalog number: 058-00361)
- Ethanol (Wako Pure Chemical Industries, catalog number: 054-00461)
- Xylene (Wako Pure Chemical Industries, catalog number: 244-00081)
- Mayer's hematoxylin solution (Muto Pure Chemicals, catalog number: 30001)
- 1% eosin Y solution (Muto Pure Chemicals, catalog number: 32001)
- Paraformaldehyde (PFA, Wako Pure Chemical Industries, catalog number: 162-16065)
- Dental cement (Fuji I, GC, catalog number: 219AFB2X00208000)
- Woodworking glue (Konishi, catalog number: 10122)
- Dibutylhydroxytoluene (BHT, Wako Pure Chemical Industries, catalog number: 047-29451)
- PGE2 (Cayman Chemicals, catalog number: 14010)
- d4-PGE2 (Cayman Chemicals, catalog number: 314010)
- Glycerol (Nacalai Tesque, catalog number: 17045-65)
- KCl (Wako Pure Chemical Industries, catalog number: 163-03545)
- MgSO4 (Nacalai Tesque, catalog number: 210-32)
- NaH2PO4 (Wako Pure Chemical Industries, catalog number: 197-09705)
- Na2HPO4·12H2O (Kanto Chemical, catalog number: 37240-00)
- CaCl2 (Kanto Chemical, catalog number: 07057-00)
- NaCl (Wako Pure Chemical Industries, catalog number: 191-01665)
- HCl (5 N, Wako Pure Chemical Industries, catalog number: 081-05435)
- NaOH solution (5 N, Wako Pure Chemical Industries, catalog number: 196-05375)
- Pentobarbital solution (see Recipes)
- 10% glycerol solution (see Recipes)
- Krebs-Ringer phosphate buffer (KRPB, see Recipes)
- 0.5-M phosphate buffer (PB, see Recipes)
- 4% PFA solution (see Recipes)
Equipment
- Pipettes (Nichipet EXII, Nichiryo, catalog numbers: 00-NPX2-20, 00-NPX2-200, 00-NPX2-1000)
- Scissors (Natsume Seisakusho, catalog number: B-12H)
- Tweezers (Y-277, Hirasawa, catalog number: 9523)
- Dental drill
- Power supply (Minitor, catalog number: C2012)
- Rotary (Minitor, catalog number: M112)
- Diamond bar (diameter 0.8 mm, Minitor, catalog number: AD1403)
- -80 °C deep freezer (CLN-50U, Nihon Freezer)
- Microsyringe pump CMA/102 (CMA Microdialysis AB, catalog number: CMA/102)
- Microsyringe (Ito Seisakusho, catalog number: MS-GAN100)
- Stereotaxic instrument (Narishige, catalog number: SR-5M-HT)
- Anchor screws (Eicom, catalog number: AN-3)
- CMA7 guide cannula (CMA Microdialysis AB, catalog number: CMA P0000137)
- CMA7 microdialysis probe (1 mm membrane length, CMA Microdialysis AB, catalog number: CMA P000082)
- Device for free-movement (Eicom, catalog number: TSU-25)
- Acrylic cage (Eicom, catalog number: FC-25)
- Ultrasonic sonicator (QSonica, catalog number: XL2000)
- Glass micropipette puller (Narishige, catalog number: PC-10)
- Solid phase column (Oasis®MAX 3 cc (60 mg) extraction cartridges, Waters, catalog number 186000367)
- LCMS8050 (Shimadzu)
- LC-30AD (Shimadzu)
- Analytical column (Capcell Pak C18 IF2, 2.1 mm inner diameter x 100 mm, 2 µm, Shiseido, catalog number: 92887)
- Benchtop centrifuge (CF15RXII, Hitachi)
- Savant SpeedVac SPD2010 (Thermo Fisher Scientific, catalog number: SPD2010A-220)
Software
- LabSolution LCMS (Shimadzu)
Procedure
Part I: Sample collection
- Collection of interstitial fluid from the hypothalamic area by means of a microdialysis method
- Administer pentobarbital solution intraperitoneally to a c57BL/6J mouse (male, at 10-25 weeks old) with a dose of 50 mg/kg body using an insulin syringe (29 gauge of needle x 12.7 mm, 0.5-ml). Tilt the mouse with its head slightly toward the ground, and insert the needle at the lower right quadrant of the abdomen and to a depth in which 1/2 of the needle length is placed in the peritoneal cavity. Use caution to avoid injection of the intestinal organs and urinary bladder.
- Attach a guide cannula to a manipulator of the stereotaxic instrument in the vertical plane (Figure 1).
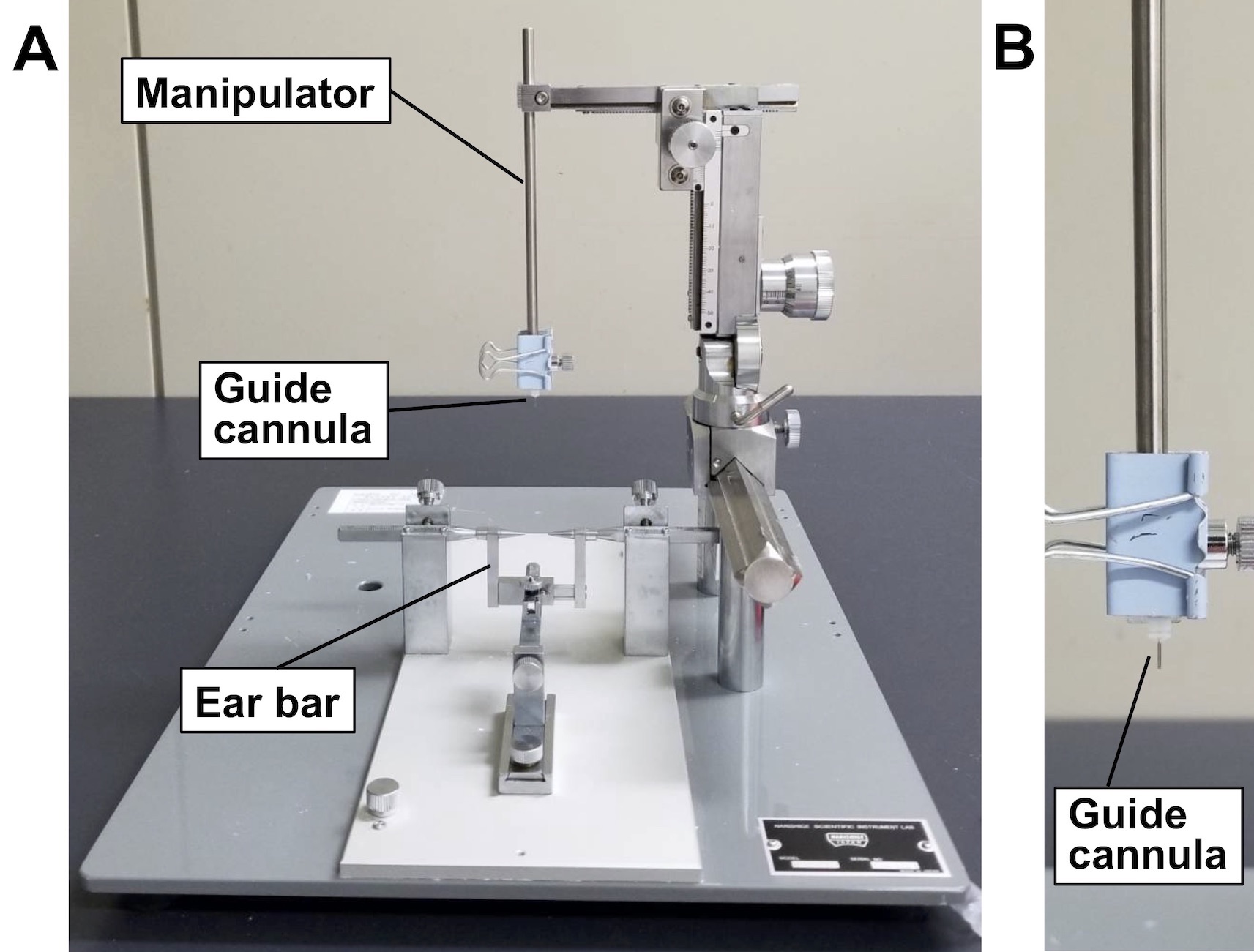
Figure 1. Attachment of a guide cannula on the stereotaxic instrument. A. Stereotaxic instrument SR-5M-HT obtained from Narishige. B. A guide cannula is attached to the tip of a manipulator of the instrument. - Under anesthesia, attach an ear bar to the mouse so that its sinciput is horizontally held.
- Before the surgery, wet the mouse head thoroughly with physiological saline to avoid the hairs adhering to scissors and disturbing the operation. Alternatively, you can shave the scalp, although this is not necessary for the following operation.
- Remove the scalp at the top of the head around the bregma with scissors in a circle of at most 3 mm in diameter so that the skull becomes visible.
- Carefully remove the periosteum and aponeurosis covering the skull using tweezers and scissors until the film disappears.
- Wipe the skull with cotton wool, and let the surface dry for about 10 min.
- The bregma is difficult to visualize when the surface is wet. Until the bregma appears, make a hole of approximately 1 mm in diameter in the posterior part of the exposed skull using a dental drill to attach an anchor screw to fasten the guide cannula. The screw should be attached near the guide cannula to be set.
- Move the manipulator just as the tip of the guide cannula comes over the bregma (Figure 2), and then record the vertical and horizontal tick marks of the stereotaxic instrument.
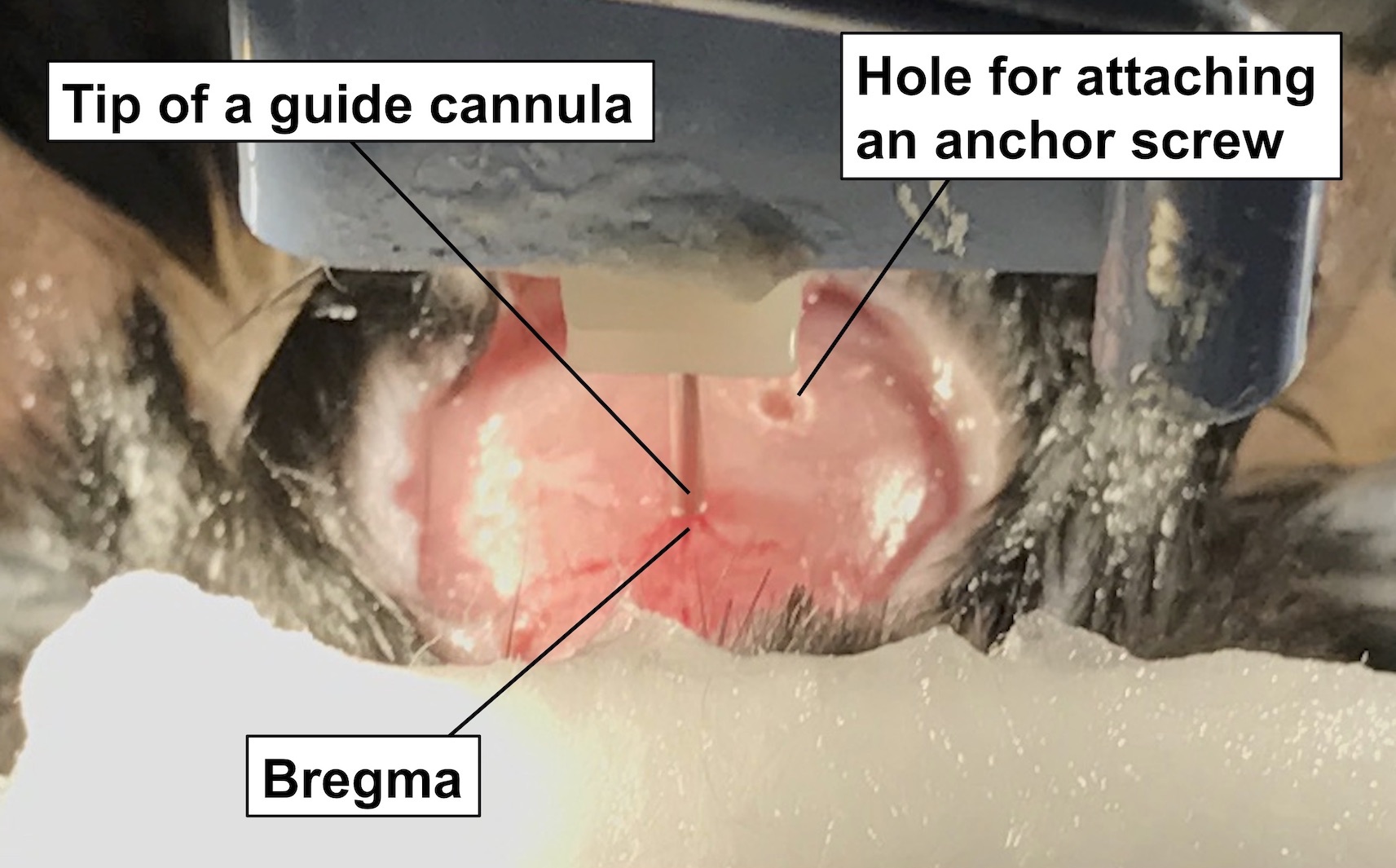
Figure 2. Setting the tip of the guide cannula onto the bregma of the skull. The photo represents the tip of a guide cannula on the bregma. - Move the tip of the guide cannula from the bregma, 0.9 mm forward, 0.1 mm right, and mark the position with oil-based ink. To drill a hole at the position, the guide cannula needs to be detached; therefore, it is recommended that the position set is marked with an oil-based ink before the cannula is removed.
- Use a dental drill to make a hole of about 1 mm in diameter at the marked point.
- Move the cannula down, touch it to the surface of the brain (at the level of the dura mater), and read the scale of the depth.
- Move the cannula further down slowly to a depth of 4.0 mm from the surface. This operation usually takes at least 1 min. Wipe the blood off with absorbent cotton when the surgical area bleeds during the operation. If bleeding does not stop, keep cotton where the blood is coming out from until it stops. If the mice bleed by more than a tenth of whole blood, usually estimated as 1/13 of the body weight, discontinuation of the operation should be considered.
- Fasten the cannula to the skull with instant adhesive temporarily.
- When the instant adhesive hardens, use dental cement to seal the gap between the guide cannula and the skull.
- When the cement is set, detach the cannula from the manipulator.
- Using a precision screwdriver, attach an anchor screw to the hole made in Step A8.
- Coat the skull and anchor screw firmly with dental cement, and then fasten the cannula with the anchor screw. Please make sure the guide cannula cannot be moved; it may come off or move when the mouse moves freely, resulting in inaccurate measurements.
- Once the cement is dry, apply woodworking glue to cover the surface of the cement. It usually takes approximately 10 and 5 min for the cement and glue to harden, respectively.
- When the glue has hardened, release the mouse to its cage.
- The next day, anesthetize the mice with pentobarbital and use tweezers to pull off the dummy probe that was inserted inside the guide cannula.
- Insert a fresh microdialysis probe, and fix it with dental cement. Before insertion, rinse the probe according to the manufacturer’s instruction. In our case, the tip of the probe was dipped in 70% EtOH solution and infused with KRPB for 5 min at a flow rate of 10 μl/min. Then, the tip of the probe was transferred into KRPB and infused with KRPB for another 5 min at the same flow rate. Probes were usually prepared on the day of use.
- Keep the mice in their cages for another 5 days to recover from postoperative damage. If the mice lose body weight of 25% or more after the operation, the experiment is not recommended due to severe damage to the brain.
- Attach the mouse to a free-moving device, transfer it to an acrylic cage, and infuse KRPB at a flow rate of 2 μl/min. The dialysate sample should be collected every hour in 0.6-ml microtubes kept on ice (Video 1).Video 1. Collection of dialysate from free-moving mice. The mouse can freely move in the acryl cage during KRPB infusion. KRPB flows from a blue tube, and dialysate samples are collected from a colorless tube. (This video was made at Kanazawa Univ. according to guidelines from the Kanazawa Univ. on Animal Care and approved by the Animal Research Ethics Board of Kanazawa University under protocols # AP-143148, AP-153511, AP-163750.)
- Two hours after initiation of infusion, administer LPS (100 μg/kg body) intraperitoneally to the mouse using an insulin syringe.
- Six hours after administration, euthanize the mouse by cervical dislocation, and isolate the brain. Fix the brain overnight at 4 °C in 4% PFA solution.
- Collect the probe, dip it in 10 ng/ml PGE2/KRPB solution, and infuse KRPB at 37 °C for 1 h at a flow rate of 2 μl/min. Store the collected dialysate sample at -30 °C until use. We confirmed that PGE2 in the dialysate is relatively stable by storage at -30 °C for at least one week.
- Embed the brain tissue fixed with 4% PFA in paraffin, and prepare brain sections attached on a glass slide.
- To remove paraffin from the sections, dip them in fresh xylene for 4 min in a glass container, and repeat this step 4 times.
- Dip the sections into absolute EtOH, followed by 95%, 90%, 80%, and 70% EtOH solution for 4 min each to remove xylene from them.
- Stain nuclei with hematoxylin for 1 min.
- After washing with tap water for 5 min, to visualize the cytoplasm, stain the sections with eosin for 40 s. Wash off extra eosin with tap water and dip the slides in 80% solution, followed by 95% EtOH and absolute ethanol for 10 s each to make them dehydrated.
- Dip them into fresh xylene for 3 min, repeat this procedure three times, and then seal the sections using a mounting agent and cover glass.
- Confirm the insertion position of the probe in the brain with a stereomicroscope (Figure 3). Hematoxylin and eosin (HE) staining makes it easy to observe the place of probe insertion.
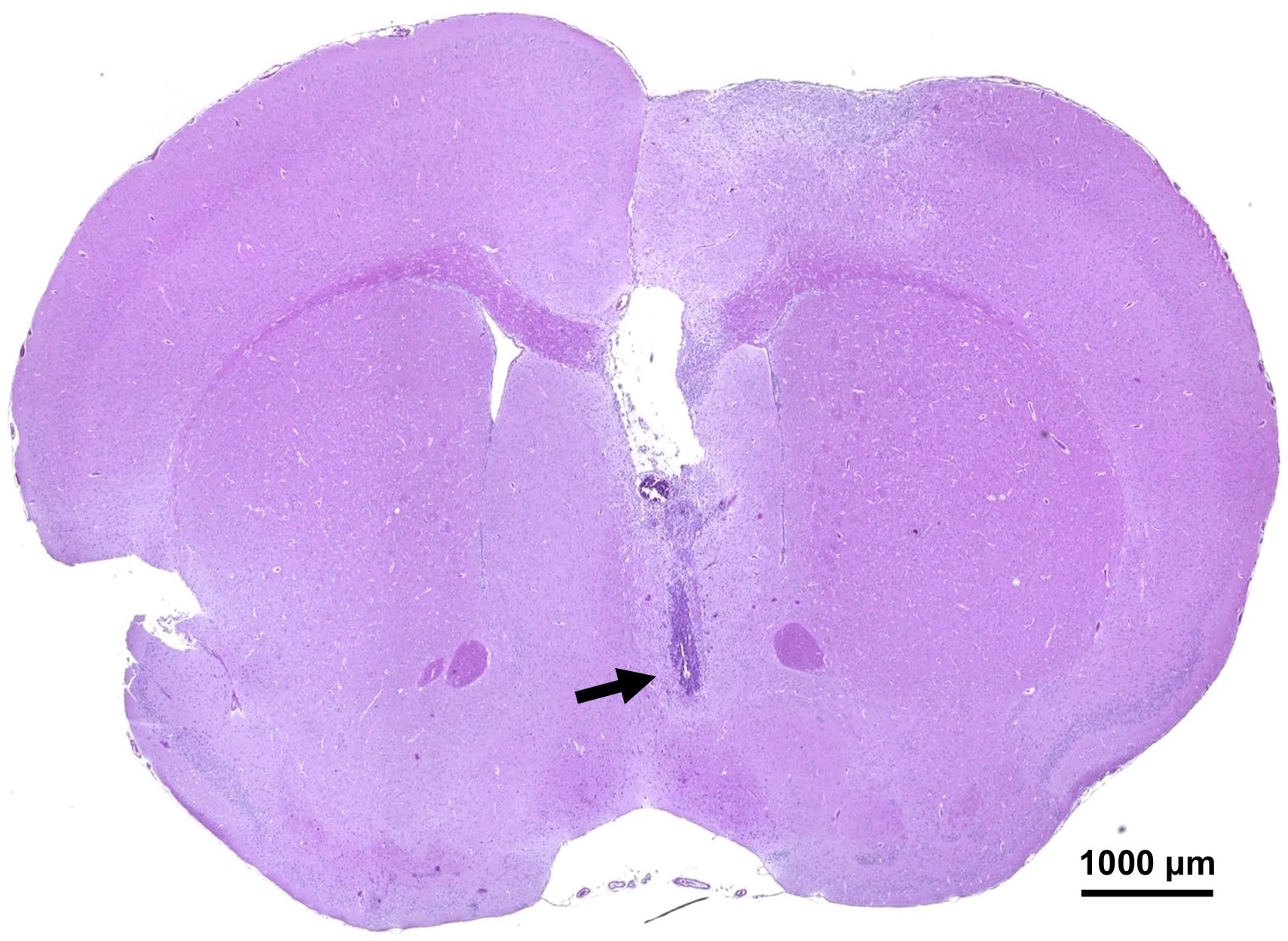
Figure 3. HE staining in the mouse brain after the microdialysis experiment. The histological image of the mouse brain coronal section is shown after microdialysis probe insertion. The arrow indicates the place of an edge of the inserted microdialysis probe. The indicated bar represents 1,000 µm.
- Collection of hypothalamic tissues
- Administer LPS (100 μg/kg body) intraperitoneally to a mouse.
- At the designated period of time, euthanize the mouse by cervical dislocation.
- Immediately, decapitate the mouse, incise the scalp and avoid damage to the brain, expose the skull, and then isolate the whole brain. The dissection can be performed on tissue papers spread on ice.
- Place the brain ventral side up on glass plates on ice to keep it relatively cold.
- Dissect out the diencephalon by coronal cuts just in front of the optic chiasma and behind the mammillary body. Remove the arcuate nuclei by another coronal cut in the middle of the optic tract, from rostral to infundibulum. Please see the previous article (Salehi et al., 2012) for detailed procedures.
- Cut the obtained brain block along the anterior commissure, and collect the ventral side of the anterior commissure as the hypothalamic tissue in a 1.5-ml microtube.
- Freeze the hypothalamic tissue immediately in liquid nitrogen, and store in a -80 °C deep freezer until use. The frozen samples can be stored for at least one week, under which PGE2 is relatively stable.
- Collection of CSF
Note: CSF was collected based on the method reported previously (Liu et al., 2012).- Administer LPS (100 μg/kg body) intraperitoneally into a mouse.
- At the designated period of time, anesthetize the mouse with pentobarbital (i.p. administration, 50 mg/kg body).
- Under anesthesia, attach the mouse head to the stereotaxic instrument with its neck tilted forward as much as possible. Take caution not to break the neck by bending it too much. Further information is provided in the previous report (Liu et al., 2012).
- Cut and remove the skin over the occipital region with scissors, and expose the dura of the cisternal region with tweezers.
- Attach a glass micropipette horizontally to the manipulator of the stereotaxic instrument.
- Insert the tip of the micropipette into the dura at a depth of approximately 1 mm, and then suck up the CSF into the micropipette. The volume of the collected CSF should be approximately 10 μl.
- Transfer the collected CSF to a 1.5-ml microtube.
- Freeze the CSF immediately in liquid nitrogen, and store in a -80 °C deep freezer until use. The frozen samples can be stored for at least one week, under which PGE2 is relatively stable.
- Interstitial fluid (The schematic figure is shown as Figure 4)
- Transfer 100 μl of dialysate sample to a 1.5-ml microtube, and add 200 μl of 1.25 ng/ml d4-PGE2 (as an internal standard) and 15 μl of 10% formic acid aqueous solution (v/v). Set the final concentration of d4-PGE2 at 0.635 ng/ml by addition of 85 µl of de-ionized water. The concentration of 0.635 ng/ml corresponds to 5 ng/ml when the sample is set to 50 μl with the mobile phase, which is high enough not to be affected by daily fluctuation of the measurement.
- Add 500 μl of ethyl acetate, and shake vigorously for 15 min.
- Spin the sample by a benchtop centrifuge for 10 min at 21,000 x g at 4 °C.
- Transfer the supernatant to a fresh 1.5-ml microtube, and evaporate the supernatant to dryness under reduced pressure at room temperature for 30 min with a Savant SpeedVac SPD2010.
- Reconstitute the obtained residues with 50 μl of 25% acetonitrile solution containing 0.075% formic acid (v/v), and dissolve them as much as possible by shaking for at least 10 min.
- Separate the undissolved materials or debris by centrifuge for 10 min at 21,000 x g at 4 °C, and then obtain the clear supernatant.
- Apply the supernatant to LC-MS/MS to determine Cisf of PGE2.
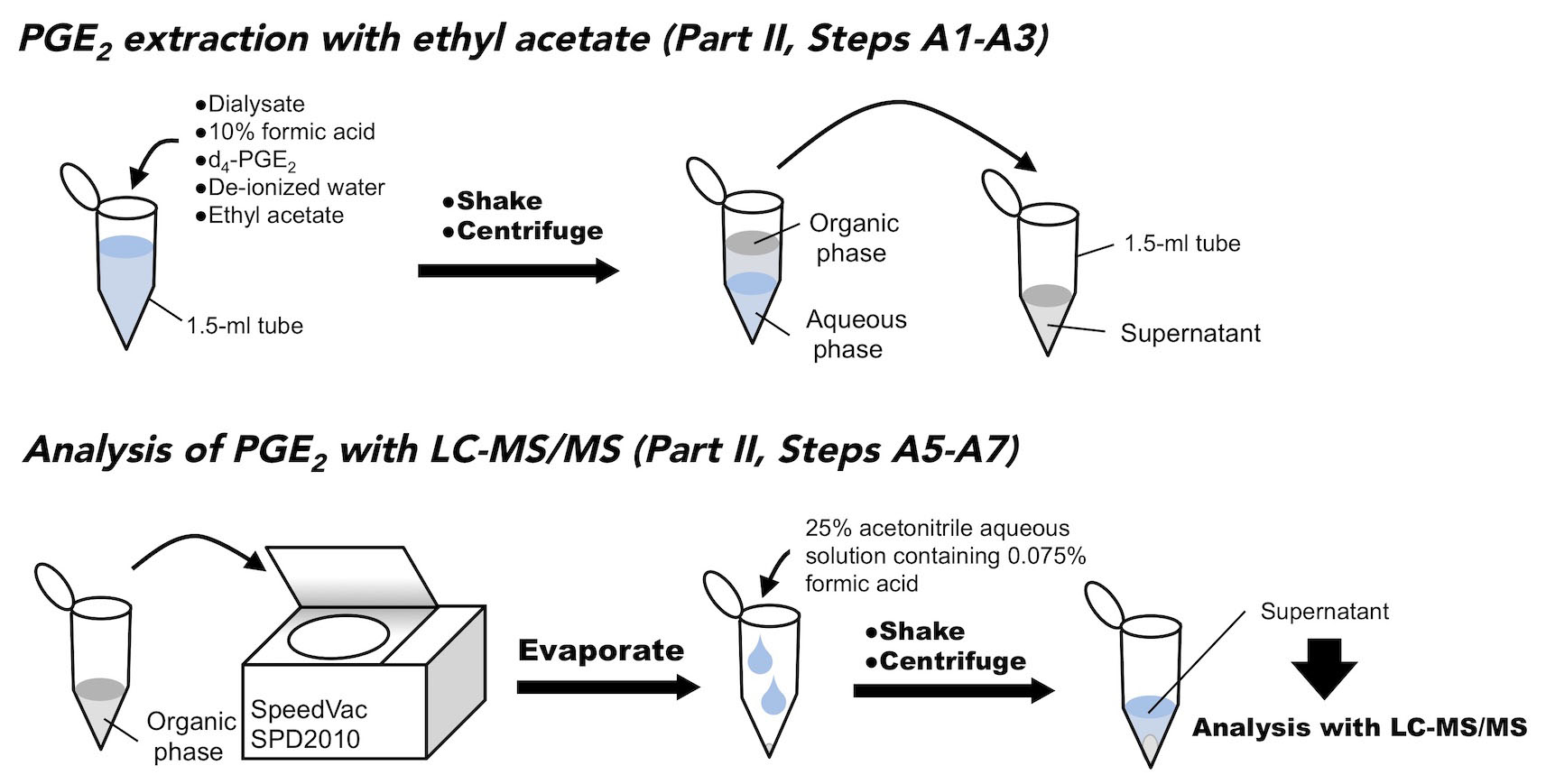
Figure 4. Schematic figure of PGE2 extraction from the dialysate - Hypothalamic tissue (The schematic figure is shown as Figure 5)
- Prepare a block of hypothalamic tissue, and weigh approximately 10 mg in a 1.5-ml microtube. Then, add 200 μl of PBS, 200 μl of 10% glycerol solution, 5 µl of 10 mg/ml BHT solution, and 10 μl of 100 ng/ml d4-PGE2 solution (to be 2.4 ng/ml at the final concentration). Homogenize the tissue, and shear deoxyribonucleotides for 10 s with an ultrasonic sonicator.
- Transfer the homogenate to a 10-ml glass conical tube, and add 2.485 ml of 25% acetonitrile solution (v/v). Shake vigorously for 5 min.
- Add 3 ml of absolute acetonitrile to a solid phase column (Oasis® MAX column cartridge), pressurize with the plunger of a 2.5 ml syringe, and pass through the column.
- Add 3 ml of 25% aqueous acetonitrile solution (v/v) to the cartridge, and pass through the column.
- Add 3 ml of the prepared homogenate to the cartridge, and pass through the column.
- Add 3 ml of 25% aqueous acetonitrile solution (v/v) to the cartridge, and pass through the column.
- Add 3 ml of absolute acetonitrile to the cartridge, and pass through the column.
- Add 1.3 ml of 1% formic acid acetonitrile solution (v/v), and collect the eluate into a 2.0-ml microtube.
- Evaporate the eluate for 1 h at room temperature under reduced pressure using a Savant SpeedVac SPD2010.
- Add 50 μl of 25% acetonitrile aqueous solution containing 0.075% formic acid (v/v), and shake for 10 min.
- Separate the supernatant with a benchtop centrifuge for 10 min at 21,000 x g at 4 °C.
- Apply the supernatant to LC-MS/MS to measure Chyp of PGE2.
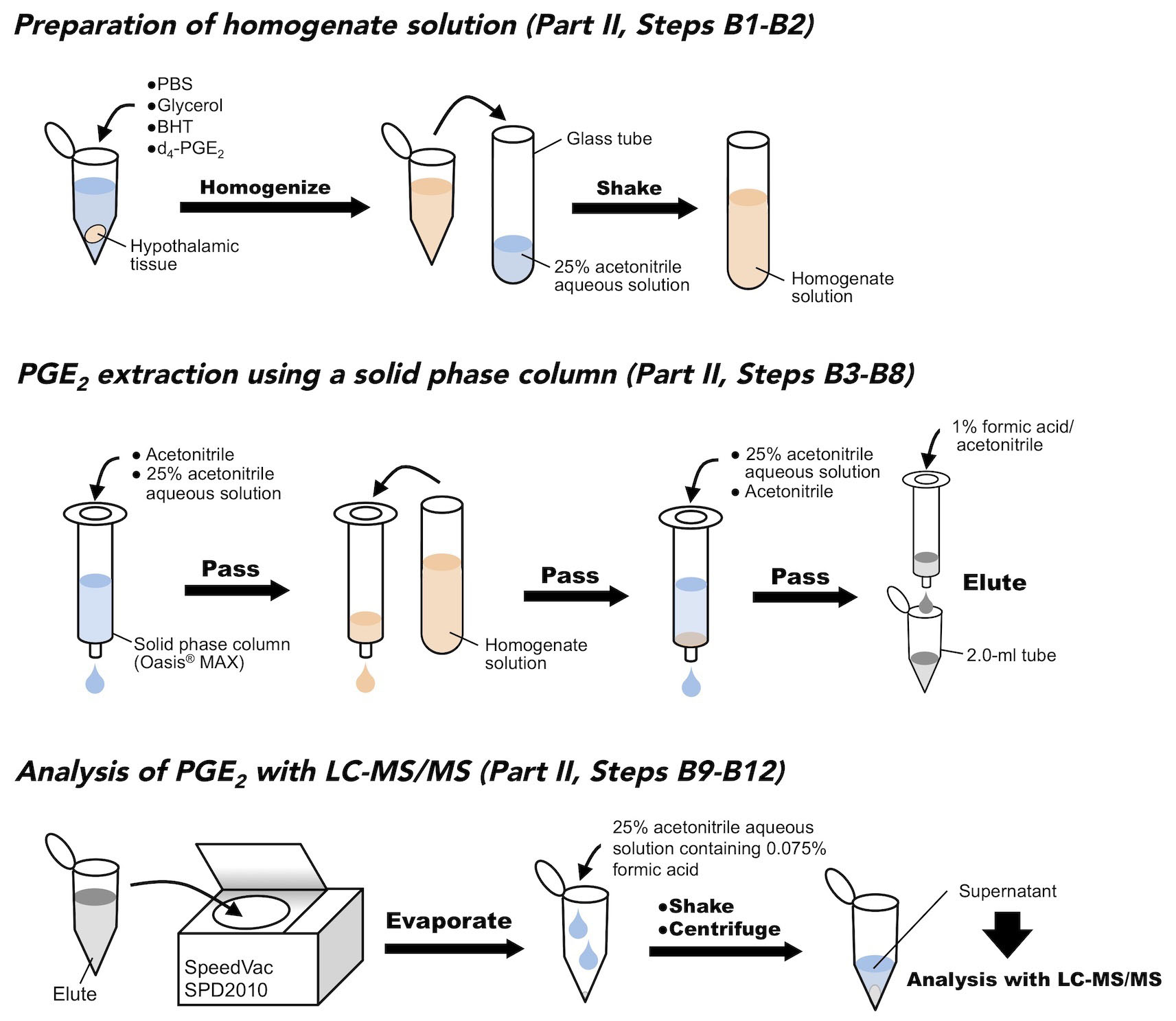
Figure 5. Schematic figure of PGE2 extraction from hypothalamic tissues - CSF
- Add 200 μl of PBS, 200 μl of 10% aqueous glycerol, 5 μl of 10 mg/ml BHT, and 10 μl of 100 ng/ml d4-PGE2 aqueous solution (to be 2.4 ng/ml at the final concentration) to a 10-ml glass conical tube containing 5 μl of CSF.
- Add 2.58 ml of 25% acetonitrile solution (v/v), and mix by vigorous shaking for 5 min.
- Subsequent procedures are as described for the hypothalamic tissue (see Procedure B).
Part III: PGE2 quantification by means of LC-MS/MS
The sample subjected to the extraction procedure is measured by LC-MS/MS. The measurement conditions are shown below. Molecular weights of PGE2 and PGD2 are identical, but they can be separated under the condition described below for LC-MS/MS analysis (Figure 6).
- Analytical column: Capcell Pak C18 IF2 (2.1 mm inner diameter x 100 mm, 2 µm)
- Mobile phase:
- Aqueous phase: 0.1% formic acid/water (v/v)
- Organic phase: acetonitrile
- Gradient of (b) concentration:
0-9 min: 15-50%
9-10 min: 50-90%
10-11 min: 90%
11-12 min: 90-15%
12-13 min: 15% - Flow rate: 0.4 ml/min
- Injection volume: 30 µl
- Q1, Q3: Mass-to-charges ratios (m/z) are set to determine PGE2 and PGE2-d4. Q1 and Q3 are set to 351.00 and 271.35 for PGE2 and 355.00 and 275.25 for PGE2-d4, respectively.
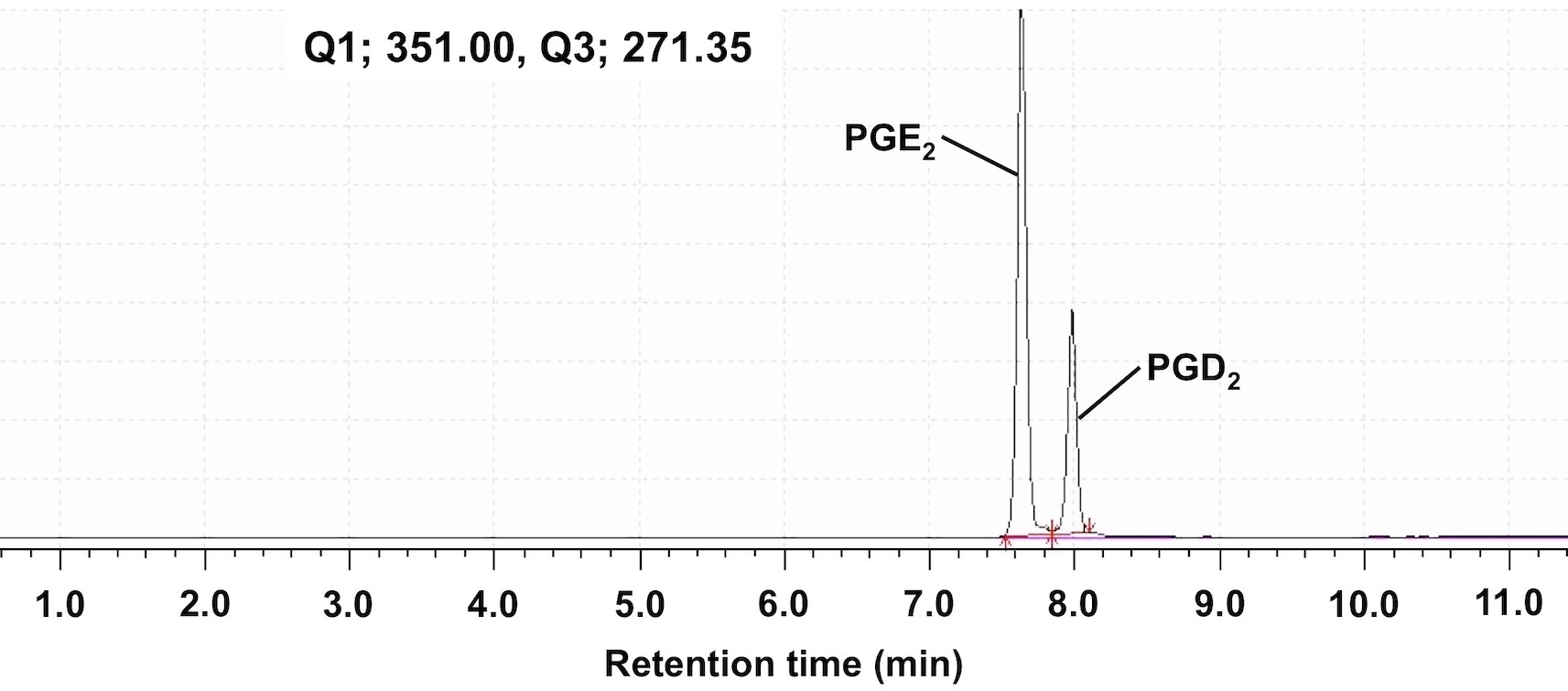
Figure 6. Chromatogram image of PGE2 and PGD2 in this protocol. PGE2 and PGD2 were detected at the same m/z Q1 (351.00) and Q3 (271.35). PGE2 and PGD2 were detected at retention times of 7.6 and 8.0 min, respectively.
Data analysis
Calculation of interstitial PGE2 concentration in the mouse hypothalamus:
The in vitro recovery rate (RR) of PGE2 concentration in the microdialysis method was calculated by the following equation,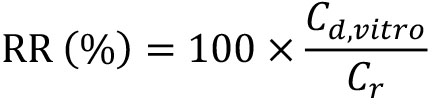
where, Cd,vitro and Cr represent the PGE2 concentration in the dialysate sample and the PGE2 concentration dissolved in KRPB, respectively. Cisf was estimated by dividing the concentration of PGE2 in the dialysate sample collected from the mouse (Cd,vivo) by RR as described below.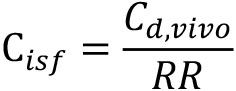
Notes
Please refer to the original publication http://www.jneurosci.org/content/38/24/5584 for examples of the expected results of this protocol.
Recipes
- Pentobarbital solution (6.48 mg/ml)
- Weigh 64.8 mg of pentobarbital sodium, and dissolve it in physiological saline
- Add sterilized physiological saline up to 10 ml
- Store the solution at room temperature under shading
- 10% glycerol solution (v/v)
Mix 100 µl of glycerol with 900 µl of de-ionized water, and store the solution at room temperature - Krebs-Ringer phosphate buffer (KRPB, total volume 100 ml; pH 7.4)
- Weigh 700.8, 17.9, 14.4, 10.8, 50.1, and 13.3 mg of NaCl powder (at a final concentration of 120 mM), KCl (2.4 mM), MgSO4 (1.2 mM), NaH2PO4 (0.9 mM), Na2HPO4·12H2O (1.4 mM), and CaCl2 (1.2 mM), respectively
- Dissolve the powders in de-ionized water
- Adjust the pH to 7.4 with 5 N HCl
- Fill them with water up to 100 ml
- Filter the solution using a 0.2-µm filter to sterilize it
- Store the solution at room temperature until use
- 0.5 M PB (pH 7.4)
- Weigh 6 g of NaH2PO4 powder, and dissolve it in 100 ml of de-ionized water (0.5 M)
- Weigh 53.7 g of Na2HPO4·12H2O powder, and dissolve it in 300 ml of de-ionized water (0.5 M)
- Adjust the pH to 7.4 by adding 0.5 M NaH2PO4 solution to 0.5 M Na2HPO4
- Store the solution at room temperature
- 4% PFA solution (w/v, total volume of 200 ml)
- Boil 150 ml of de-ionized water using a microwave oven
- Add 120 µl of 5 N NaOH
- Add 8 g of PFA powder
- After PFA is dissolved, add 40 ml of 0.5 M PB
- Fill with de-ionized water to 200 ml
- Store the solution at 4 °C until use
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI, 15H04755, and 15K15181) from the Japan Society for the Promotion of Science (No. 055). Part of the experiment described was also supported by the Japan Society for the Promotion of Science and the Smoking Research Foundation [Grant 055 to T.N.]. We thank Drs. Okura and Deguchi at Teikyo University for their kind suggestions and instructions on how to perform the experiments.
Competing interests
The authors have no conflicts of interest to disclose.
Ethics
All animal experiments were performed in accordance with the requirements of the Kanazawa University Institutional Animal Care and Use Committee (permit numbers AP-143148, AP-153511, and AP-163750).
References
- Cao, H., Xiao, L., Park, G., Wang, X., Azim, A. C., Christman, J. W. and van Breemen, R. B. (2008). An improved LC-MS/MS method for the quantification of prostaglandins E2 and D2 production in biological fluids. Anal Biochem 372(1): 41-51.
- Chang, H. Y., Locker, J., Lu, R. and Schuster, V. L. (2010). Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice. Circulation 121(4): 529-536.
- Choi, K., Zhuang, H., Crain, B. and Dore, S. (2008). Expression and localization of prostaglandin transporter in Alzheimer disease brains and age-matched controls. J Neuroimmunol 195(1-2): 81-87.
- Gerozissis, K., De Saint Hilaire, Z., Orosco, M., Rouch, C. and Nicolaidis, S. (1995). Changes in hypothalamic prostaglandin E2 may predict the occurrence of sleep or wakefulness as assessed by parallel EEG and microdialysis in the rat. Brain Res 689(2): 239-244.
- Hosotani, R., Inoue, W., Takemiya, T., Yamagata, K., Kobayashi, S. and Matsumura, K. (2015). Prostaglandin transporter in the rat brain: its localization and induction by lipopolysaccharide. Temperature 2(3): 425-434.
- Ivanov, A. I. and Romanovsky, A. A. (2004). Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci 9: 1977-1993.
- Ivanov, A. I., Scheck, A. C. and Romanovsky, A. A. (2003). Expression of genes controlling transport and catabolism of prostaglandin E2 in lipopolysaccharide fever. Am J Physiol Regul Integr Comp Physiol. 284(3): R698-R706.
- Kanai, N., Lu, R., Satriano, J. A., Bao, Y., Wolkoff, A. W. and Schuster, V. L. (1995). Identification and characterization of a prostaglandin transporter. Science 268(5212): 866-869.
- Kao, C. H., Kao, T. Y., Huang, W. T. and Lin, M. T. (2007). Lipopolysaccharide- and glutamate-induced hypothalamic hydroxyl radical elevation and fever can be suppressed by N-methyl-D-aspartate-receptor antagonists. J Pharmacol Sci 104(2): 130-136.
- Kao, T. Y., Huang, W. T., Chang, C. P. and Lin, M. T. (2007). Aspirin may exert its antipyresis by inhibiting the N-methyl-D-aspartate receptor-dependent hydroxyl radical pathways in the hypothalamus. J Pharmacol Sci 103(3): 293-298.
- Kis, B., Isse, T., Snipes, J. A., Chen, L., Yamashita, H., Ueta, Y. and Busija, D. W. (2006). Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol 100(4): 1392-1399.
- Liu, M., Kuhel, D. G., Shen, L., Hui, D. Y. and Woods, S. C. (2012). Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am J Physiol Regul Integr Comp Physiol 303(9): R903-R908.
- Nakamura, Y., Nakanishi, T., Shimada, H., J., S., Aotani, R., Maruyama, S., Higuchi, K., Okura, T., Deguchi, Y. and Tamai, I. (2018). Prostaglandin transporter OATP2A1/SLCO2A1 is essential for body temperature regulation during fever. J Neurosci 38(24): 5584-5595.
- Nakanishi, T. and Tamai, I. (2018). Roles of organic anion transporting polypeptide 2A1 (OATP2A1/SLCO2A1) in regulating the pathophysiological actions of prostaglandins. AAPS J 20(1): 13.
- Nakanishi, T., Hasegawa, Y., Mimura, R., Wakayama, T., Uetoko, Y., Komori, H., Akanuma, S., Hosoya, K. and Tamai, I. (2015). Prostaglandin transporter (PGT/SLCO2A1) protects the lung from bleomycin-induced fibrosis. PLoS One 10(4): e0123895.
- Pradelles, P., Grassi, J. and Maclouf, J. (1985). Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Anal Chem 57(7): 1170-1173.
- Schmidt, R., Coste, O. and Geisslinger, G. (2005). LC-MS/MS-analysis of prostaglandin E2 and D2 in microdialysis samples of rats. J Chromatogr B Analyt Technol Biomed Life Sci 826(1-2): 188-197.
- Schuster, V. L. (2002). Prostaglandin transport. Prostaglandins Other Lipid Mediat 68-69: 633-647.
- Sehic, E., Szekely, M., Ungar, A. L., Oladehin, A. and Blatteis, C. M. (1996). Hypothalamic prostaglandin E2 during lipopolysaccharide-induced fever in guinea pigs. Brain Res Bull 39(6): 391-399.
- Shimada, H., Nakamura, Y., Nakanishi, T. and Tamai, I. (2015). OATP2A1/SLCO2A1-mediated prostaglandin E loading into intracellular acidic compartments of macrophages contributes to exocytotic secretion. Biochem Pharmacol 98(4): 629-638.
- Tachikawa, M., Ozeki, G., Higuchi, T., Akanuma, S., Tsuji, K. and Hosoya, K. (2012). Role of the blood-cerebrospinal fluid barrier transporter as a cerebral clearance system for prostaglandin E2 produced in the brain. J Neurochem 123(5): 750-760.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Nakamura, Y., Sakaguchi, T., Tamai, I. and Nakanishi, T. (2019). Quantification of Prostaglandin E2 Concentration in Interstitial Fluid from the Hypothalamic Region of Free-moving Mice. Bio-protocol 9(15): e3324. DOI: 10.21769/BioProtoc.3324.
- Nakamura, Y., Nakanishi, T., Shimada, H., J., S., Aotani, R., Maruyama, S., Higuchi, K., Okura, T., Deguchi, Y. and Tamai, I. (2018). Prostaglandin transporter OATP2A1/SLCO2A1 is essential for body temperature regulation during fever. J Neurosci 38(24): 5584-5595.
Category
Neuroscience > Nervous system disorders > Animal model
Biochemistry > Other compound > Small molecular
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









