- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Morphological Evaluation of Wound Healing Events in the Excisional Wound Healing Model in Rats
Published: Vol 9, Iss 13, Jul 5, 2019 DOI: 10.21769/BioProtoc.3285 Views: 12876
Reviewed by: Gal HaimovichChaitali BasoleAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cryopreservation Method for Preventing Freeze-Fracture of Small Muscle Samples
Namrata Ghag [...] Nashwa Cheema
Jan 5, 2025 1932 Views

An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei
Jeremy R. Pearson [...] Eduardo Rosa-Molinar
Apr 20, 2025 2504 Views

Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation
Mi Rim Lee [...] Yun-Hee Kim
Oct 5, 2025 1705 Views
Abstract
Skin wound healing is a complex process involving different events such as blood coagulation, inflammation, new blood vessels formation, and extracellular matrix deposition. These events can be observed by using histology techniques. However, the lack of the standardization of such parameters impacts on the reproducibility of results. Here, we describe a protocol to perform macroscopic and microscopic analyses of the events that occur during skin wound healing using the experimental model of excisional wounds in rats.
Keywords: Rat excisional woundBackground
Skin wounds can be induced by extrinsic factors such as surgical incision, accidental injury or trauma, or by intrinsic factors such as those produced by infection and chronic ulcers. The skin wound healing is a complex process involving cellular, molecular and humoral components to tissue reestablishment after injury. In healthy adult mammals, it involves an early inflammatory phase, characterized by infiltration of leucocytes, with subsequent formation of granulation tissue (new tissue formed), epithelization, and matrix remodeling (Balbino et al., 2005; Estevão et al., 2017). An accurate assessment of wound healing events is critical to reproducible data, with histological studies being the gold standard in this area of knowledge. Here, we describe a protocol using an excisional wound healing model in rats to demonstrate the wound creation, wound closure evaluation and qualitative analysis of microscopic events (inflammation, extracellular matrix deposition, angiogenesis, and epithelialization) in skin wounds.
Materials and Reagents
- Glass slides (Micro Slides, VWR, catalog number: 48311-703)
- Coverslip slides (IMEB, catalog number: CG1-2450)
- Wistar rats (Rattus norvergicus albinus), 250-300 g, and 90-100 days old
- Paraffin (Fisher Scientific, catalog number: AC416770020)
- Ketamine hydrochloride (Sigma, catalog number: K113)
- Xylazine hydrochloride (Sigma, catalog number: K4138)
- Formaldehyde (Fisher Scientific, catalog number: SF100-4)
- Ethanol (Fisher Scientific, catalog number: BP2818100)
- Xylene (Fisher Scientific, catalog number: X3S-4)
- Xylene-based mounting medium (Source Mount, catalog number: 9277722)
- Hematoxylin (Merck, catalog number: 517-28-2)
- Potassium alum (Merck, catalog number: 7784-24-9)
- Mercury oxide (Merck, catalog number: 21908-53-2)
- Eosin (Merck, catalog number: 15086-94-9)
- Phloxine B (Merck, catalog number: 18472-87-2)
- Acetic Acid (Merck, catalog number: 64-19-7)
- Sodium phosphate dibasic (Merck, catalog number: 7558-79-4)
- Sodium phosphate monobasic (Merck, catalog number: 7558-80-7)
- Phosphotungstic acid hydrate (Merck, catalog number: 12501-23-4)
- Chromotrope 2R (Merck, catalog number: 4197-07-4)
- Fast green FCF (Merck, catalog number 2353-45-9)
- Harris Hematoxylin (see Recipes)
- Eosin (stock solution) (see Recipes)
- Eosin phloxine (see Recipes)
- 10% buffered formaldehyde (see Recipes)
- Gomori (see Recipes)
Equipment
- Digital caliper (Digimess, King Tools, catalog number: 502.150BL)
- Hair removal machine (Wahl and Toshico)
- Tweezers 14 cm (Golgan, Fibra cirúrgica, catalog number: 003439)
- Straight Metzenbaum Scissors (WPI, catalog number: 501747)
- 8 mm diameter circular biopsy punch (Kruuse, catalog number: 273693)
- 10 mm diameter circular biopsy punch (Integra Miltex, catalog number: 12-460-406)
- LEICA® manual microtome (LEICA, model: M2125RT)
- Trinocular Microscope with HD camera (LEICA, model: DM500)
- Histological Bath (Lupetec, model: BH2015)
Procedure
- Excisional skin wounds in rats (Figure 1)
- Anesthetize the animal with an intramuscular injection with a mixture of ketamine and xylazine (20 mg/kg and 100 mg/kg, respectively) or following the requirements from the local ethics committee.
- Shave the dorsal region and gently remove two fragments from the skin using an 8 mm diameter circular biopsy punch (the distance between wounds should be approximately 1.5 cm).
Note: This procedure should be handled carefully to not damage the muscular layer below the skin. - Measure the wound area immediately using a digital caliper and repeat the procedure on Days 3, 7, 14 and 21 after wound creation.
Note: This time-course corresponds virtually with phases of skin healing processes.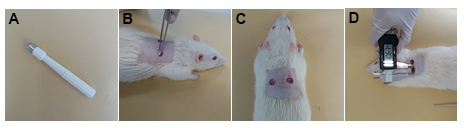
Figure 1. Skin wound creation in rats. A. 8-mm diameter biopsy punch. B-C. Remove two fragments from the dorsal skin. D. Wound area measurement using a digital caliper.
- Histological evaluation of wounds
- Euthanize the animals on Days 3, 7, 14 and 21 after wound creation. This procedure must be in agreement with protocols approved by the local ethical committee.
- Collect the skin fragments using a 10 mm punch. It should cover the entire wound area.
- Immediately fix the skin fragments in 10% buffered formaldehyde. After 6 h in fixation solution, cleaves the tissues in half using a razor and keep the tissues in fixation solution for 24 h. It is crucial to establish the cleave orientation for all wounds (craniocaudal or later-lateral direction), as showing in Figure 2.
Note: For longer-term storage, after 24 h transfer the fragments to 70% ethanol. - Use routine procedures for processing paraffin embedding tissues (dehydration, clearing and wax infiltration).
- Dehydration
90% ethanol: 30 s
100% ethanol I: 30 s
100% ethanol II: 30 s - Clearing (Diaphanization)
Xylol I: 30 s
Xylol II: 30 s - Wax infiltration (Inclusion in paraffin) in a regulated oven at 59 °C
Paraffin I: 30 s
Paraffin II: 30 s - Embedding
The infiltrated tissue should be placed into the base mold with skin sectioned surface facing down (Figure 2C).
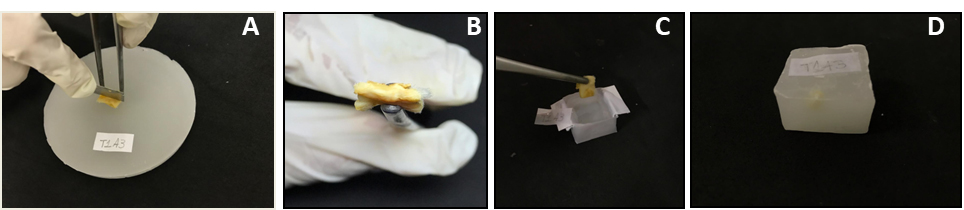
Figure 2. Cleavage and embedding wound tissues. A. Cleavage of paraffin infiltrated tissue; B. Cleaved surface; C. Placing the cleaved surface tissue in a base mold; D. Wax block. - Dehydration
- Sectioning of paraffin-embedded tissue
- Place the wax block on the microtome with its surface parallel to the blade.
- Cut sections at a thickness of about 4-5 µm.
- Use tweezers to pick up the sectioned tissues and transfer to the 39 °C water bath.
- Take the floating sections using glass slides (Figure 3).
- Slides can be stored in racks in an upright position, then dried in an oven, however not exceed 65 °C.
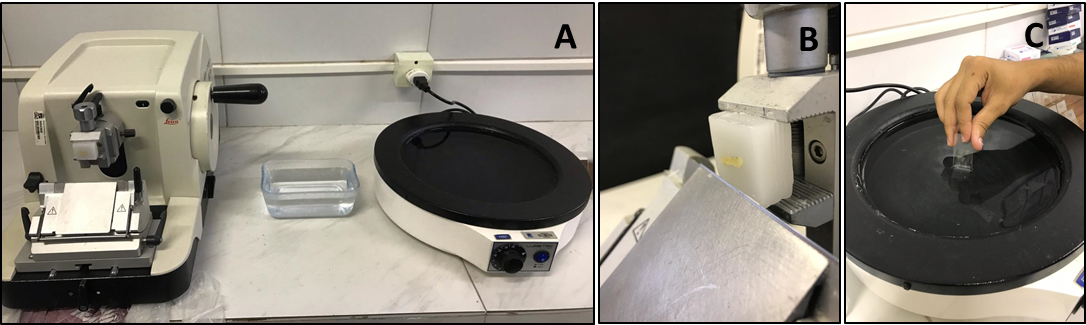
Figure 3. Sectioning procedure using microtome. A. Manual microtome and histological bath. B. Place and adjust the wax block. C. Collect the sectioned tissue with a clean and identified glass slide. - Hematoxylin and Eosin (H&E) and Gomori’s trichrome staining
- Hematoxylin-Eosin staining
See preparation of solutions (Recipes): Harris Hematoxylin and Eosin Phloxine
Technique:Xylene I and xylene II 5 min each 100% ethanol 1 min 90% ethanol 1 min 70% ethanol 3 min Rinse in distilled water 1 min Hematoxylin 30 s to 1 min 30 s Tap water 10 min Eosin phloxine 1-3 min 70% ethanol count 10 times 90% ethanol count 10 times 100% ethanol I 1 min 100% ethanol II 1 min Xylene I 3 min Xylene II 3 min - Gomori trichrome staining
Xylene I and xylene II 5 min each 100% ethanol 1 min 90% ethanol 1 min 70% ethanol 3 min Rinse in distilled water 1 min Harris Hematoxylin 30 s to 1 min 30 s Rinse under tap water 10 min Gomori trichrome dye 15-20 min Rinse in distilled water with 0.5% acetic acid 2 min 95% ethanol I count 10 times 95% ethanol II 1 min 100% ethanol I 1 min 100% ethanol II 1 min Xylene I 3 min Xylene II 3 min
- Hematoxylin-Eosin staining
- The morphological analyses are performed on new tissue in formation and, at later time-points, on the formed cicatricial tissue. Six fields per slice should be evaluated to encompass the borders (area of boundary between the intact connective tissue and the one in formation) and the center of the wound or the scar. For each parameter, five slices are analyzed.
- The qualitative histology studies should be performed by analyzing the stained tissues using a specific score for each parameter, according to described below:
Inflammation
Evaluate the cellular infiltration (polymorphonuclear and mononuclear) in HE-stained sections using the 10x objective (Table 1 and Figure 4).
Table 1. Morphological scores of inflammation in H&E-stained histological sections of excisional rat wounds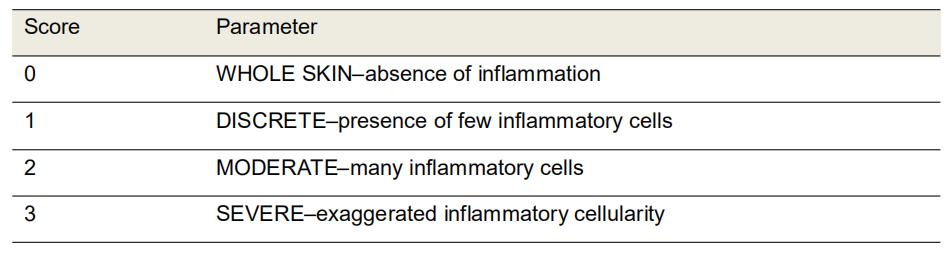
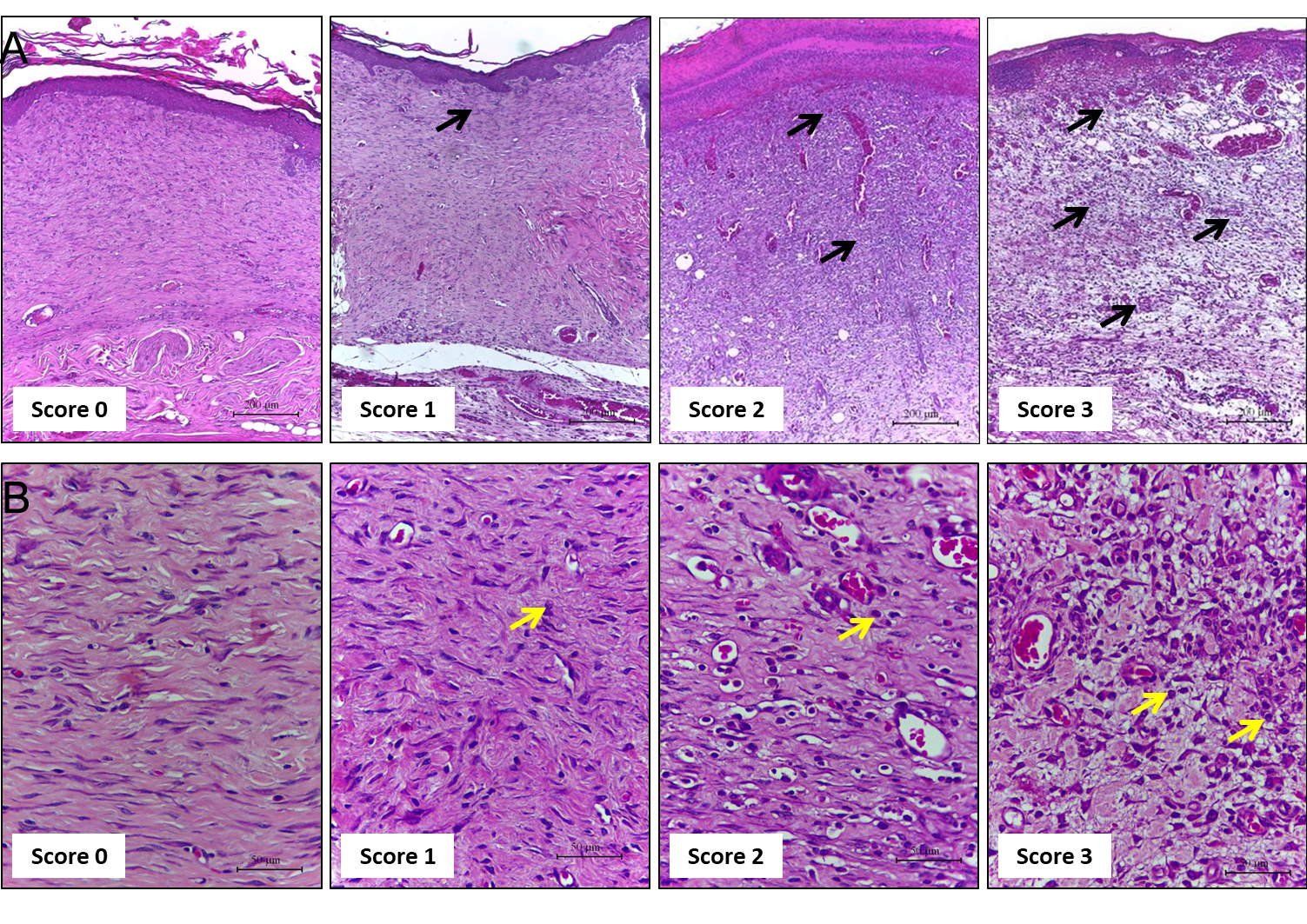
Figure 4. Representative photomicrographs of sections stained with H & E showing the inflammation score. Score 0: skin; Score 1: the wound of Days 21 and 14; Score 2: on Day 7, score 3: on Day 3. Inflammatory cells indicated by black and yellow arrows at different concentrations according to the healing time. A. Scale bars: 200 μm B. Scale bars: 50 μm.
Scab
Evaluate the amount of scab formed in the wound tissue by HE-stained sections analysis using the 4x objective (Table 2 and Figure 5).
Table 2. Morphological scores of scab in H&E-stained histological sections of excisional rats wounds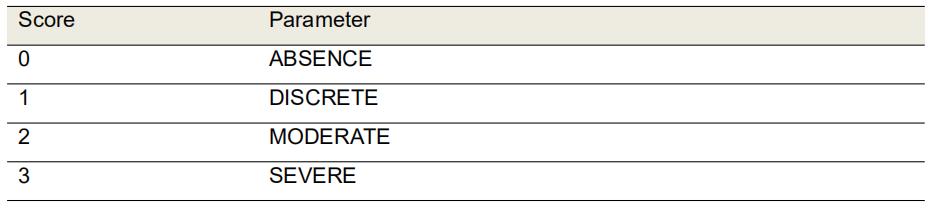

Figure 5. Representative photomicrographs of H&E stained sections showing the scores of scab. Scores 0: on Day 21; Score 1: on Day 14, Score 2: on Day 7 or 3, Score 3: on Day 3 or 7. Scale bars: 500 μm.
Extracellular matrix deposition
Evaluate the amount of extracellular matrix deposited, and the thickness of collagen fibers in Gomori’s trichrome-stained sections using the 10x objective (Table 3 and Figure 6).
Table 3. Morphological scores of extracellular matrix deposition in Gomori’s trichrome-stained histological sections of excisional rat wounds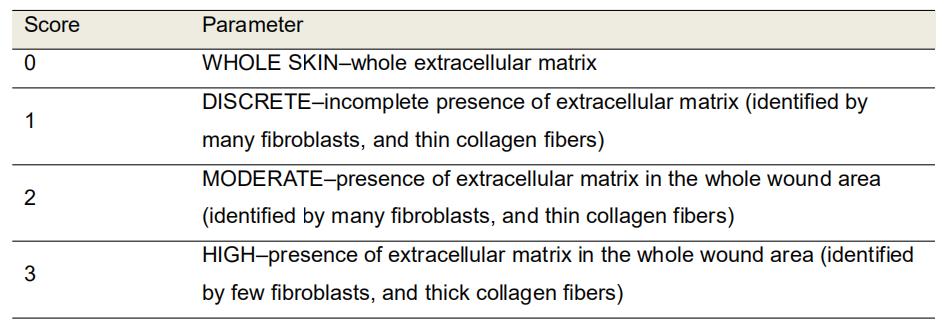
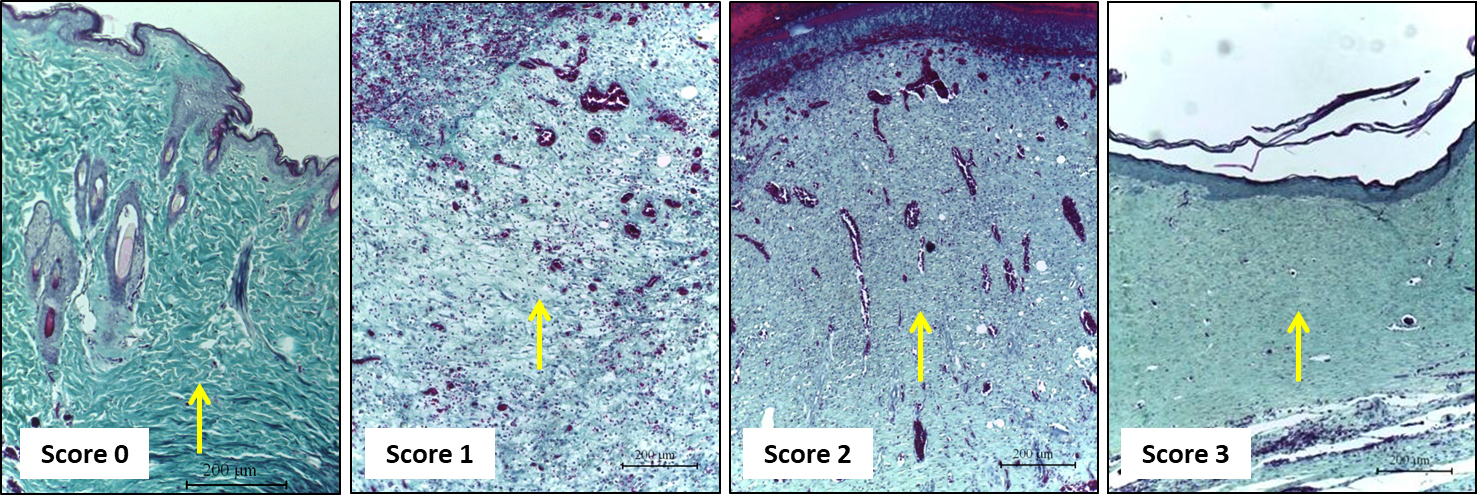
Figure 6. Representative photomicrographs of Gomori’s trichrome-stained sections showing the score of extracellular matrix deposition. Score 0: skin; Score 1: on Day 3; Score 2: on Day 7, Score 3: on Day 14 and Day 21. Scale bars: 200 μm.
Vascularization
Evaluate the amount of blood vessels in the new tissue formed in HE-stained sections using the 40x objective (Table 4 and Figure 7).
Table 4. Morphological scores of vascularization in H&E-stained histological sections of excisional rats wounds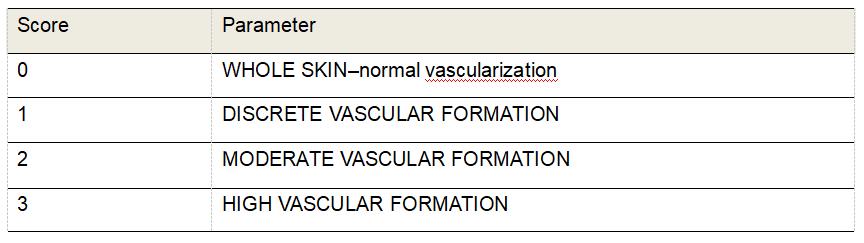

Figure 7. Representative photomicrographs of H&E stained sections showing the score of vascularization. Score 0: whole skin; Scores 1: on Day 21; Score 2: on Day 14, Score 3: on Days 3 and 7. Scale bars: 50 μm.
Epithelialization
Evaluate the new epithelial layer formed in HE-stained sections using the 10x objective (Table 5 and Figure 8).
Table 5. Morphological scores of epithelialization in H&E-stained histological sections of excisional rat wounds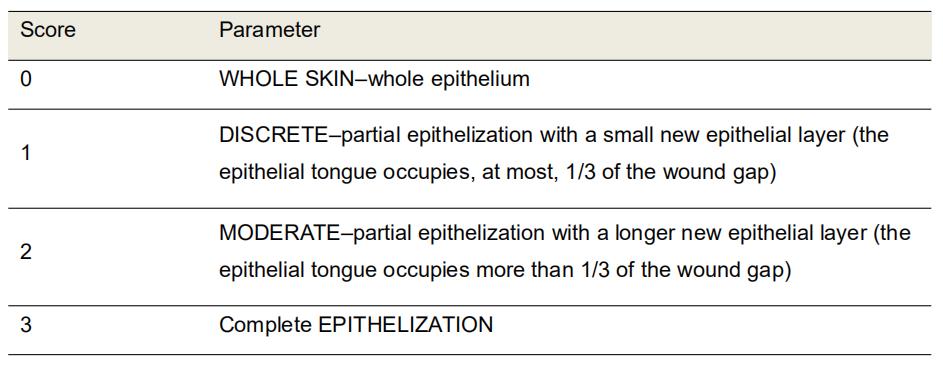

Figure 8. Representative photomicrographs of H&E stained sections showing the wound epithelialization score. Scores 0: on Day 3; Score 1: on Day 7, Score 2: on Day 14, Score 3: on Day 21. Scale bars: 200 μm.
Data analysis
For each parameter, the data analysis is performed using the mean of the score from each slice analyzed (Figure 9). 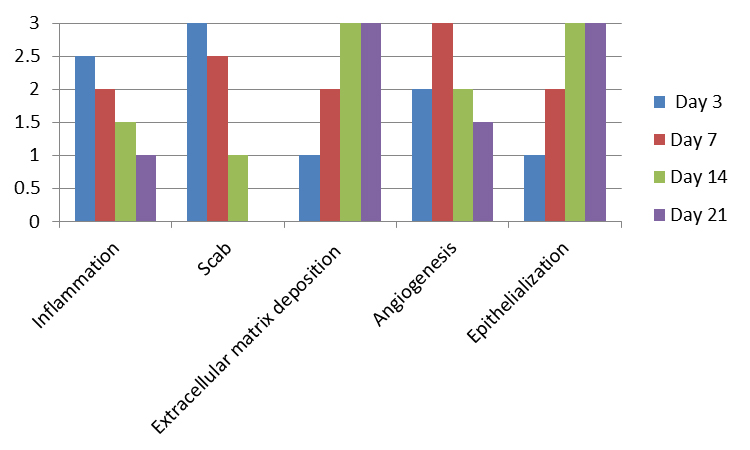
Figure 9. Predictable scores for each parameter on Days 3, 7, 14, and 21 after wound confection in healthy Wistar rats. Inflammation: It occurs at the beginning of the healing process with plasma extravasation, platelet aggregation, clot formation and recruitment of inflammatory (polymorphonuclear and mononuclear) cells to the wound site. Scab: Formed from cellular debris, fibrin, and red blood cells; remain as the epithelial tongue fills the injured site with epithelium. Extracellular matrix deposition: In a second phase, the fibroblasts are in charge of producing collagen fibers and filling the injured area, along with leucocytes and blood vessels, forming the granulation tissue. These fibers will be remodeling and polymerizing until the end of the healing, becoming increasingly dense and organized. Angiogenesis: accompanies the stage of inflammation and fibroplasia, peaking at 5-7 days. Epithelialization: In excisional wounds, these cells depart from the edges of the injured epithelium towards the center of the wound until they are joined and cover the whole site of the epithelium (Balbino et al., 2005; Abbas et al., 2010; Moreira et al., 2015).
Notes
- For reproducibility, the measurement of the wound area must be performed by a single observer throughout the experimental time.
- For histological analysis, after collection of the wound tissue, place the wound on a piece of filter paper to avoid tissue folding during the fixation process in 10% buffered formaldehyde. Then proceed to conventional paraffin embedding processes.
- The cicatricial process can last for months in the remodeling phase, being able to be evaluated in time-points longer than 21 days, according to the experimental aim.
- The pictures indicated for each score correspond to cutaneous wounds of 8 mm that cure spontaneously in healthy rats.
- See Castro-Souza et al. (2017) for details on how to analyze the wound area.
- See Moreira et al. (2015) for details on excisional skin wounds.
Recipes
- Harris Hematoxylin
Dissolve hematoxylin in alcohol; Dissolve the potassium alum in the heated water (do not boil); mix the two solutions; boil quickly (1 min); slowly add the mercury oxide; let it simmer until it turns purple; remove from heating and put in cold water bath; Add acetic acid; Store in an amber bottle and filter before useHematoxylin 5.0 g Alcohol 96% 50 ml Potassium alum 100 g Distilled water 1,000 ml Mercury oxide 2.5 g Glacial acetic acid 4 ml - Eosin (stock solution)
Eosin Y 1.0 g Distilled water 100 ml - Eosin Phloxine
Aqueous Eosin 1% 100 ml Ethanol 95% 780 ml Phloxine B 1% in distilled water 10 ml Glacial acetic acid 4 ml - 10% buffered formaldehyde
Phosphate buffer, 0.2 M, pH 7.2-7.4Na2HPO4 (sodium phosphate dibasic) 11.155 g NaH2PO4 sodium phosphate monobasic) 2.767 g Distilled water 400 ml Adjust the pH and fill the volume to 500 ml 37% formaldehyde 100 ml Phosphate buffer 900 ml - Gomori
Chromótrope 2R 0.6 g Fast greem FCF 0.3 g Phosphotungstic acid hydrate 0.8 g Glacial acetic acid 1.0 ml Distilled water 100 ml
Acknowledgments
This work was supported by Conselho Nacional de Pesquisa/CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES, Fundação de Amparo à Ciência e Tecnologia de Pernambuco- FACEPE, Programa de Biociência Animal da UFRPE, Recife, PE, Brazil. LRME holds a CAPES PNPD scholarship; LSB and JEN hold CNPq Research Fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics
The project proposal was approved by the Animal Ethics Committee of the Universidade Federal Rural University de Pernambuco (license no 57/2017). At the end of the experiment the animals were euthanized, prepared and discarded complied with the guidelines established by our local Institutional Animal Welfare Committee.
References
- Abbas, A., Kumar, V., Fausto, N (2010). Robbins & Cotran -Patologia - Bases Patológicas das Doenças. 8a ed. Rio de Janeiro: Elsevier. 1557p.
- Balbino, C. A., Pereira, L. M. and Curi, R. (2005). Mechanisms involved in wound healing: a revision. Rev Bras Cienc Farm 41(1): 27-51.
- Castro Souza Junior Neto, J., Estevao, L. R., Baratella-Evencio, L., Vieira, M. G., Simoes, R. S., Florencio-Silva, R., Evencio-Luz, L. and Evencio-Neto, J. (2017). Mast cell concentration and skin wound contraction in rats treated with Ximenia americana L. Acta Cir Bras 32(2): 148-156.
- Estevão, L. R. M., Simões, R. S., Cassini-Vieira, P., Canesso, M. C. C., Barcelos, L. D. S., Rachid, M. A., Camara, C. and Evêncio-Neto, J. (2017). Schinus terebinthifolius Raddi (Aroeira) leaves oil attenuates inflammatory responses in cutaneous wound healing in mice. Acta Cir Bras 32(9): 726-735.
- Moreira, C. F., Cassini-Vieira, P., Silva, M. F. and Barcelos, L.S. (2015). Skin wound healing model - excisional wounding and assessment of lesion area. Bio-protocol 5(22): e1661.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Estevão, L. R. D. M., Cassini-Vieira, P., Leite, A. G. B., Bulhões, A. A. V. D. C., Barcelos, L. D. S. and Evêncio-Neto, J. (2019). Morphological Evaluation of Wound Healing Events in the Excisional Wound Healing Model in Rats. Bio-protocol 9(13): e3285. DOI: 10.21769/BioProtoc.3285.
Category
Immunology > Animal model > Rat
Cell Biology > Tissue analysis > Injury model
Cell Biology > Tissue analysis > Histomorphology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link













