- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Indole-3-acetic Acid (IAA) Production in Klebsiella by LC-MS/MS and the Salkowski Method
Published: Vol 9, Iss 9, May 5, 2019 DOI: 10.21769/BioProtoc.3230 Views: 24011
Reviewed by: Dennis J NürnbergManish Kumar PatelDarrell Cockburn

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bacterial Pathogen-mediated Suppression of Host Trafficking to Lysosomes: Fluorescence Microscopy-based DQ-Red BSA Analysis
Mădălina Mocăniță [...] Vanessa M. D'Costa
Mar 5, 2024 2895 Views

Purification of Native Dentilisin Complex from Treponema denticola by Preparative Continuous Polyacrylamide Gel Electrophoresis and Functional Analysis by Gelatin Zymography
Pachiyappan Kamarajan [...] Yvonne L. Kapila
Apr 5, 2024 2087 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1655 Views
Abstract
Many rhizobacteria isolated from plant rhizosphere produce various phytohormones in the form of secondary metabolites, the most common of which is Indole-3-acetic acid (IAA). Here, we detail analytical protocols of IAA detection and quantification, in vitro and in situ, as recently applied to Klebsiella SGM 81, a rhizobacterium isolated from the rhizosphere of Dianthus caryophyllus (a commercially important flower across the globe). Specifically, we describe a detailed protocol for a colorimetric assay using the Salkowski reagent method, which can be used to screen for the presence of Indole compounds. To further detect and quantify IAA, a highly accurate analytical approach of LC-MS/MS is used. To detect the presence of IAA around the root system of Dianthus caryophyllus, in situ staining of plant roots is done using Salkowski reagent.
Keywords: Indole-3-acetic acidBackground
The bacterial auxin in the form of Indole-3 Acetic Acid (IAA) is a product of L-tryptophan metabolized by bacteria (Lynch, 1985). The group of bacteria known as plant growth promoting rhizobacteria (PGPR) specifically residing in the vicinity of the roots depend on tryptophan being present in the root exudates of plants (Kravchenko et al., 2004; Kamilova et al., 2006). These PGPR use IAA as a signal to interact with plant roots and to colonize the plant parts. This signaling feature of IAA is thought to effect on the physiology of the bacteria (Spaepen et al., 2007).
Different methods are found in the literature to detect the biosynthesis of IAA. Gordon and Weber (1951) were the first to provide a colorimetric assay using Salkowski reagent for the detection of IAA. This method has since been widely used for detecting IAA from microorganisms. Salkowski reagent is a mixture of 0.5 M ferric chloride (FeCl3 ) and 35% perchloric acid (HClO4) which upon reaction with IAA yields pink color, due to IAA complex formation with and reduction of Fe3+ (Kamnev et al., 2001). The color developed by positive reaction indicates the presence of various indole compounds as a product of tryptophan metabolism. Apart from the colorimetric assay, other methods for IAA estimation from bacteria and plant are High Performance Liquid Chromatography (HPLC) (Perrig et al., 2007), Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometric (LC-ESI-MS/MS) (Chiwocha et al., 2003), and by High Performance Thin Layer Chromatography HPTLC (Goswami et al., 2015). Liquid Chromatography (LC) is the preferred approach to determine the concentration of IAA and to confirm its purity with high accuracy and standardization. LC coupled with various mass spectrometry detectors are powerful tools for IAA analysis. Because of the high sensitivity and selectivity, Mass Spectrometry detectors are most commonly coupled with LC. One of the important benefits of LC-MS is that analysis and separation of compounds can be achieved in a continuous manner eliminating the step of purification (Kallenbach et al., 2009).
Materials and Reagents
- 100-1,000 μl pipette tips (Sigma-Aldrich)
- Test tube racks (Sigma-Aldrich, catalog number: Z334146)
- Screw-cap tubes and caps (Sigma-Aldrich, catalog number: AXYSCT10MLS)
- Pyrex test tube 20 ml (Sigma-Aldrich, catalog number: CLS980016)
- UV quartz cuvette semi-micro square, 1.4 ml, PTFE stopper (Sigma-Aldrich, catalog number: Z276707)
- Sterile syringe filters with a pore size of 0.2 μm (Fisher Scientific, catalog number: 720-1230)
- Centrifuge tube with screw cap, capacity 10 ml (Sigma-Aldrich, catalog number: SIAL301NN10R)
- Pyrex glass serological pipettes, capacity 5 ml (Sigma-Aldrich, catalog number: Z653829)
- Amber storage bottles (Sigma-Aldrich, Supelco, catalog number: 23230-U)
- Klebsiella SGM 81 strain (isolated from the rhizosphere of Dianthus caryophyllus)
- Distilled water
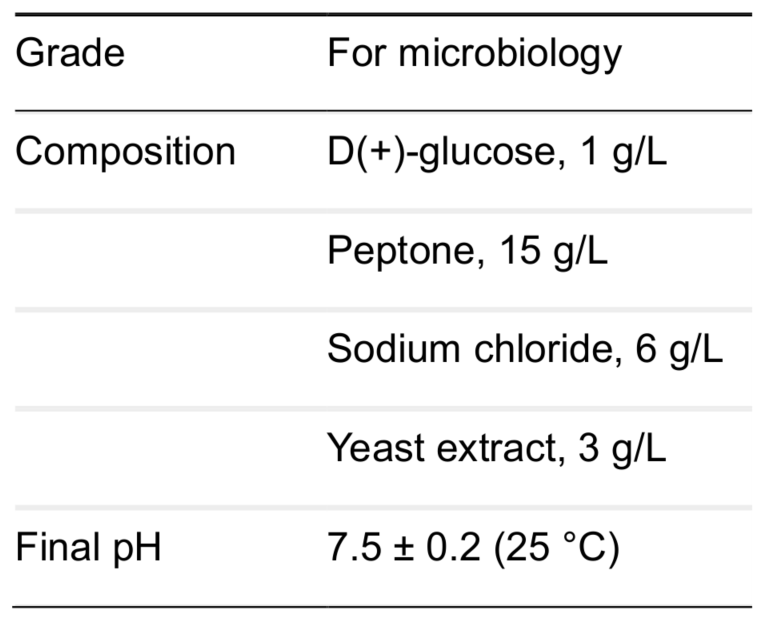
- Nutrient broth (Microbiology grade) (Sigma-Aldrich, catalog number: 70122-500G), its composition and final pH see below:
- FeCl3 reagent grade (Sigma-Aldrich, CAS: 7705-08-0)
- Perchloric acid 70% (Sigma-Aldrich, CAS: 7601-90-3)
- Methanol (laboratory grade) (Sigma-Aldrich, CAS: 67-56-1)
- HCl (ACS grade) (Sigma-Aldrich, CAS: 7647-01-0)
- L-tryptophan (traceCERT grade) (Sigma-Aldrich, CAS: 73-22-3)
- Acetic acid glacial (Sigma-Aldrich, CAS: 64-19-7)
- Ethyl acetate (grade anhydrous) (Sigma-Aldrich, CAS: 141-78-6)
- Indole-3-Acetic acid (IAA) (Sigma-Aldrich, CAS: 87-51-4)
- Murashige and Skoog medium (Sigma-Aldrich)
- Acetonitrile
- Ammonium formate
- CH3CN
- Formic acid
- Ethanol
- Agarose
- Culture media (see Recipes)
- Salkowski reagent (see Recipes)
Equipment
- Spatula (Thermo Fisher, catalog number: F36711-0012)
- Nichipet Eco pipette, 100-1,000 μl volume (Sigma-Aldrich, catalog number: Z710199)
- Measuring cylinder (Sigma-Aldrich, catalog number: Z324361-2EA)
- 250 ml conical flask (Sigma-Aldrich, catalog number: Z723088-1EA)
- Rotary Vacuum Flash Evaporator (Buchhi Type) (Jain Scientific Glass, 1188)
- Balance
- Agilent 1100 LC system and an ABSciex 6500 Qtrap MS [Chromatography was on a Phenomenex Luna C18 column (100 mm x 2 mm x 3 μm)]
- UV-spectrophotometer (Systronics, 166)
- Microcentrifuge (Thermo scientific, Pico 17)
- Magnetic stirrer (Remi, 1 MLH)
- Vortex cyclo mixer (Remi, CM 101)
Software
- Analyst 1.6.1 (AB Sciex)
Procedure
- IAA screening and quantification from Klebsiella SGM 81 using Salkowski reagent method
- Culture preparation
- Take preculture medium (without tryptophan) and inoculate it with a single colony of SGM 81 strain, or a loopful of glycerol stock of the strain.
- Incubate the inoculated pre-culture medium at 30 °C, 120 rpm overnight. This will result in the young culture of the Klebsiella SGM 81 strain which can be used for inoculating IAA production medium (test media).
- Add 100 μl of the young culture in test medium and vortex it to get a uniform suspension.
- Incubate it under dark conditions (by wrapping the container with newspaper/aluminum foil) at 30 °C in shaking condition at 120 rpm.
- Incubation lasts for 24-96 h in dark condition depending on IAA production ability of the strain. Klebsiella SGM 81 produces IAA till 72 h and subsequent decrease in the production in recorded after that.
- Indole quantification using Salkowaski reagent
- After 24 h, take out 1.5 ml of sample culture broth and transfer to an Eppendorf tube.
- Centrifuge at 16,278 x g for 5 min using a microcentrifuge.
- Carefully withdraw 1 ml supernatant and transfer to a new test tube.
- Mix equal volume (1 ml) of Salkowski reagent, vortex gently and incubate the reaction at 30 °C in a dark condition for 30 min.
- In a new test tube replace the supernatant with 1 ml of control uninoculated medium and mix with 1 ml of Salkowski reagent. This serves as blank.
- The presence of IAA is detected by measuring pink color development after 30 min (some strains may develop red color as shown in Figure 1).
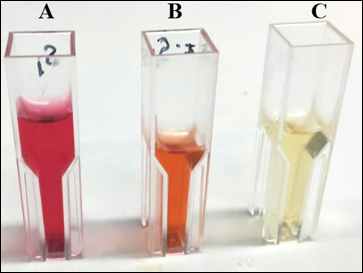
Figure 1. Color development due to Indole-Salkowski reagent reaction. A. Klebsiella SGM 81. B. SGM09. C. Control uninoculated media. - Use uninoculated medium to set the blank. Measure the color intensity spectrophotometrically at 536 nm using cuvette.
- Compare the optical density of the test sample with a standard IAA curve (10-100 μg·ml-1) to calculate the concentration.
- Repeat the process at similar intervals until the optical density begins to decrease, indicating no further production of IAA.
- Culture preparation
- Analysis of IAA by liquid chromatography-mass spectrometry (LC-MS/MS)
- Sample preparation
- Prepare 100 ml nutrient broth medium supplemented with 0.5 g of L-tryptophan in a 250 ml Erlenmeyer flask.
- Add 100 μl of the young culture in test medium and vortex it to get a uniform suspension.
- Incubate it under dark conditions (by wrapping the container with newspaper/aluminum foil) at 30 °C in shaking condition at 120 rpm for 72 h.
- To separate the cells, centrifuge at 4 °C for 20 min at 2,800 x g and collect 1,000 ml supernatant.
- Transfer the supernatant to a 250 ml screw-cap glass bottle. Acidify it by adding 2-3 drops of 1 N concentrated HCl to reach pH 2.5-3.
- To extract IAA, add double the volume of ethyl acetate to the acidified supernatant and shake vigorously for 5 min (alternatively, a separating funnel can be used). Let the mixture stand at room temperature for 10 min to get the top layer of ethyl acetate. Use this layer for further treatment.
- In a rotary evaporator, set water bath temperature to boiling temperature of ethyl acetate i.e., 77.1 °C (alternatively, ethyl acetate phase can be vacuum dried in a rotational evaporator at 40 °C). The time for drying of the solvent system varies according to the sample volume.
- Transfer the ethyl acetate layer to the round bottom flask and adjust its rotation to avoid any bumping of the liquid sample. “Bumping is the phenomenon in chemistry where homogenous liquids boiled in a test tube or other container will super heat and, upon nucleation rapid boiling will expel the liquid from the container (Wikipedia)”–This would leave the IAA escaping the flask into the condenser tube attached with the round bottom flask.
- Upon complete evaporation of the liquid ethyl acetate, pure IAA is left behind in the crystalline form attached to the bottom of the rotary flask.
- Switch off all the units of the rotary evaporator and remove the round bottom flask. Re-dissolve the crystalline IAA in 5 ml of 20% methanol and store at -20 °C for future use.
- LC-MS/MS analysis
- Filter the stored methanol extract (100 µl) using sterile syringe filters of 0.2 µm so as to separate insoluble particles and larger compounds if any. This is the final analysis sample.
- Phenomenex Luna C18(2) column (100 mm x 2 mm x 3 µm) at temperature of 50 °C is used as chromatography system.
- A gradient solvent system of 10% solvent A (2% acetonitrile, 10 mM ammonium formate, pH 4.2) to 90% solvent B (94.9% CH3CN, 5% H2O, 0.1% formic acid) is used for the column over 10 min at a flow rate of 250 ml/min.
- Wash the column with 90% solvent B for 3 min and then re-equilibrate with 90% solvent A for 6 min.
- Use 10 µl injections to load the sample for analysis.
- The MS is configured with a Turbo Spray Ion Drive source where the source temperature is set to 500 °C and the ion spray voltage to 5,500 V.
- Analyze IAA by Multiple Reaction Monitoring (MRM) in positive mode using a transition of 176 > 130 with collision energy of 20 eV. Transition of 176 > 130 means the specific pairs of mass to charge (m/z) values associated to the precursor and fragment ions. In our case the Q1 is set to a value of 176 amu and the Q3 to 130 amu, the collision energy was 20 mV.
- Set the declustering potential, exit potential and collision cell exit potential at 30 V, 10 V and 10 V respectively.
- Sample preparation
- In Situ Salkowski staining
- Prepare Murashige and Skoog (MSM) basal salt medium with 0.8% agarose, devoid of plant hormone supplements.
- Sterilize the D. caryophyllus seeds using three-step procedure: a 1 min wash in 70% ethanol, followed by a 4 min wash in 20% NaClO, and a final rinse in sterile distilled water 3 times.
- Allow the D. caryophyllus seeds to germinate on the MS media by incubating the plates at 25 °C in 14 h light and 10 h dark cycles in a plant culture room, until the root length reaches 2 cm (approximately six days).
- Treat the germinated plant roots by immersing the root tips into 5 ml of the bacterial suspension with titre 105 CFU ml-1 in the universal tube.
- Transfer the treated plants onto new MS agar medium devoid of IAA and sucrose in square Petri plates (120 mm x 120 mm x 15 mm).
- Again, incubate the plates at 25 °C in 14 h light and 10 h dark cycles in a plant culture room.
- After two weeks of infection, stain the treated roots with Salkowski reagent.
- To stain the roots, add 400 μl of Salkowski reagent on each root and observe any visible color change.
- The development of pink color as shown in the figure below indicates the presence of proximal IAA on and around the roots (Figure 2).

Figure 2. In situ Salkowski staining on plant roots treated with Klebsiella SGM 81 of 105 CFU ml-1 titre
Data analysis
- Take the optical density of the samples at 536 nm and graphically calculate the concentration of IAA based on the standard curve. IAA estimation from Klebsiella SGM 81 strain, using Salkowski reagent shows a maximum yield to 215 μg·ml-1 detected after 72 h with 0.15% tryptophan (Figure 1a in Gang et al., 2018).
- For LC-MS/MS analysis, confirmation of the identity in the samples is done by Enhanced Product Ion scans.
- Data acquisition and analysis is done with Analyst 1.6.1 (AB Sciex).
- Quantification is carried out by comparing the test sample data with control standard IAA.
- Overlapping retention times during LC (5.90 min and 5.92 min) and identical m/z ratios (130.1) between a commercial IAA standard and sample supernatant showed Klebsiella SGM 81 to produce substantial amounts (960 μg·ml-1 after 72 h) homogeneous IAA (Gang et al., 2018).
- Development of pink color on the Salkowski treated roots indicates the presence of bacterial IAA proximal to the roots (Figure 3a in Gang et al., 2018).
Notes
- The Salkowski reagent method discussed in this paper is shown using nutrient broth liquid medium (supplemented with L-tryptophan) which is non-specific liquid medium for most gram-positive and gram-negative bacteria. However other media like Yeast extract mannitol (YEM), Pikovskaya’s broth (supplemented with L-tryptophan) can be used if IAA production and quantification is to be done from Rhizobium or phosphate solubilizing bacteria respectively.
- The preculture preparation is important to normalize the initial cell count especially when a comparative study of IAA quantification is carried out for the optimization process.
- Salkowski reagent method quantifies the overall Indole present in the sample. To get the purity and conjugants of Indole-3-acetic acid (IAA), other analytical methods like HPLC, LC-MS/MS, HPTLC etc. can be used.
Recipes
- Culture media
- Making use of measuring cylinder, measure 100 ml distilled water and transfer it into a 250 ml Erlenmeyer flask or screw-capped glass bottle. Two other flasks of the same media, with and without tryptophan will be used as control and pre-culture media (used to activate the SGM 81 culture to yield young culture) respectively
- For simple liquid nutrient broth medium, weigh 1.3 g of Nutrient broth and dissolve it in 100 ml distilled water. Stir it properly so as to mix the powder in distilled water properly
- Weigh 0.15 g of L-tryptophan (0.15% w/v) and add to the prepared nutrient broth flask. This is liquid nutrient broth with tryptophan supplement
- Autoclave the prepared media at 121 °C for 15 min
- Allow the media to cool down and come to room temperature before inoculation so as to ensure the survival of inoculum
- Salkowski reagent
- 0.5 M of 100 ml ferric chloride (dissolve 8.125 g of FeCl3 in 100 ml of distilled water)
- To dilute readily available perchloric acid, measure 24.5 ml (v/v) of distilled water in a measuring cylinder and add 24.5 ml (v/v) of concentrated acid to it
- Add 1 ml (v/v) of 0.5 M ferric chloride solution to 49 ml of 35% perchloric
- Mix well and store in a dark brown bottle at room temperature. It can be used for further experiments if stored under described conditions
Acknowledgments
We are thankful to the Department of Science and Technology, India for the financial assistance under INSPIRE Fellowship 2014-15 (IF 140042). This work was also supported by the BBSRC (BB/003608/1), UK. At Imperial College London, we thank Mark Bennett for helping with LC-MS/MS analysis. The protocol is adapted from Gang et al. (2018).
Competing interests
We declare no conflict of interest.
References
- Chiwocha, S. D., Abrams, S. R., Ambrose, S. J., Cutler, A. J., Loewen, M., Ross, A. R. and Kermode, A. R. (2003). A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35(3): 405-417.
- Gang, S., Saraf, M., Waite, C. J., Buck, M. and Schumacher, J. (2018). Mutualism between Klebsiella SGM 81 and Dianthus caryophyllus in modulating root plasticity and rhizospheric bacterial density. Plant Soil 424: 273-288.
- Gordon, S. A. and Weber, R. P. (1951). Colorimetric estimation of indoleacetic acid. Plant Physiol 26(1): 192-195.
- Goswami, D., Thakker, J. N. and Dhandhukia, P. C. (2015). Simultaneous detection and quantification of indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) produced by rhizobacteria from l-tryptophan (Trp) using HPTLC. J Microbiol Methods 110: 7-14.
- Kallenbach, M., Baldwin, I. T. and Bonaventure, G. (2009). A rapid and sensitive method for the simultaneous analysis of aliphatic and polar molecules containing free carboxyl groups in plant extracts by LC-MS/MS. Plant Methods 5: 17.
- Kamilova, F., Kravchenko, L. V., Shaposhnikov, A. I., Azarova, T., Makarova, N. and Lugtenberg, B. (2006). Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact 19(3): 250-256.
- Kamnev, A., Shchelochkov, A., Perfiliev, Y. D. Tarantilis, P. A., and Polissiou, M. G. (2001). Spectroscopic investigation of indole-3-acetic acid interaction with iron (III). J Mol Struct 563:565-572.
- Kravchenko, L., Azarova, T., Makarova, N. and Tikhonovich, I. (2004). The effect of tryptophan present in plant root exudates on the phytostimulating activity of rhizobacteria. Microbiology 73:156-158.
- Lynch, J. (1985). Origin, nature and biological activity of aliphatic substances and growth hormones found in soil. In: Soil Organic Matter and Biological Activity. Springer 151-174.
- Perrig, D., Boiero, M. L., Masciarelli, O. A., Penna, C., Ruiz, O. A., Cassan, F. D. and Luna, M. V. (2007). Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol 75(5): 1143-1150.
- Spaepen, S., Vanderleyden, J. and Remans, R. (2007). Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31(4): 425-448.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gang, S., Sharma, S., Saraf, M., Buck, M. and Schumacher, J. (2019). Analysis of Indole-3-acetic Acid (IAA) Production in Klebsiella by LC-MS/MS and the Salkowski Method. Bio-protocol 9(9): e3230. DOI: 10.21769/BioProtoc.3230.
Category
Microbiology > Microbe-host interactions > Bacterium
Biochemistry > Other compound > Plant hormone
Plant Science > Plant biochemistry > Plant hormone
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









