- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adhesive and Cohesive Peel Force Measurement of Human Airway Mucus
Published: Vol 9, Iss 13, Jul 5, 2019 DOI: 10.21769/BioProtoc.3287 Views: 6326
Reviewed by: Andrea PuharSourav S PatnaikSongjie Cai

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Adoptive Transfer of Monocytes Sorted from Bone Marrow
Damya Laoui [...] Jo A Van Ginderachter
Jan 5, 2019 9068 Views

Safety Profiling of Tumor-targeted T Cell–Bispecific Antibodies with Alveolus Lung- and Colon-on-Chip
S. Jordan Kerns [...] Lauriane Cabon
Jan 5, 2023 3678 Views

In Vitro Assay to Examine Osteoclast Resorptive Activity Under Estrogen Withdrawal
Cara Fiorino [...] Rene E. Harrison
Jan 5, 2025 1656 Views
Abstract
In health, the high-speed airflow associated with cough represents a vital backup mechanism for clearing accumulated mucus from our airways. However, alterations in the mucus layer in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) leads to the mucus layer adhered to the airway surfaces, representing the nidus of chronic lung infection. To understand what is different about diseased mucus and why cough clearance is defective, there is a need for techniques to quantify the strength of the interactions limiting the ability of airflow to strip mucus from the airway surface (i.e., adhesive strength) or tear mucus apart (i.e., cohesive strength). To overcome the issues with measuring these properties in a soft (i.e., low elastic modulus) mucus layer, we present here novel peel-testing technologies capable of quantifying the mucus adhesive strength on cultured airway cells and cohesive strength of excised mucus samples. While this protocol focuses on measurements of airway mucus, this approach can easily be adapted to measuring adhesive/cohesive properties of other soft biological materials.
Keywords: Lung diseaseBackground
The healthy airway mucosal barrier represents a powerful innate immune system designed to protect the pulmonary surfaces from the persistent onslaught of inhaled infectious and noxious substances. During times of airway infection, high-velocity airflow (i.e., cough) represents an effective backup mechanism to clear accumulated mucus. However, abnormalities in mucus properties, which characterize several airway diseases, including cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and asthma, lead to a reduction in airflow-mediated cough clearance (Button et al., 2016).
To fully elucidate why such mucus fails to clear with cough-induced shear forces, it is necessary to understand the biophysical interactions between airway mucus and airway surfaces. The high-speed airflow associated with cough can remove mucus from airway surfaces through at least two distinct mechanisms: 1) “disadhesion”, whereupon the mucus layer is physically detached from the underlying airway surface as a result of overcoming the “adhesive” interactions between mucus and the airway cell surface; and 2) “tearing”, where chunks of mucus are broken off adherent mucus masses as a result of overcoming mucus-mucus cohesive bonds and interactions.
There are various material science approaches, such as “peel” testers, to quantify the strength of the interaction between one material and another. To initiate a test with these devices, the user “connects” one end of the test material to a movable arm, which then measures the force required to peel the material off a stationary surface. However, the challenge with measuring the mucus adhesive and cohesive strength is that mucus is a fragile material with elastic modulus typically < 10 Pa (Hill et al., 2014) therefore making it impossible to directly connect to a peel device. Furthermore, to measure adhesion strength of mucus to the airway epithelial surface, it is necessary to perform studies in a suitable environment to prevent evaporation of the thin (< 100 μm) mucus layer (Matsui et al., 1998). To overcome these limitations, we have developed a novel peel force technique to quantify both the adhesive and cohesive strengths of airway mucus. Key to this technique, described in detail in this protocol, is the use of a mucus-binding mesh which allows for the peeling of the mucus layer off of the airway surface (i.e., adhesive strength) or another a fixed mucus-binding mesh (i.e., cohesive strength) (Figure 1A). The mucus binding mesh utilized in this protocol is made from a standard laboratory cellulose wipe (Kimwipe). These mucus-binding meshes are very strong, flexible, and as anyone who has blown their nose into tissue paper knows, binds very strongly to mucus. Finally, extensive testing has shown that these meshes are not cytotoxic and do not damage the airway epithelial cells that they are laid upon (Button et al., 2018). Below, we show the construction and application of the mucus-binding mesh-based approach to measure mucus adhesion and cohesion, when connected to a peel test device (Figure 1B).
Using the protocol, we have previously shown that both adhesive and cohesive properties of mucus are sensitive to changes in mucus concentration associated with CF and COPD (Button et al., 2018). In addition to understanding the role of diseased mucus on these biophysical properties, the approach described here can also be useful for identifying/screening therapies designed to improve mucus clearance in subjects with muco-obstructive diseases. Additionally, these measurements can be performed on human-derived mucus samples (i.e., expectorated sputum and isolated airway mucus), potentially valuable for assessment of therapeutic intervention and disease progression. Indeed, we have utilized these techniques to assess the effect of several agents (i.e., mucolytics) which can reduce the adhesive and cohesive strength of CF/COPD mucus (Button et al., 2018). Finally, while this protocol is written to describe the measurement of airway mucus properties, this approach should easily be adapted to measuring adhesive/cohesive properties of other mucus samples (GI, cervical, etc.) as well as other fragile biological or synthetic materials.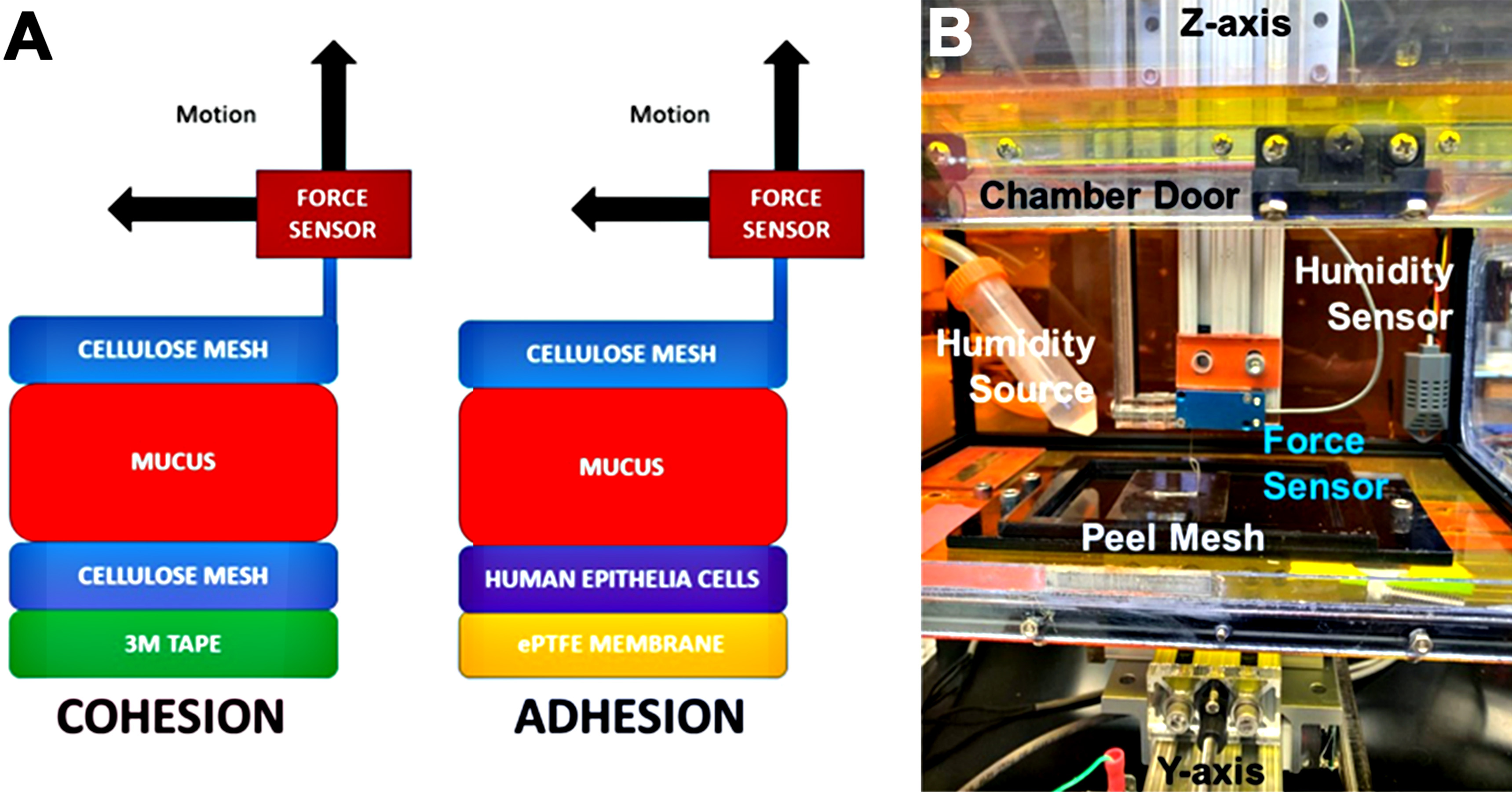
Figure 1. Overview of the adhesion and cohesion technique. A. Assembly diagram of the systems for measuring mucus cohesion (left) and adhesion (right). B. Picture of the actual peel test device described in this protocol.
Materials and Reagents
- Kimwipe cellulose wipe (Kimberly Clark, catalog number: 34256)
- Sylgard 184 (Dow Corning, catalog number: 4019862)
- Magnetic strips (McMaster Carr, catalog number: 5699K68)
- Glass plates (McMaster Carr, catalog number: 8476k23)
- Polyethylene double-sided medical adhesive (3M, catalog number: 1552)
- Spin concentrator (Ultra 10K) (Amicon, catalog number: UFC901024)
- 50 ml conical tube (Corning, catalog number: 352070)
- 6-well plate (Corning Costar, catalog number: 3516)
- Well-differentiated human airway epithelial cultures (Fulcher et al., 2005)
- Airway mucus samples (Button et al., 2012)
- Hexane (Sigma-Aldrich, catalog number: 293253)
- 1x Phosphate buffered saline (PBS) containing calcium and magnesium (Gibco, catalog number:14190144)
- 100% Isopropanol (Fisher Scientific, catalog number: A416-1)
- 30-mm Millicell culture inserts, hydrophilic PTFE (Millipore, catalog number: PICM03050)
- Air-liquid interface (ALI) cell culture media (Randell et al., 2011) (or commercially available from Stemcell Technologies, catalog number: 05001)
Equipment
- Custom-designed 3-axis peel-tester (see text below)
- Aluminum extrusions (80/20 Incorporated, catalog number: 1515-LS)
- Linear bearings (80/20 Incorporated, catalog number: 6415)
- Precision leadscrews (McMaster-Carr, catalog number: 6350K689)
- DC servo Motors (Rhino, catalog number: RMCS-22)
- Magnetic position sensor (AMS, catalog number: AS5311)
- Microcontroller running Labview Interface for Arduino (Arduino, UNO)
- Force sensor (Transducer Technologies, GSO family) (model depends on sensitivity required)
- Acrylic box (McMaster-Carr, catalog number: 8774K12)
- Stable Micro Systems TA.XT2i Texture Analyzer (in lieu of a custom peel-tester)
- CO2 laser cutter (Full Spectrum Laser/Epilog/etc.) (optional, see below)
- UV sterilizer (Thermo)
- Ultrasonic humidifier (Holmes, catalog number: HM500TG1)
- Humidity sensor and controller (Image, catalog number: WH8040)
- Positive-displacement pipetters (Gilson)
- Accusprayer with 2.0-micron head (3M, catalog number: 166609)
- High-humidity tissue culture incubator (Nuaire, catalog number: NU-4850)
Software
- National Instruments Labview motor control and data acquisition software (available upon request)
- Microsoft Excel (or Equivalent)
Procedure
- Material Preparation
- Human airway primary cell cultures
Protocols described here utilize well-differentiated human bronchial epithelial (HBE) cultures with endogenously-accumulated mucus. Isolated primary large airway cells were plated 250,000 cells per square centimeter on 30-mm Millicell membranes (24 mm inner membrane diameter) using previously published techniques (Fulcher et al., 2005) and ALI cell culture media (Randell et al., 2011). Cultures were maintained in a standard tissue culture incubator (37 °C, 5% CO2, > 95% humidity) for the duration of differentiation into mucus-secreting and ciliated cells (~4 weeks) and media was changed three times weekly. Because mucus is not cleared from airway surfaces in culture, airway surface mucus concentrations can be controlled by varying culture-washing intervals. Intact cells with endogenous mucus are utilized for all mucus adhesion measurements. - Mucus harvesting
Mucus for the cohesion peel experiments was harvested from well-differentiated HBE cultures using methods previously described (Button et al., 2018).- Briefly, a large number (between 24 and 96) of HBE cultures on 30-mm supports are left to accumulate mucus for up to 4 weeks. On the day of the cohesive measurements, lavage the mucus by incubating the apical surface with a small volume of PBS (50 μl/cm2) for 30 min at 37 °C.
- Then carefully remove mucus samples from the culture using a positive-displacement pipetter (Gilson) and pooled. Spin-concentrate the dilute mucus samples (Ultra 10K; Amicon) at 4,000 x g (4 °C) to the desired mucus concentration. Determine the mucus concentration at each step as previously described (Button et al., 2012). In these studies, mucus samples were used on the same day as prepared and never frozen.
Note: The actual measurement does not require prior knowledge about the concentration. However, the cohesive/adhesive strength of biopolymers is expected to be sensitive to overall concentration, the user’s downstream analysis will likely need this information.
- Silicon elastomer preparation
- A volume of Sylgard 184 (~100 ml/m2 surface area sprayed) parts A and B are mixed by gentle stirring to minimize bubble formation at a standard 1:10 ratio and allowed to cure for a minimum of 30 min and a maximum of 1 h.
- After the initial cure period, mix the silicone at approximately 1:1 with hexane in a 50 ml conical tube.
- Mix sample thoroughly by stiring samples for 1 min prior to spraying.
- Human airway primary cell cultures
- Equipment Preparation
- Adhesion/cohesion peel test device (Custom designed device)
Shown in Figure 1B, the custom-made peel machine utilized in our studies was constructed using off-the-shelf aluminum extrusions and linear bearings (80/20 Incorporated) coupled to precision leadscrews. The cartesian XY + Z robot configuration is a similar configuration to a knee-type mill in such that the Z-axis is coupled directly to the base while the X-axis travels atop the Y-axis coupled to the base. On top of the XY axes is attached a box with enough access doors. Torque through the X-, Y-, and Z-axis leadscrews were provided by DC servo motors. The step and directional control of each motor is managed through a microcontroller (Arduino UNO running Labview Interface for Arduino (LIFA) embedded code package) with custom-written Labview interface code.
To detect the force applied at the peel tip, we utilized a custom-designed force sensor which was described previously (Holden et al., 2012). The heart of this sensor is an aluminum thin-beam four-bar linkage (180 micron thick) coupled to an IR-LED dual-beam optical interrupter. This sensor is capable of reliably measuring forces from 0.1 to 100 mN.
Note: While the sensor utilized in these studies has an excellent dynamic range for mucus adhesion/cohesion studies (over 4 orders of magnitude), an equivalent commercial off-the-shelf sensor such as Transducer Technologies GSO family of sensors can be utilized to measure forces between the ranges of 10 to 1,000 gram-force (i.e., 9.8 mN to 9.8 N), depending on the material being tested.
To measure submicron motor positions and peel velocity during peeling, a non-contact magnetic position sensor (AS5311; AMS) is affixed to each axis bearing mount with a corresponding pole-pair magnetic tape (305 mm long). Signals from the AS5311 are sent to the Labview software which generates an Excel file of force vs. distance.
To prevent mucus dehydration, which could significantly affect both adhesion and cohesion measurement, the humidity in the sample chamber is maintained > 80% by a commercially available ultrasonic humidifier controlled through a PID and humidity sensor. The humidifier only uses distilled deionized water and the output of the humidifier is funneled into a 12.7 mm internal diameter tube running into the side of the sample chamber. Inside the chamber, the humidity tubing is placed into the top of a 50-ml tube in order to trap any potential condensation, but allow humidity to escape. - Adhesion/cohesion peel test device (commercially available alternative device)
The system utilized for our previous studies employed the above custom-made peel testing devices. However, there are a number of commercially available devices which might be able to be easily adapted for to the mesh-based mucus adhesion and cohesion studies. For example, the Stable Micro Systems TA.XT2i Texture Analyzer has previously been used to measure biopolymer adhesion (Repka et al., 2005). Here, the key to successful measurements is the identification of a device capable of 1) implementing the peeling and static meshes, 2) possessing an appropriate force sensor (i.e., load cell) with sensitivity for these measurements (typically < 100 mN), and 3) the ability to regulate the sample humidity. - Force sensor calibration and peel machine validation
Prior to adhesion/cohesion studies, calibrate the force sensor using one or more of the following complementary approaches.- A series of known weights covering the range of analysis (1 to 100 mM) can be put on the sensor tip and determine the force.
- Laser-cut calibration tape (2125 Series; 3M) can be peeled off a clean steel plate, following the ASTM-D3330 standard protocol for peel testers for validation (Reference 6).
- Finally, as an overall validation of the system, peel a water-saturated mesh off a static mesh (such as those used in the cohesion studies, below). Here, the force required to do this is twice the surface tension of water (i.e., 1.4 mN/cm) as it creates two water-covered surfaces (Button et al., 2018).
- Adhesion/cohesion peel test device (Custom designed device)
- Adhesion/cohesion “peel” mesh construction
- As described in the background section, the key to making adhesive and cohesive measurements with the low elastic modulus mucus is the use of a “mesh” that binds avidly to the mucus and is connected to the force sensor. In these studies, the mucus-binding peel mesh was prepared from a cellulose mesh (Kimberly Clark). This mesh is laser cut to specific dimensions (see below).
- To strengthen the fragile end of the cellulose mesh connected to the force sensor and to prevent wicking of water away the “sample” end of the mesh, coat one end of the mesh with a silicone elastomer prior to laser cutting as shown in Figure 2.

Figure 2. Silicone spray-coated mesh prior to laser cutting - Here, drape the Kimwipe over two parallel 50 mm wide magnetic steel plates (~3 mm thick) which are themselves mounted roughly 200 mm apart (the distance of the area to be coated with silicone elastomer).
- Spray the silicone elastomer onto the exposed Kimwipe mesh using a spray gun in a well-ventilated area or fume hood. Here, the Kimwipe is infiltrated with silicone everywhere except where covered by the magnetic strip.
- Gently remove each Kimwipe from the magnetic jig and then hang to dry. The demarcation line between the silicone-infiltrated and silicone-free area created during this spray process should be visible, clean, and straight (see Figure 2). After drying, the silicon layer should be between 10-20 μm thick.
- After being allowed to cure an additional 24-48 h at room temperature each Kimwipe is pre-damped with distilled water to remove creases and is laid flat onto a sheet of aluminum and laser-cut to the desired dimensions. In our studies, we produced peel meshes with a silicone-free area of 5 mm by 20 mm and a “tail” of silicone-impregnated mesh approximately 3 mm by 30 mm long (Figure 3)
Note: As mucus binds poorly to the silicon-impregnated mesh, it is vital to ensure that the part in contact with the mucus (i.e., non-tail portion) be free of silicone coating.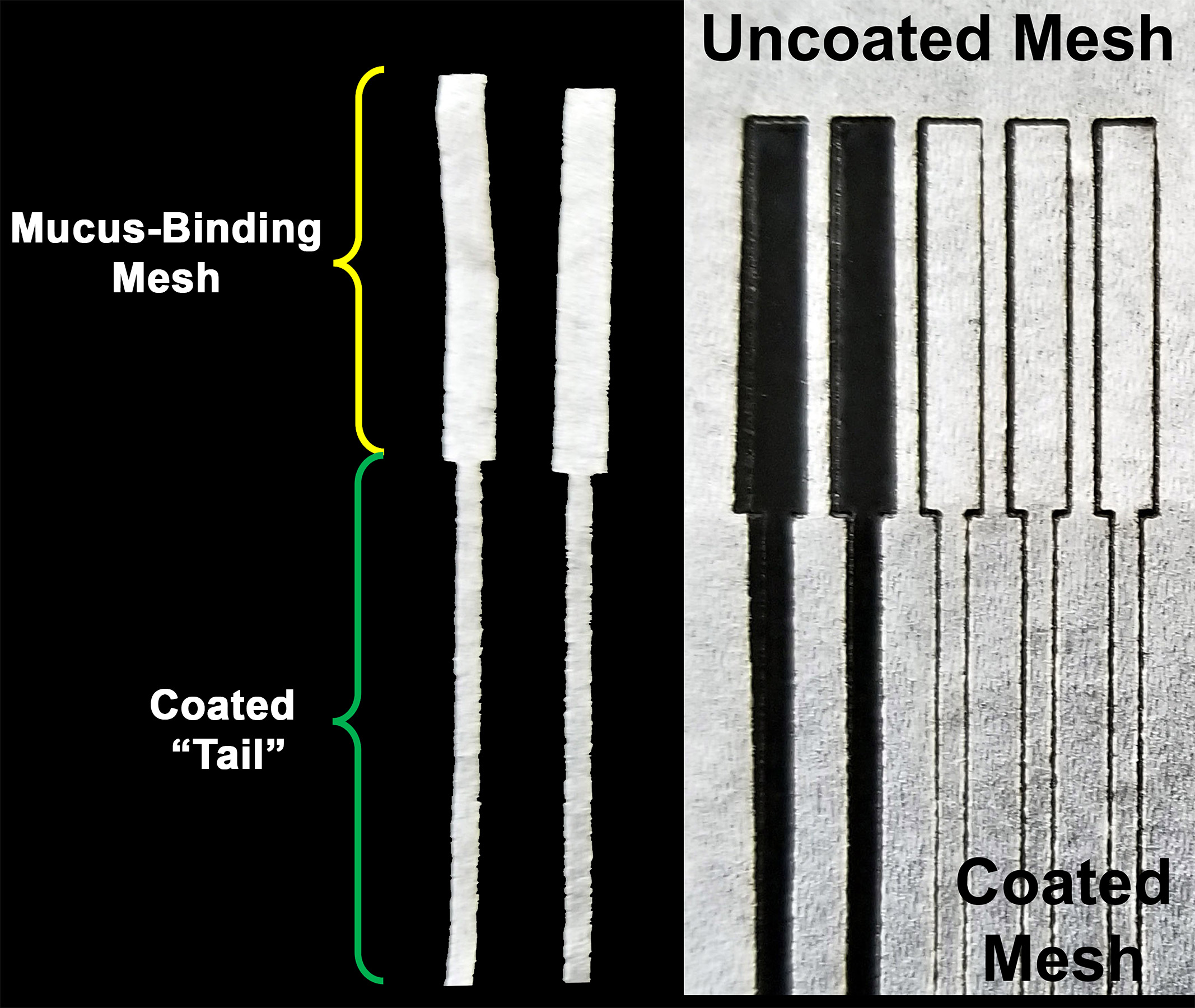
Figure 3. Laser-cut peel meshes. Note region coated with silicon on the “tail” (longer thin portion). - Place the laser cut meshes between two glass plates assisted by 100% isopropanol to get them to lay flat. These meshes in glass plates where then UV sterilized as well as dried in the process overnight.
Note: In the absence of a laser cutter, strips of meshes can be cut using a scalpel or razor blade to the desired sizes. However, the key is to produce meshes with accurate widths as the adhesion/cohesion force is normalized to the width (see Data analysis, below).
- Construction of the cohesion static mesh
- Determine mucus cohesive strength by measuring the force required to tear mucus apart. Here, it is necessary to have a stationary layer of mucus that does not simply detach during the peeling process. To do this, a mucus layer is sandwitched between two mucus-binding meshes (one peel mesh, as described above, and one affixed to the bottom of the measurement chamber using 3M tape–see Figure 1A).
- The static bottom mesh is made using a Kimwipe sheet with double-sided medical grade adhesive tape (3M #1552) on one side
- Then laser-cut the mesh/adhesive tape combo into the desired size. Here, we cut the bottom mesh to be slightly bigger than the peel mesh (here, 7 mm by 14 mm) to ensure that the peel mesh is always covered with mucus.
- During the study, these meshes are adhered to the bottom of the peel tester’s sample chamber using the double-sided tape side of the mesh.
- Mucus adhesion assay
- Airway cultures with either accumulated or exogenous mucus will be utilized to measure mucus adhesion. To ensure sterility, this procedure is best performed in a sterile tissue culture hood.
- To ensure for proper mesh/mucus binding, the silicone-free area of the mesh is slowly lowered onto the apical surface of airway cultures. This is easiest to do starting at the free end and slowing lowering the rest of the mesh until the entire mesh is in contact with the cells.
Note: Because mesh/tail interactions with the wall of the Millipore support (i.e., plastic walls) can affect the force values during peeling, it is important to ensure that the mesh is placed in the center of the culture (see Figure 4). - Once positioned, carefully drape the “tail” over the side of the culture to ensure that this area is not in contact with the cells.
- To ensure for efficient mucus-mesh binding, the cultures should be returned to tissue culture incubator for a minimum of 30-minute prior analysis. If incubation times are less, then poor mucus-mesh binding might result in significantly variable data during the peeling process.
Note: If all the components are sterile, then the mesh can be left in contact with the cultures indefinitely. - Directly prior to an assay, place the plate with the culture into the chamber box and hold in place (using tape or magnets). Given the very small volume of mucus covering the airway surface (on the order of a few microliters) and that adhesion (and cohesion) force is proportional to mucus concentration, it is important that the humidity be maintained to values of > 80%.
- Position the mesh “tail” under the force sensor either manually or using the motorized stage. It is critical that the mesh in contact with the cell culture is not moved during this step as this can result in a failed study (as the mucus has already been peeled).
- Connect the silicone-infiltrated “tail” end of the embedded mesh to the force sensor on the wire end-effector as shown in Figure 4. This can be done by clips, or as shown here, a small amount of hot glue.
- Once connected, the Z-axis is initially tensioned to optimize the angle of peel while not over-tensioning to the point of peel initiation (as denoted by an increase in the force measured by the force sensor.)
- Once properly positioned, zero the force and position sensors.
- Program the parameters of the peel experiment, including speed and peel distance, into the software before initiating a peel.
- During a peel, move the X-axis at the same velocity as the Z-axis to maintain the peel tip at a 90-degree angle with regards to the peel mesh (Video 1).
- Record the peel positions and force sensor data during the peel (at 5,000 samples per second) into an Excel file during the assay.
- Terminate the movement once the peeling has reached the end of the peel mesh.

Figure 4. Peel mesh placement on an airway culture for adhesion strength measurementsVideo 1. Adhesion Test. This video shows the peeling of the mesh (with embedded mucus) from the airway culture cell surface during an adhesion strength measurement. (HBE cells from normal donors and CF patients were obtained from the University of North Carolina Cystic Fibrosis Tissue Culture Core under the auspices of protocols approved by the UNC Institutional Review Board using previously reported methodologies.)
- Mucus Cohesion assay
- For cohesion measurements, adhere the double-sided tape of the static mesh (prepared in Protocol D, above) to the bottom of the peel testing device (Figure 5). Here, it is important that this mesh be placed parallel to the X-axis of the peel machine as off-axis peeling can potentially alter the force recorded.
- Carefully place a test sample of mucus onto the bottom mesh and a bent pipette tip can then be used spread the layer to ensure complete mesh coverage.
Note: Our previous studies with airway mucus (and expectorated sputum) suggest that cohesive strength is insensitive to mucus thickness up to 2 mm. In our previous studies, we typically used a volume of ~5 μl/cm2 mesh surface area (i.e., 0.5 mm thick). Depending on the mucus concentration, thin layers (i.e., < 1 mm) are important to minimize possible slippage of the mesh during the peel maneuver. - Then gently place the corresponding sized peel mesh on top of the lower mesh and sample.
- Incubate the sample and meshes for at least 30 min in the well-humidified sample chamber.
- As with the adhesion measurement, the mesh “tail” is positioned under the force sensor and connected to the sensor (Figure 5) before the Z-axis is tensioned to optimize the angle of peel while not over-tensioning.
- Once properly positioned, zero the force and position sensors.
- Data recording the force of peeling at the desired rate is recorded in to an Excel file (Video 2).
- Terminate the movement once the peeling has reached the end of the peel mesh.
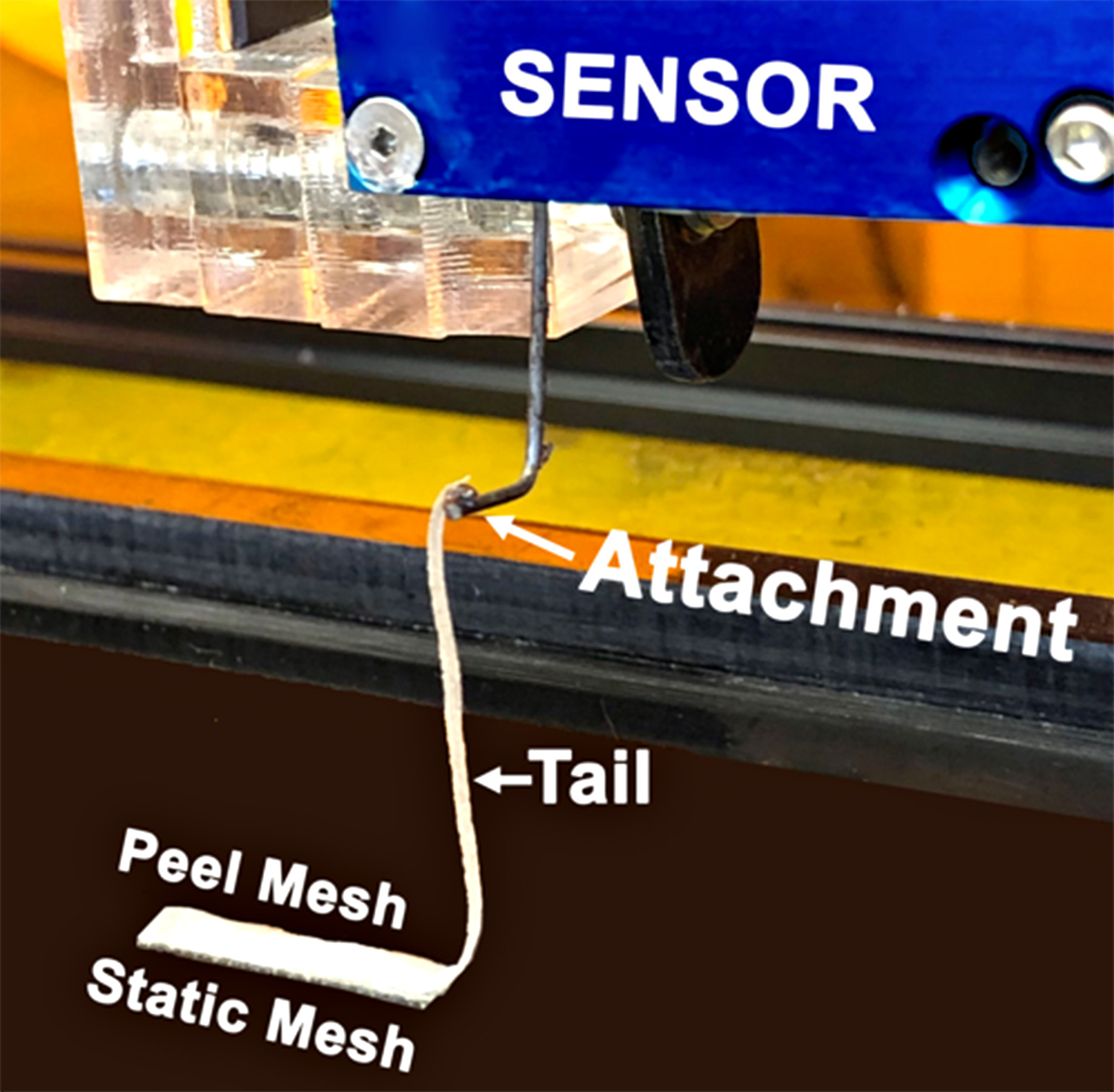
Figure 5. Setup for mucus cohesive strength measurements. Note the placement of the force sensor effector directly above the end of the peel mesh.Video 2. Cohesion peeling. This video shows the peeling of the top mesh (peel mesh) from the bottom mesh (static mesh) during a cohesive strength measurement. (HBE cells from normal donors and CF patients were obtained from the University of North Carolina Cystic Fibrosis Tissue Culture Core under the auspices of protocols approved by the UNC Institutional Review Board using previously reported methodologies.)
Data analysis
For each adhesion and cohesion study, the Labview software generates an Excel file of measured force vs. distance peeled. Figure 6 shows an example-tracing of the resulting cohesion data (the data from adhesion studies are similar). Because the force obtained is directly proportional to the cross-sectional width of the mesh, the results should be divided by the mesh width of the mesh used, allowing for comparable results between experiments. For example, in Figure 6, the data is plotted as mN per cm width. This is particularly important for hand-cut peel meshes.
There are typically two identifiable regions, an initial peel fracture crack peak followed by a region of steady-state peeling. These regions are shown in Figure 6. The boundary between these regions is determined by the location of a positive change in slope following the initial peak (observed at 1 mm peel distance in Figure 6). Note that the variations in the data during the steady-state peeling is commonly observed and is associated with the nature heterogeneities in the real biological mucus samples. The data obtained during the steady-state peeling can then averaged into a single value per assay (for this example, the cohesive strength was 2.61 ± 0.24 mN/cm) (Button et al., 2018). The technical variability of this device/measurement is quite low, allowing us to measure the surface tension of water quite accurately (see Calibration Info in Section C). However, based upon previous studies, we recommend that between 3 and 6 replicate measurements are averaged, in order to overcome the natural biological variability cultured mucus (or expectorated sputum). We have previously used these techniques to produce data demonstrating that both the adhesive and cohesive strength of mucus is highly dependent on mucus concentration and peeling velocity (see Button et al., 2018). 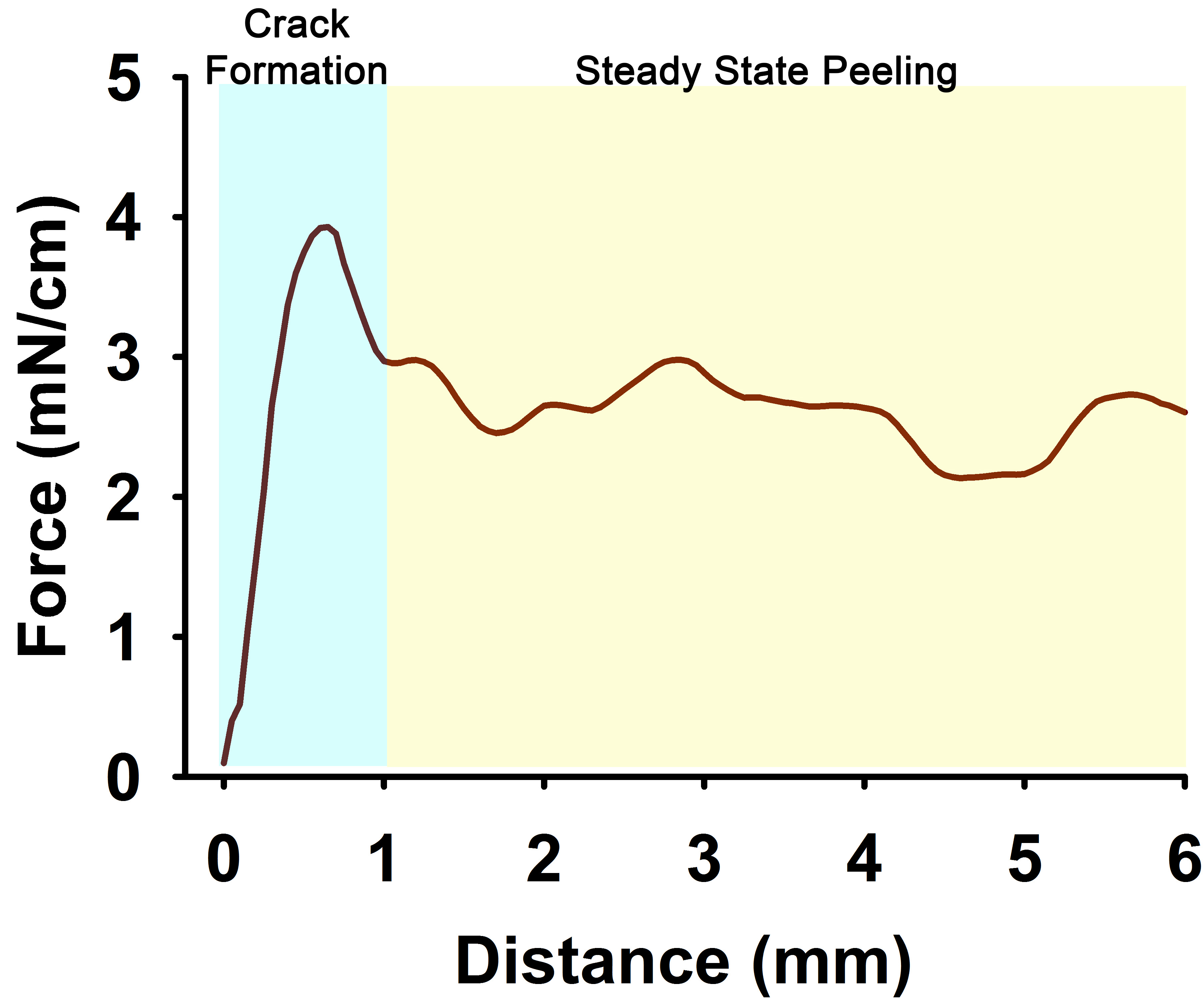
Figure 6. Example of cohesive force sensor data (plotted as mN/cm width) vs. distance of mucus torn at a rate of 100 μm/s
Acknowledgments
We would like to thank Drs. Michael Rubinstein and Richard C. Boucher for assistance with the study design. We also acknowledge the UNC Marsico Lung Institute Tissue Core for cell culture. This project was funded by the Cystic Fibrosis Foundation (Grants BUTTON07XX0, RUBINS09XX0, and BOUCHE15R0), NIH (Grants R01HL125280, R01HL136961, P01HL108808, and P30DK065988), and NSF (Grants EFMA-1830957 and DMR-1121107). This protocol was adapted from Button et al. (2018).
Competing interests
None for any authors listed.
Ethics
HBE cells from normal donors and CF patients were obtained from the University of North Carolina Cystic Fibrosis Tissue Culture Core under the auspices of protocols approved by the UNC Institutional Review Board using previously reported methodologies.
References
- Button, B., Anderson, W. H. and Boucher, R. C. (2016). Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann Am Thorac Soc 13 Suppl 2: S156-162.
- Button, B., Cai, L. H., Ehre, C., Kesimer, M., Hill, D. B., Sheehan, J. K., Boucher, R. C. and Rubinstein, M. (2012). A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337(6097): 937-941.
- Button, B., Goodell, H. P., Atieh, E., Chen, Y. C., Williams, R., Shenoy, S., Lackey, E., Shenkute, N. T., Cai, L. H., Dennis, R. G., Boucher, R. C. and Rubinstein, M. (2018). Roles of mucus adhesion and cohesion in cough clearance. Proc Natl Acad Sci U S A 115(49): 12501-12506.
- Fulcher, M. L., Gabriel, S., Burns, K. A., Yankaskas, J. R. and Randell, S. H. (2005). Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107: 183-206.
- Hill, D. B., Vasquez, P. A., Mellnik, J., McKinley, S. A., Vose, A., Mu, F., Henderson, A. G., Donaldson, S. H., Alexis, N. E., Boucher, R. C. and Forest, M. G. (2014). A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS One 9(2): e87681.
- Holden, J. K., Nguyen, R. H., Francisco, E. M., Zhang, Z., Dennis, R. G. and Tommerdahl, M. (2012). A novel device for the study of somatosensory information processing. J Neurosci Methods 204(2): 215-220.
- International, A. (2010). Active standard ASTM D3330/D3330M-04 (2010), standard test method for peel adhesion of pressure-sensitive tape.
- Matsui, H., Grubb, B. R., Tarran, R., Randell, S. H., Gatzy, J. T., Davis, C. W. and Boucher, R. C. (1998). Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95(7): 1005-1015.
- Repka, M. A., Gutta, K., Prodduturi, S., Munjal, M. and Stodghill, S. P. (2005). Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur J Pharm Biopharm 59(1): 189-196.
- Randell, S. H., Fulcher, M. L., O’Neal, W. and Olsen, J. C. (2011). Primary epithelial cell models for cystic fibrosis research. In Cystic Fibrosis (pp. 285-310). Humana Press.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Goodell, H. P., Shenoy, S. K., Shenkute, N. T., Lackey, E., Dennis, R. G. and Button, B. (2019). Adhesive and Cohesive Peel Force Measurement of Human Airway Mucus. Bio-protocol 9(13): e3287. DOI: 10.21769/BioProtoc.3287.
Category
Biochemistry > Protein > Stability
Cell Biology > Cell-based analysis > Extracellular microenvironment
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










