- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Detection of Antigen-specific T cells in Spleens of Vaccinated Mice Applying 3[H]-Thymidine Incorporation Assay and Luminex Multiple Cytokine Analysis Technology
Published: Vol 9, Iss 11, Jun 5, 2019 DOI: 10.21769/BioProtoc.3252 Views: 12068
Reviewed by: Alexandros AlexandratosWoojong LeeMichela Perego

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2431 Views

Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood
Ilyssa E. Ramos [...] James M. Cherry
Nov 20, 2025 2379 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 268 Views
Abstract
For many infectious diseases T cells are an important part of naturally acquired protective immune responses, and inducing these by vaccination has been the aim of much research. Here, we describe a protocol for the analysis of vaccine-induced antigen-specific immune responses. For this purpose, cells of whole spleens obtained from vaccinated BALB/c mice were ex vivo stimulated with the antigen incorporated in the vaccine. Evaluation and characterization of vaccine-induced adaptive T cell responses was performed by assaying spleen cell proliferation through radioactive 3[H]-thymidine incorporation and multiplex cytokine analysis of IL-2, IFN-γ and TNFα in supernatants from spleen cell suspensions. This protocol can be very useful as a starting point for assessing vaccine-induced memory T cell populations in pre-clinical studies.
Keywords: Splenocyte culturesBackground
T cells have an essential role in protection against a variety of infections by controlling or delaying the onset of disease. Thus, vaccine development for these infections is focused on generating protective T-cell responses (Seder and Hill, 2000). Therefore, selecting T-cell readouts to measure in pre-clinical vaccines studies to assess immunogenicity would aid progress on T cell vaccines. Ideally, a T cell–vaccine would induce long-lived memory T cells, characterized as central memory (TCM) within lymphoid tissues, i.e., lymph nodes and spleen, and effector memory (TEM) within peripheral tissues. Memory T cells when encounter the previously given antigen via vaccination they are clonally expanded in order to differentiate into effector cells. A key mechanism by which T cells mediate their effector function is through the simultaneous production of IFN-γ, TNFα and IL-2 (Darrah et al., 2007). IFN-γ synergize with TNFα to promote killing of pathogens through activation of macrophages, whereas IL-2 strongly enhances the expansion of T cells leading to more efficient effector responses. Therefore, among the most common ways to assess the existence of vaccine-induced cell effector populations is to measure spleen cell proliferation and IL-2, TNFα and IFN-γ production upon ex vivo stimulation with the antigens that have been incorporated in the vaccine (Seder et al., 2008). Cell proliferation encompasses DNA synthesis, mitosis and an increase in cell number. The 3[H]-thymidine incorporation assay allows the measurement of 3[H]-thymidine into DNA during the S phase of the cell cycle as a quantitative measure of new DNA synthesis and yields statistically significant data even in the presence of low number of divided cells. This is achieved due to the linear relationship between the count rates and the rate of DNA synthesis (Ahern et al., 1976). Thus, this method has been a gold standard for measuring cell proliferation in mixed lymphocyte cultures. If the goal of the study is not only to determine DNA synthesis but also to characterize the fraction of cells in the S phase, then CFSE labeling stands out as a versatile alternative. Despite the fact that the 3[H]-thymidine incorporation assay is a radioactive method, it is still considered one of the best and most reliable methods providing statistically significant data. On the other hand, there are various established methods for assessing cytokine production. Typically, ELISA and ELISpot assays are the gold standard methods for quantifying antigen-specific cellular responses after vaccination but they are limited only to a single parameter readout (Ranieri et al., 2014). Intracellular cytokine staining and analysis by flow cytometry is another method of choice as it allows both multi-parameter cytokine analysis and phenotyping of cells (Freer and Rindi, 2013). However, it is characterized by many disadvantages. Among them are that it is labor intensive, it costs more per sample and requires a large number of cells (Freer and Rindi, 2013). On the other hand, multiplex arrays allow multiple cytokine analysis using different fluorescent beads that depending on the kit used a maximum of 80 cytokines can be investigated. The cytometric bead array system and the Luminex multi-analyte profiling (xMAP) technology both employ beads sets which are distinguishable under flow cytometry in the absence of Luminex machine. Moreover, they have been used in various pre-clinical studies to explore cytokines profiles induced by vaccination, since different samples can be used, i.e., serum or cell-supernatant (Flaxman and Ewer, 2018). Based on the above, we describe a detailed protocol for assessing activation and effector functions of antigen-specific spleen cell populations using 3[H]-thymidine incorporation and Luminex assays.
Materials and Reagents
- Pipettes tips: 0.5-10 μl, 10-200 μl, 200-1,000 μl (Greiner Bio-One, catalog numbers: 771291, 739290, 740290)
- 50 ml sterile conical tube (Thermo Fischer Scientific, catalog number: 339652)
- 15 ml sterile conical tube (Thermo Fischer Scientific, catalog number: 339650)
- Disposable sterile pipettes: 2 ml, 5 ml, 10 ml (Greiner Bio-One, catalog numbers: 710180, 606180, 607180)
- 96-well sterile cell culture U-bottom plate (Greiner Bio-One, catalog number: 650180)
- 24-well sterile cell culture plate (Greiner Bio-One, catalog number: 662160)
- 5 ml syringe (Terumo, catalog number: SS+05S21381)
- 70 μm Nylon Cell strainer (Falcon, catalog number: 352350)
- Petri dishes (Greiner Bio-One, catalog number: 664102)
- Eppendorf tubes (Greiner Bio-One, catalog number: 616201)
- Glass fiber filtermat (PerkinElmer, catalog number:1205-401)
- Sample Bags for Microbeta (PerkinElmer, catalog number: 1405-432)
- 0.22 μm filter (Merck Millipore, catalog number: SLGV004SL)
- BALB/c female mice, 6-8 weeks old (Department of Animal Models for Biomedical Research, Hellenic Pasteur Institute)
- Antigens of interest. For the purposes of the present protocol re-stimulation of spleen cells was conducted with multi-epitope peptides (i.e., CPA160-198 and EF-21-137) contained in experimental vaccines against parasite Leishmania developed in our laboratory
- RPMI-1640 (Biowest, catalog number: L0501-500)
- HEPES (Biowest, catalog number: L0180-100)
- Penicillin-streptomycin (100x) (Biowest, catalog number: L0022-100)
- L-glutamine (100x) (Biowest, catalog number: X0550-100)
- Fetal bovine serum Premium (FBS) (catalog number: LSB-015S)
- Trypan blue for vital staining (B.D.H., catalog number: 34078)
- Ammonium chloride (NH4Cl), ACS reagent, ≥ 99.5% (Sigma-Aldrich, catalog number: 213330)
- Potassium bicarbonate (KHCO3), ACS reagent, ≥ 99.7% (Sigma-Aldrich, catalog number: 237205)
- Hydrochloric acid (HCl) solution, 1 M (Sigma-Aldrich, catalog number 150696)
- Ethylenediaminetetraacetic acid disodium salt dehydrate (Na2EDTA), ACS reagent, 99.0-101.0% (Sigma-Aldrich, catalog number: E4884)
- Sodium chloride 99.9% (NaCl) (Applichem, catalog number: 381659)
- Potassium chloride (KCl), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 746336)
- Sodium phosphate dibasic (Na2HPO4), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 795410)
- Potassium phosphate monobasic (KH2PO4), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 795488)
- Sodium hydroxide (NaOH) solution, 1 M (Sigma-Aldrich, catalog number 79724)
- Concanavalin A from Canavalia ensiformis (Sigma-Aldrich, catalog number C5275)
- Thymidine, [methyl-3H] (PerkinElmer, catalog number: NET27X001MC)
- Scintillation fluid (PerkinElmer, catalog number: 1200-439)
- Milliplex® MAP Kit (MerckMillipore Corporation, catalog number: MCYTOMAG-70K)
- (Optional) Array Mouse Th1/Th2 Cytokine Kit (BD Biosciences, catalog number: 551287); LEGENDplex Mouse Th1/Th2 Panel (8-plex) (Biolegend, catalog number: 740750); FlowCytomix mouse Th1/Th2 10plex (Bender MedSystems, catalog number: BMS720FF)
- Ammonium chloride lysis solution (ACK, pH 7.2) (see Recipes, page 10)
- Phosphate-buffered saline, 10x (PBS, pH 7.2) (see Recipes, page 10)
- Complete RPMI medium (see Recipes, page 10)
- Concanavalin A stock solution (see Recipes, page 10)
- 0.4% (w/v) Trypan blue exclusion dye (see Recipes, page 10)
Equipment
- Dressing scissor straight sharp with blunt forceps, skylar dressing forcep and forcep full curved, all kept sterile with 70% ethanol
- Gilson Pipettes (P-10, P-20, P-200, P-1000)
- Multichannel pipette (Nichiryo, Nichipet 7000)
- Neubauer counting chamber (Paul Marienfeld GmbH & Co.)
- CO2 incubator (New Brunswick, Galaxy 170S)
- Laminar flow hood (Telstar, Bio-II-A)
- Refrigerated centrifuge (Centurion Scientific Ltd, PrO-Research)
- Light microscope (Olympus, BH)
- Water bath (LabTech, LSB-015S)
- Combi cell harvester (Skatron Instruments, 11025/11028)
- Microplate scintillation and luminescence counter (Wallac, Microbeta 1450 Trilux)
- Luminex® 200TM System with xPONENT® Software (Life Technologies, Novex®)
Software
- Cytokine production data were analyzed using the xPONENT® software (Luminex)
- Statistical tests were performed using GraphPad Prism v6 software
Note: The different groups were compared using One-way ANOVA with the Tukey’s multiple comparison test, comparing all groups with the non-vaccinated mice group.
Procedure
- Isolation of white-blood cells from spleen
- Take the spleen from each vaccinated mouse at pre-determined time points under aseptic conditions.
Note: The time points selected for the evaluation of vaccination-induced cellular immune responses vary according to the vaccination protocol followed in each lab. Usually, spleens are obtained at the end of vaccination protocol (i.e., 2 weeks post last injection) in order to detect the existence of antigen-specific memory and effector T cell populations. - Under a sterile biosafety cabinet, put each spleen into a Petri dish containing 5 ml of complete RPMI medium.
- Using a circular motion, press spleen against the bottom of the dish with the plunger of a 5 ml syringe until mostly fibrous tissue remains (Figure 1).
- Filter cells to avoid tissue debris by passing cell suspension through a 70 μm Nylon Cell strainer into a 15 ml sterile conical tube (Figure 1).
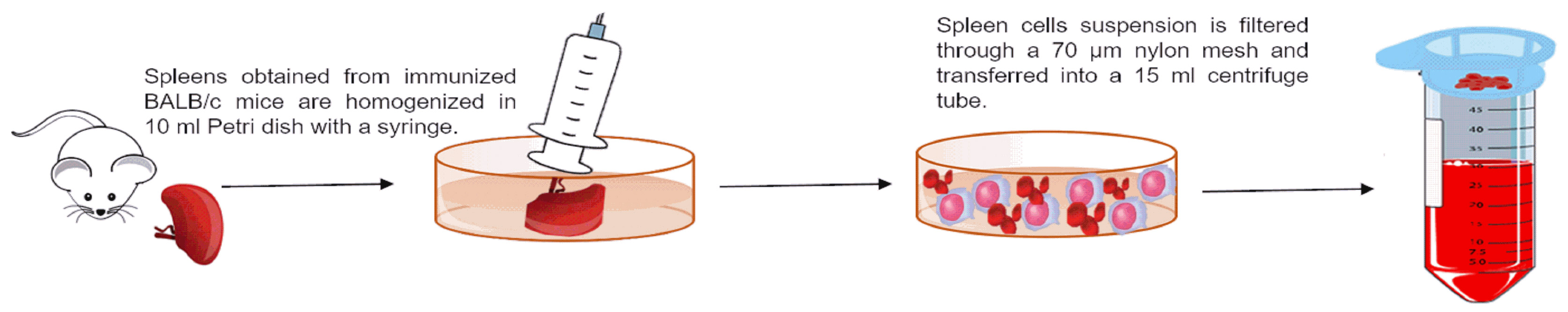
Figure 1. Representative procedure of spleen cells isolation - Wash the spleen cells suspension with complete RPMI medium by centrifugation at 350 x g for 10 min at 4 °C.
- Discard the supernatant and resuspend cells in 3 ml of ice-cold ACK to lyse red blood cells.
- Incubate for 5 min at room temperature with occasional shaking.
- Stop lysis reaction by adding complete RPMI medium to a final volume of 10 ml.
- Wash twice the cells with complete RPMI medium by centrifugation at 350 x g for 10 min at 4 °C.
- Discard the supernatant and resuspend cells in 5 ml of complete RPMI medium.
- Mix 10 to 20 μl of cell suspension to Trypan blue exclusion dye in an Eppendorf tube to a final dilution of cells of 1:10 or 1:20. Allow mixture to incubate about for 3 min at room temperature. Apply a drop of trypan blue/cell mixture to a Neubauer chamber.
Note: Cells should be re-suspended in serum-free medium since serum proteins stain with Trypan blue and produce misleading results. Also, the final dilution of cells in Trypan blue dye depends on the approximate number of cells present. If the sample is not diluted enough, the cells will be too crowded and difficult to count. If it is too dilute, the sample size will not be enough to make strong inferences about the concentration in the original mixture. By performing a redundant test on a second chamber, the results can be compared. Thus, the aliquot should contain a convenient number of cells to count in a Neubauer. Usually, 1:10 final dilution of cells is suitable when a spleen is dispersed to a 5 ml RPMI. - Count separately the unstained (viable) and stained (nonviable) cells under a light microscope.
Note: Trypan blue exclusion test is based on the principle that living cells possess intact membranes that exclude certain dyes such as Trypan blue, whereas dead cells do not. Thus, a viable cell will have a clear cytoplasm whereas a nonviable will have a blue cytoplasm. Also, caution is needed to the time the cells are left with the dye since longer incubation periods will lead to cell death and reduced viability counts. For that reason, cells should be counted within 3 to 5 min of mixing with Trypan blue. - Determine cells per ml by the following calculations:
Cells/ml = average count per square x dilution factor x 104
Total cells = cells/ml x total original volume of cell suspension from which sample was taken
Routinely, one mouse spleen gives about 1 x 108 splenocytes.
Note: Splenocytes recoveries vary significantly with age, gender and strain of mice. - Resuspend cells in complete RPMI medium-10% FBS at a final concentration of 2 x 106 cells/ml.
- Take the spleen from each vaccinated mouse at pre-determined time points under aseptic conditions.
- Proliferation assay
- For the assay incorporate the following controls:
- No antigen stimulation (negative control; splenocytes in complete RPMI-10% FBS only).
- Positive control with a lymphocyte mitogen (splenocytes with the mitogen Concanavalin A).
- Prepare 2-fold dilution series on the antigens of choice in 15 ml conical tubes using complete RPMI-10% FBS. The recommended dilutions are: 20, 10 and 5 μg/ml.
- Plate cells in a 96-well U-bottom cell culture plate. Consider 2 x 105 splenocytes per well (i.e., 100 μl of splenocyte suspension). Each condition should be assayed in triplicate.
Note: When using unfractionated splenocytes from recently primed mice, usually 1 x 105-5 x 105 cells are plated per well in order to have a significant proliferation assay. In this case, unfractionated splenocytes contain accessory cells such as macrophages, dendritic cells and B cells that serve as antigen-presenting cells of the added antigen as well as a source of secondary signals to responder T cells, thus enhancing their proliferative response. - Add 100 μl of diluted antigens or ConA per well to a final volume of 200 μl per well. Final antigen concentrations should correspond to 10, 5 and 2.5 μg/ml for antigens and 3 μg/ml for ConA.
- To negative controls add 100 μl of complete RPMI-10% FBS.
- Incubate for 72 h at 37 °C, 5% CO2, 95% humidity.
- Add 25 μl of diluted [3H]-Thymidine to complete RPMI per well corresponding to 0.5 μCi per well. [3H]-Thymidine should be added gently as a drop, without mixing in the well so as not to disrupt cell clusters (Figure 2).
Note: [3H]-Thymidine is a radioactive material. All the procedures need to be done with dedicated incubators, hoods and pipettes in certified laboratories. Consult material safety data for proper handling instructions and get training to work with radioactive materials. - Incubate for 18 h at 37 °C, 5% CO2, 95% humidity.
Note: Incubation time is dependent on cell number used. - Lyse cells and harvest their labeled DNA onto glass-fiber filtermats using a semi-automated cell-harvester. Let dry filtermats at a dry oven for about 30 min at 60 °C.
- Put filters into bags and add scintillation fluid with a disposable plastic pipette.
- Seal bags and count [3H]-Thymidine incorporation in a beta-scintillation counter.
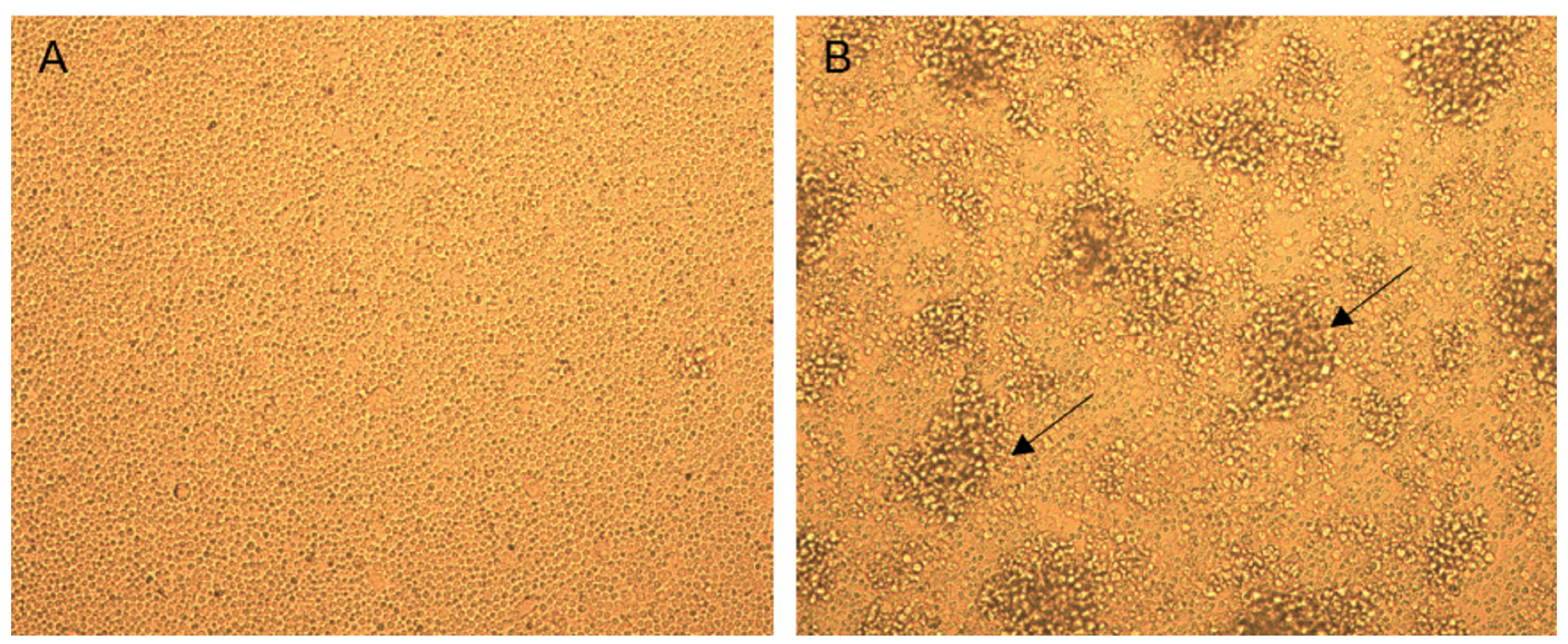
Figure 2. Representative images of A) spleen cell cultures that do not proliferate (complete medium only) and B) spleen cell cultures that proliferate in response to Concanavalin A. Proliferating T cell clusters are indicated by arrows. Magnification 20x.
- For the assay incorporate the following controls:
- Cytokine detection
- Dilute antigens in complete RPMI -10% FBS beginning from as described above.
- Plate cells in a 24-well cell culture plate. Consider 4 x 106 splenocytes per well (i.e., resuspend cells so as 4 x 106 cells would be in 500 μl of complete RPMI-10% FBS). Each condition should be assayed at least in duplicate.
- Add 500 μl of diluted antigens per well to a final volume of 1 ml per well. Final antigen concentrations correspond to 10 μg/ml.
- To negative controls add 500 μl of complete RPMI-10% FBS.
- Incubate for 72 h at 37 °C, 5% CO2, 95% humidity.
- Collect supernatants to Eppendorf tubes and centrifuge at 2,330 x g for 5 min at 4 °C to remove cellular debris.
- Collect cell-free supernatants into new Eppendorf tubes and store at -70 °C until used.
- On the day of analysis, thaw samples at room temperature. Measure cytokine production with the mouse 5-plex Luminex panel (IL-2, IFN-γ, TNF-α, IL-4, and IL-10) following exactly the outlined protocol in the Milliplex manual.
Note: Avoid multiple (> 2) thawing-freezing cycles since it would cause protein degradation leading to cytokine production underestimation. Also, in some cases culture supernatants may require a dilution in the case of samples with cytokine concentrations above the dynamic range of the assay (range over which there is a linear relationship between the cytokine concentration and the absorbance reading). - Run the samples on the Luminex® 200TM system with xPONENT® software according to the manufacturer’s instructions.
Note: In the case of not having a Luminex multi-analyte profiling system, a cytometric bead array that it is compatible with flow cytometers can be used as an alternative, i.e., Cytometric Bead Array Mouse Th1/Th2 Cytokine Kit (BD Biosciences), LEGENDplex Mouse Th1/Th2 Panel (8-plex) (Biolegend), FlowCytomix mouse Th1/Th2 10plex (Bender MedSystems).
Data analysis
Lymphoproliferation assay:
- [3H]-Thymidine incorporation is given as counts per minute (cpm) (Figure 3A). Compute the data as the difference in cpm of stimulated (experimental or ConA pulsed) and control (medium) cultures. This is done by subtracting mean of cpm from triplicate control cultures from the arithmetic mean of the cpm from corresponding stimulated cultures. The results are referred to as “∆cpm” (Table 1, Figure 3B).
- Otherwise, compute the data as the ratio of cpm of stimulated and control cultures. This is done by dividing the arithmetic mean from stimulated cultures by the arithmetic mean of cpm from control cultures. The results are referred to “SI” stimulation index (Table1, Figure 3C).
Note: SI computation has the disadvantage that small changes in background values will result in large changes in SI and should be interpreted with caution.
Table 1. [3H]-Thymidine incorporation is given as counts per minute (cpm) from one representative experiment. Spleen cells obtained from immunized mice were treated with either complete medium, 3 and 6 μg/ml ConA or 10, 20 and 40 μg/ml CPA160-189 or 5 μg/ml N-LiEF-2. Data are means ± S.D. from three replicates for each condition.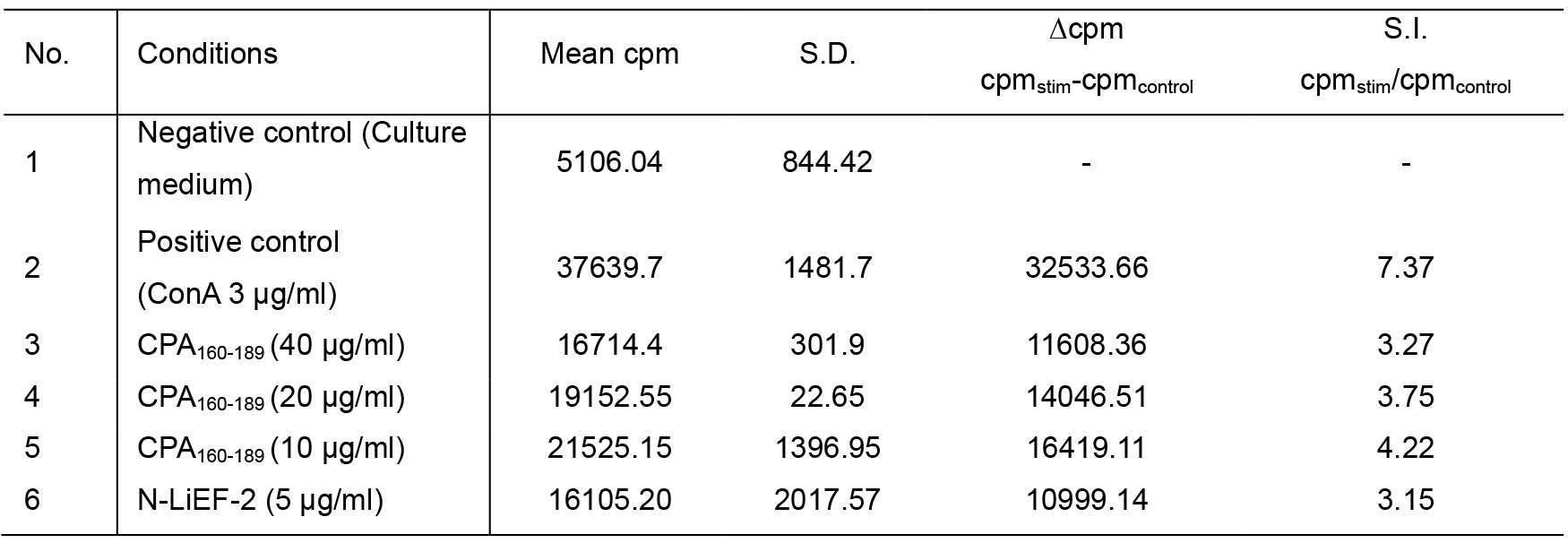
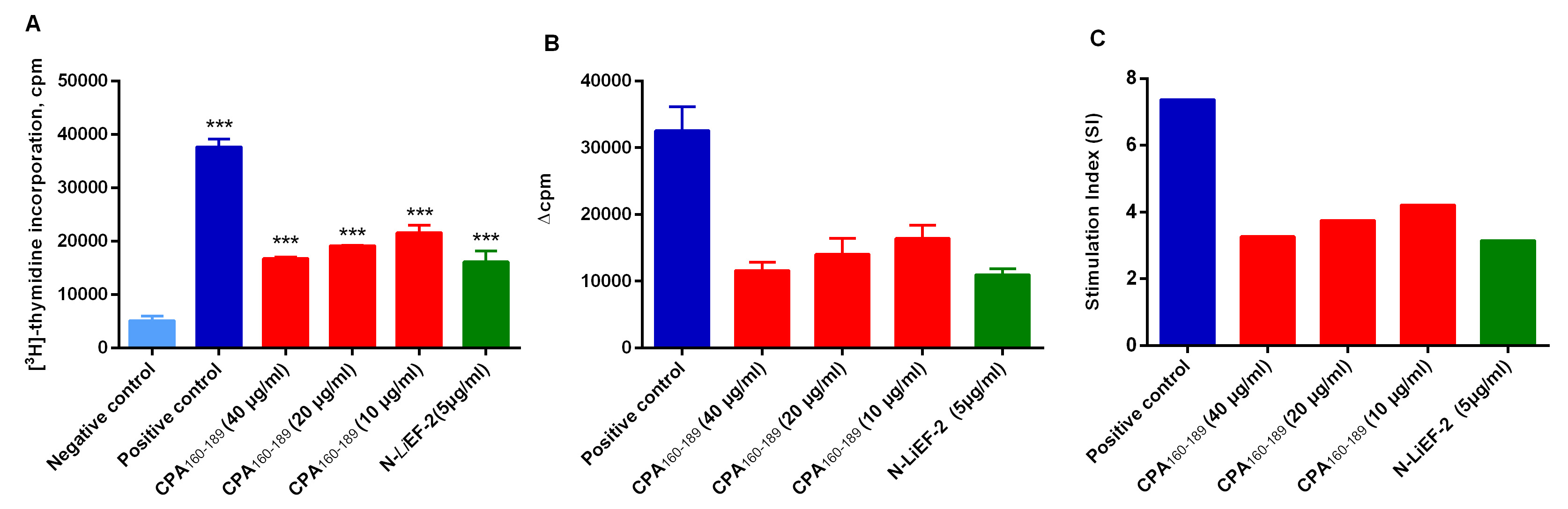
Figure 3. Typical graphs of splenocytes proliferation depicted as A) [3H]-Thymidine incorporation, B) Δcpm and C) Stimulation index (SI). Spleen cells obtained from immunized mice were treated with either complete medium, 3 and 6 μg/ml ConA or 10, 20 and 40 μg/ml CPA160-189 or 12.5 μg/ml CPA160-189. Data are means ± S.D. from three replicates for each condition.
Cytokine detection:
For cytokine analysis, for each sample is calculated the mean of the negative control that is considered the background value (spleen cells in complete medium only) (Table 2). Then, the mean of cytokine production in the presence of antigenic stimulus for each sample is calculated (Table 2). In order to estimate antigen-specific cytokine production, the mean background value is subtracted from the mean value obtained after stimulation (Figure 4).
Table 2. Representative experiment of cytokine production. Spleen cells obtained from non-immunized and immunized mice were treated with either complete medium, or 5 μg/ml N-LiEF-2. Data are means ± S.D. from three replicates for each condition.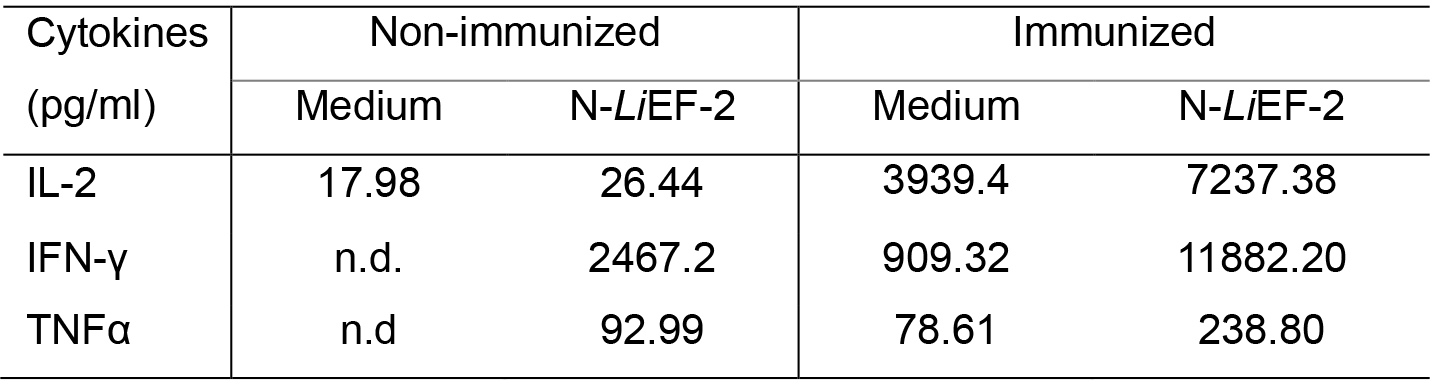
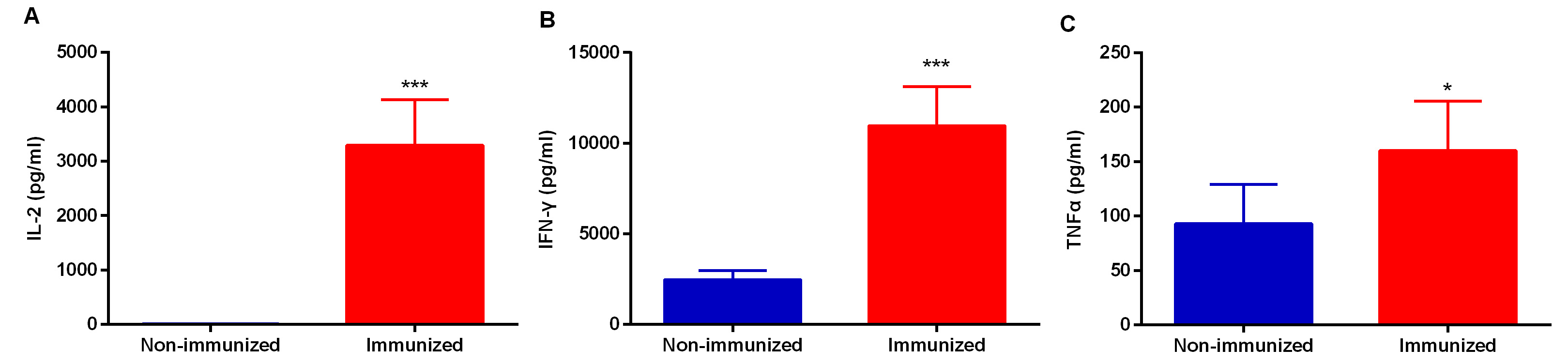
Figure 4. Representative figure demonstrating antigen (N-LiEF-2)-specific IL-2, IFN-γ and TNFα production after splenocytes ex vivo stimulation with complete culture medium or 5 μg/ml N-LiEF-2 for 72 h. Data are means ± S.D. from three replicates for each condition.
Notes
- Proliferative assays should be used solely as general indicators of T cell reactivity, since this method only detects dividing cells instead of measuring true effector T cell function. Since the majority of T cells contained in splenocyte culture respond to the added stimuli and thus produce IL-2 upon activation, differences in responsiveness in a proliferative assay in part reflect differences in IL-2 by the responding T cells. Thus, proliferative assays become more meaningful when combined with cytokine detection assays.
- Parameters affecting the magnitude of spleen cell proliferative responses as well as cytokine production are cell concentration and density, the source and amount of serum, type and concentration of stimuli/mitogen, mouse strain and the duration of culture. The microculture system is especially sensitive to cell and mitogen concentration due to the small volumes used in culture. Thus, accuracy in cell counting and in mitogen dilution is critical for obtaining optimal results. Moreover, due to the variability of serum, several lots should be tested keeping all the other conditions (cell concentration, duration of culture and mitogen concentration) stable and the one which gives the lowest control stimulation and the best support of mitogen stimulation should be chosen for use. Also, as is the case with serum, several lots of ConA should be tested and in various concentrations by keeping the cell number stable, in order to choose the one that gives the maximal cell stimulation.
Recipes
- Ammonium chloride lysis solution (ACK, pH 7.2)
- Dissolve 8.29 g NH4Cl, 1 g KHCO3 and 37.2 mg Na2EDTA to 900 ml distilled water
- Adjust pH to 7.2 with 1 M HCl and bring volume to 1 L with distilled water
- Filter sterilize with 0.22 μm filter
- Store at 4 °C
- Phosphate-buffered saline, 10x (PBS, pH 7.2)
- Dissolve 80 g NaCl, 2 g KCl, 11.5 g Na2HPO4 and 2 g KH2PO4 to 800 ml distilled water
- Adjust pH to 7.2 with 1 M HCl or 1 M NaOH depending on the acquired pH and bring volume to 1 L with distilled water
- Filter sterilize with 0.22 μm filter
- Store at 4 °C
- Complete RPMI medium
- L-glutamine stock
Supplied as 100 ml at 200 mM concentration (100x) stored at -20 °C
Thaw and disperse into 5 ml aliquots
Store at -20 °C until used - Penicillin-streptomycin
Supplied as 100 ml at 10,000 U/ml-10,000 μg/ml concentration (100x) stored at -20 °C
Thaw and disperse into 5 ml aliquots
Store at -20 °C until used - Preparation of complete medium
Add 5 ml L-glutamine, 5 ml penicillin-streptomycin and 5 ml HEPES to 500 ml RPMI-1640 medium
Store at 4 °C
When needed, take the appropriate volume of complete medium and add FBS to a final concentration of 10% (v/v) - Fetal bovine serum (FBS)
Thaw and dispense into 35 ml aliquots
Heat-inactivate at 56 °C in a water-bath under constant agitation
Store at -20 °C until used
- L-glutamine stock
- Concanavalin A stock solution
Add 1 ml PBS to the Concanavalin A powder to a final concentration of 5 mg/ml
Aliquot in volumes of 50 μl
Store at -20 °C till 3 years
Note: Concanavalin A is a mannose-binding plant lectin that has been primarily typified as a potent T cell mitogen serving as positive control in proliferation assays. Alternatively, PHA can substitute Concanavalin A. PMA/ionomycin stimulation induces polyclonal stimulation i.e., myeloid cells, B and T cells, and this works better when purified lymphocytes are used. - 0.4% (w/v) Trypan blue exclusion dye
Dissolve 0.4 g to 100 ml PBS
Filter sterilize
Acknowledgments
This work was supported by the Action “KRIPIS I” (MIS 450598) co-financed by European Union and the Greek Ministry of Education and Religion Affairs under the Operational Strategic Reference Framework (NSFR 2007-2013) and the General Secretariat of Research and Technology (GSRT). The protocols were adapted from Agallou et al. (2014 and 2018).
The proliferation assay was originally developed by Strong et al. (1973) and has over the years been modified by several groups to detect antigen-specific proliferation.
Competing interests
Both authors declare that they have no conflicts of interest.
Ethics
The experimental protocol has been positively evaluated by the Institutional Protocol Evaluation Committee and was licensed by the Official Veterinary Authorities of Attikis Prefecture. The maintenance of laboratory mice and in vivo studies were performed in SPF (Specific Pathogens Free) conditions at the approved establishments of Department of Animal Models for Biomedical Research, Hellenic Pasteur Institute under the registered codes EL25BIO011, EL25BIO012 and EL25BIO013. Animals were housed at room temperature 22 ± 2 °C, relative humidity 40-70% and 12 h light/12 h dark cycle. All procedures complied to PD 56/2013 and European Directive 2010/63/EU, welfare and ethical use of laboratory animals based on 3+1R: Replacement, Reduction, Refinement and Respect and the guidelines of PREPARE (Planning Research and Experimental Procedures on Animals: Recommendations for Excellence), ARRIVEs and ARRIGE (Association for Responsible Research and Innovation in Genome Editing). Animals’ welfare was assessed by the competent users and was supervised daily by the members of Institutional Welfare Body.
References
- Agallou, M., Athanasiou, E., Koutsoni, O., Dotsika, E. and Karagouni, E. (2014). Experimental validation of multi-epitope peptides including promising mhc class i- and ii-restricted epitopes of four known Leishmania infantum proteins. Front Immunol 5: 268.
- Agallou, M., Pantazi, E., Tsiftsaki, E., Toubanaki, D. K., Gaitanaki, C., Smirlis, D. and Karagouni, E. (2018). Induction of protective cellular immune responses against experimental visceral leishmaniasis mediated by dendritic cells pulsed with the N-terminal domain of Leishmania infantum elongation factor-2 and CpG oligodeoxynucleotides. Mol Immunol 103: 7-20.
- Ahern, T., Taylor, G. A. and Sanderson, C. J. (1976). An evaluation of an assay for DNA synthesis in lymphocytes with [3H]thymidine and harvesting on to glass fibre filter discs. J Immunol Methods 10(4): 329-336.
- Darrah, P. A., Patel, D. T., De Luca, P. M., Lindsay, R. W., Davey, D. F., Flynn, B. J., Hoff, S. T., Andersen, P., Reed, S. G., Morris, S. L., Roederer, M. and Seder, R. A. (2007). Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13(7): 843-850.
- Flaxman, A. and Ewer, K. J. (2018). Methods for measuring T-cell memory to vaccination: From mouse to man. Vaccines (Basel) 6(3).
- Freer, G. and Rindi, L. (2013). Intracellular cytokine detection by fluorescence-activated flow cytometry: basic principles and recent advances. Methods 61(1): 30-38.
- Ranieri, E., Popescu, I. and Gigante, M. (2014). CTL ELISPOT assay. Methods Mol Biol 1186: 75-86.
- Seder, R. A. and Hill, A. V. (2000). Vaccines against intracellular infections requiring cellular immunity. Nature 406(6797): 793-798.
- Seder, R. A., Darrah, P. A. and Roederer, M. (2008). T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8(4): 247-258.
- Strong, D. M., Ahmed, A. A., Thurman, G. B. and Sell, K. W. (1973). In vitro stimulation of murine spleen cells using a microculture system and a multiple automated sample harvester. J Immunol Methods 2(3): 279-291.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Agallou, M. and Karagouni, E. (2019). Detection of Antigen-specific T cells in Spleens of Vaccinated Mice Applying 3[H]-Thymidine Incorporation Assay and Luminex Multiple Cytokine Analysis Technology. Bio-protocol 9(11): e3252. DOI: 10.21769/BioProtoc.3252.
Category
Immunology > Immune cell function > Cytokine
Immunology > Animal model > Mouse
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











