- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Root Exudate Concentration in the Rhizosphere Using 13C Labeling
Published: Vol 9, Iss 9, May 5, 2019 DOI: 10.21769/BioProtoc.3228 Views: 7543
Reviewed by: Joëlle SchläpferVenkatasalam ShanmugabalajiLaura Zanin

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Dark Respiration Measurement from Arabidopsis Shoots
Jose P. Fonseca [...] Kirankumar S. Mysore
Oct 5, 2021 4182 Views
Abstract
One of the most remarkable metabolic features of plant roots is their ability to secrete a wide range of compounds into the rhizosphere, defined as the volume of soil around living roots. Around 5%-21% of total photosynthetically fixed carbon is transferred into the rhizosphere through root exudates. Until recently, studies on the quantity and quality of root exudates were conducted mostly under axenic or monoxenic in vitro conditions. Today, in situ assays are required to provide a better understanding of root exudates dynamics and role in plant-microbe interactions. By incubating plants with 13CO2 in situ for one week and quantifying 13C enrichment from the root-adhering soil using mass spectrometry, we were able to determine root exudate levels. Indeed, labeled substrate 13CO2 is converted into organic carbon via plant photosynthesis and transferred into the soil through root exudation. We assume that all 13C increases above natural abundance are mainly derived from exudates produced by 13C-labeled plants.
Keywords: Root exudatesBackground
Through the exudation of a wide variety of compounds, plants communicate with the soil microbial community in their immediate vicinity, cope with herbivores, foster beneficial symbioses, change the chemical and physical properties of the soil, and inhibit the growth of competing plant species. Understanding how plants differ quantitatively in their root exudate patterns, according to their genotypes and traits as well as to environmental parameters and the soil microbial community is an exciting research challenge. This objective could be achieved by quantifying root exudate content using 13CO2 plant-labeling coupled with a 13C enrichment measurement from the root-adhering soil using mass spectrometry. This protocol was set up by using different plant species such as Poaceae, legumes (Medicago truncatula), Arabidopsis thaliana, and Monocotyledon plants such as maize and wheat. As rhizodeposition also depends on plant species, the collection period was after 10 weeks for Poaceae and 4 weeks for the rest. This method can be used with other species, for which the collection period must be set up according to what is known about the studied plant.
Materials and Reagents
- 1 mm mesh size for soil sieving (Fisherbrand tamis, France)
- Plastic pots for plant growing (7 x 7 x 6.4, black pots, France)
- 5 x 8 mm “Ultra Clean” tin capsules (Elemental Microanalysis, Okehampton, UK, catalog number: D1034)
- In the case of calcareous soils: 5 x 8 mm silver capsules (Elemental Microanalysis, Okehampton, UK, catalog number: D2009)
- Soil and seeds for plant growing
The soil used to set up this protocol was luvisol with no added nitrogen source collected at La Côte Saint-André (Isère, France) and which was continuously harvested with maize. It was a loamy soil composed of 16.2% clay, 45.4% loam and 28.4% sand, with a total nitrogen content of 1.9 g·kg-1 - Reference materials for 13C measurements: IAEA-CH3 (cellulose), IAEA-CH6 (sucrose) (Coplen et al., 2006) and partially 13C-labelled material [e.g., D-Glucose (3-13C, 99%)] (Eurisotop, Saint-Aubin, France, catalog number: CLM-1393-0.25)
- Calibration material for carbon (C) concentration measurements: aspartic acid (Elemental Microanalysis, Okehampton, UK, catalog number: B2042)
- Distilled water
- Liquid N2
- Pure 13CO2 (> 99% atom 13C; purchased from Cortec Net, Paris, France)
- In the case of calcareous soils: hydrochloric acid (HCl) 37% (CAS number: 7647-01-0, e.g., Sigma-Aldrich, Saint-Louis, MO, USA, catalog number: 30721-M)
Equipment
- 50 ml beaker
- Spatula and forceps
- Desiccator (Freeze-dryer) (Alpha 1-4 LSC, Martin Christ®, Osterode am Harz, Germany)
- Mixer mill grinder (Mixer Mill MM 200, Retsch®, Haan, Germany)
- Laboratory balance with a resolution of 0.01 mg or better (XP6 microbalance, Mettler Toledo, Greifensee, Switzerland)
- Isotope ratio mass spectrometer (Isoprime 100, Elementar UK Ltd., Cheadle, UK), coupled in continuous flow with an elemental analyzer (vario PyroCube in CN mode, Elementar Analysensysteme, Lagenselbold, Germany, or FlashEA 1112, ThermoElectron, Waltham, MA, USA)
- Growth chamber equipped for automatic control of light, temperature, moisture, evapotranspiration, irrigation and CO2 concentration
Software
- Isoprime 100 isotope ratio mass spectrometer operating software: Ionvantage® for Isoprime, build 1.6.1.0 (Elementar Analysensysteme, Lagenselbold, Germany)
- Elemental analyzer operating software (pyrocube Software v4.0.1 was used in this protocol, Elementar Analysensysteme, Lagenselbold, Germany)
Procedure
- Sieve the soil (1 mm mesh size) and adjust it to 0.17 g of water per gram of dry weight. Afterward, place 170 g of dry weight into pots. Place one seed per pot and do six replicates per plant species and six replicates for unplanted-soil (bulk soil).
- Place the pots (six replicates per plant and six replicates for bulk soil) in a growth chamber and set up the day-night period, light intensity and daily temperature according to the studied plant (Guyonnet et al., 2018b). For Brasica napus, Arabidopsis thaliana, Triticum aestivum, Medicago truncatula and Poaceae plants, for example, the day-night period is set at 8 h/16 h, respectively; light intensity is 13.5 klux; maximum daily temperatures ranges from 20 to 22 °C. Control and adjust soil moisture by weighing pots using a laboratory balance and adding distilled water every 2 days (Figure 1).
- Three weeks after sowing seeds, inject pure 13CO2 to three replicates per plant and bulk soil during active photosynthesis, according to Haichar et al. (2008 and 2012), in order to maintain a 13CO2 level at 350 μl·L-1 for one week. Control 12CO2 (soil respiration) and 13CO2 concentrations in the growth chamber. The isotope excess of 13C atoms in the chamber must be maintained at > 80% during the first 10 days and > 90% thereafter.
- Cultivate the rest of the plants (three replicates per plant) and bulk soil (three replicates for unplanted soil) without 13C-labeling in order to determine 13C natural abundance in soil and plants.
- At the end of labeling, separate the root system from the root-adhering soil (RAS) by shaking each plant and collect the root-adhering soil. Carefully separate the remaining fine roots from the RAS and freeze the RAS immediately in liquid N2 before storing at -80 °C (Figure 1).
- Lyophilise the RAS during 48 h and grind to a fine powder (~100 µm particle size; visual verification is sufficient) using a mixer mill to homogenize before measuring 13C enrichment (Figure 2A). The duration of the grinding step should be adapted to each type of soil (approximately 1 min at 30 Hz; repeat if needed until a fine powder is obtained).
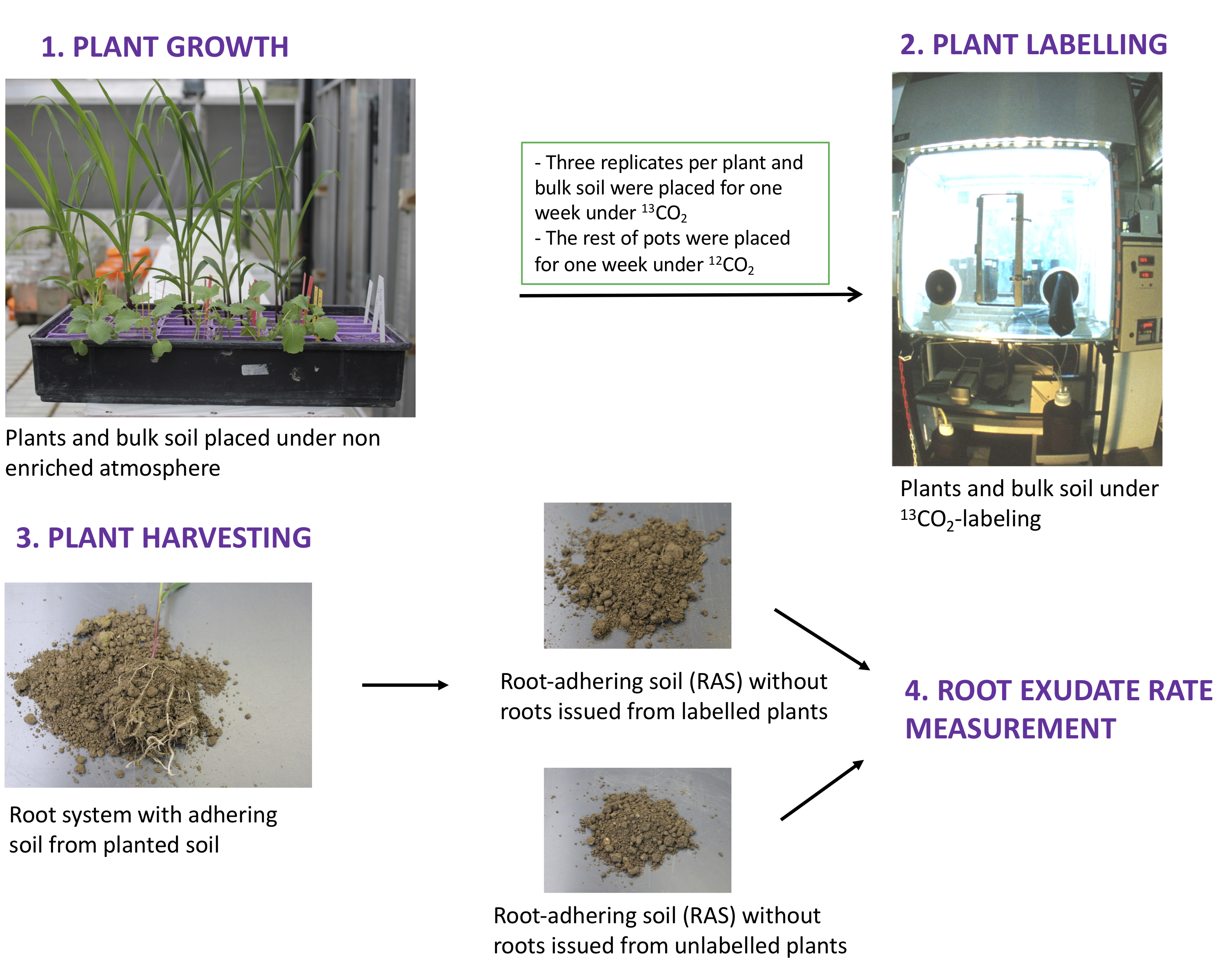
Figure 1. Plant growth, labeling and harvesting in order to measure root exudation rate - Weigh 8 mg (adjust the target mass to obtain a sufficient amount of carbon, commonly > 10 μg of C depending on the C concentration of the sample and IRMS sensitivity) of lyophilized RAS samples into 5 x 8 mm “Ultra Clean” tin capsules to the nearest 0.01 mg (Figure 2B).
Note: In calcareous soils, inorganic carbon (carbonates) can distort the 13C measurement of organic carbon. In this case, remove inorganic C by acid fumigation. Weigh soil samples into silver capsules instead of tin. Add a small amount (50-150 μl) of distilled water in each capsule to moisten the soil. Place each open capsule in a desiccator with a beaker of fuming (37%) hydrochloric acid (HCl) during 6 to 8 h. Dry at 60 °C. Crimp the capsule and place it in a new tin capsule. - Carefully seal the tin capsules using forceps into a cubical or spherical shape (Figure 2C).
- Measure the 13C/12C ratio and C concentration of each sample by EA-IRMS (Figure 2D) after optimizing the source parameters and calibration according to good practice (e.g., Dunn and Carter, 2018). Intersperse reference materials with the samples to allow for the normalization of the data and quality control.
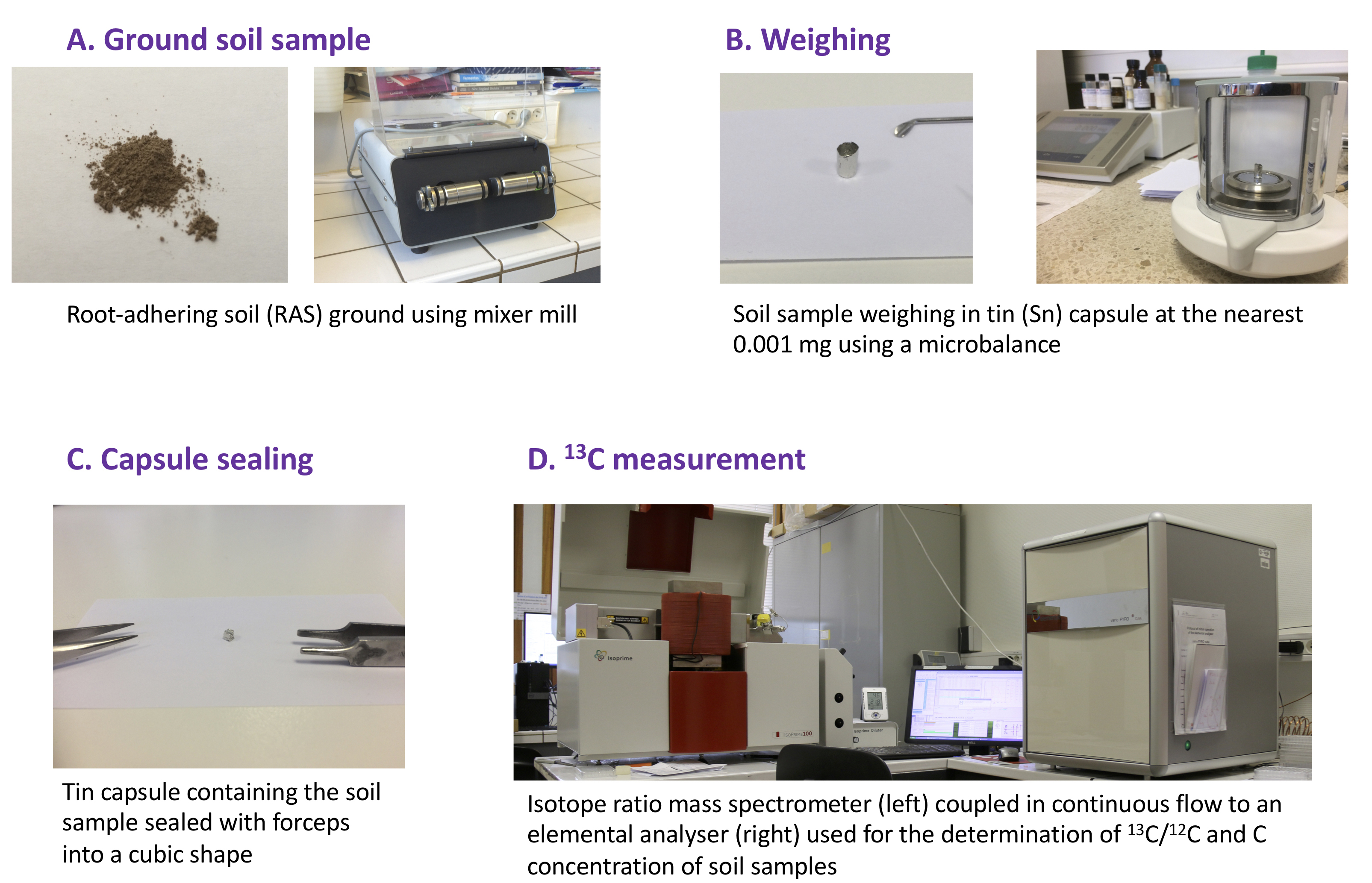
Figure 2. Scheme of soil sample preparation for the 13C/12C ratio measure using Elemental Analyser-Isotope Ratio Mass Spectrometer (EA-IRMS)
Data analysis
- Apply blank corrections and normalize the C concentrations and 13C/12C ratio with the measured values of reference and calibration materials (Coplen et al., 2006; Dunn and Carter, 2018).
- C isotope composition is measured by IRMS as a 13C/12C ratio, expressed by the instrument software as δ13C in ‰:δ13C = [(13C/12C)sample/(13C/12C)PDB - 1] x 103
with (13C/12C)sample, the 13C/12C ratio of the soil sample, (13C/12C)PDB is the 13C/12C ratio of PDB (Pee Dee Belemnite, the international standard used to anchor the δ13C scale at 0‰) and equals 0.0112372. - Calculate the 13C concentration in RAS using the equation: [13C] = [C] x [(δ13Ct - δ13Ct-0) - (δ13Ccontrol - δ13Ccontrol-0)] x (13C/12C)PDB/103
where,
[13C] estimates the concentration of exudates in the soil or the assimilation by microorganisms present in the soil in mg of 13C by kg of soil (equivalent to dry weight),
[C] is the carbon concentration of the soil in mg·kg-1,
δ13Ct is the C isotope composition of organic carbon in the planted soil with 13C-labeling,
δ13Ct-0 is the C isotope composition of organic carbon in the planted soil without 13C-labeling,
δ13Ccontrol is the C isotope composition of the organic carbon in of the bulk soil with 13C-labeling,
δ13Ccontrol-0 is the C isotope composition of the organic carbon in the bulk soil with 13C-labeling.
Note: δ13Ct-0 and δ13Ccontrol-0 (i.e., without 13C-labeling) are determined in order to take into account the 13C natural abundance in bulk and planted soils. In the majority of experiments, these values will be identical and can therefore be removed from the equation in step 3.
Example of calculations:
For the soil planted with Bromus erectus with 13C-labeling (δ13Ct), a δ13C of 480.5‰ was measured for the root-adhering soil. The δ13C of the bulk soil with 13C-labeling (δ13Ccontrol) was -5.5‰. Without 13C-labeling, the δ13C of the root-adhering soil (δ13Ct-0) was equal to -22.5 ± 0.3 ‰ and the δ13C of the bulk soil (δ13Ccontrol-0) was -22.7 ± 0.2 ‰. The C concentration ([C]) of the soil was 1.67 ± 0.2 % (16.7 x 103 mg·kg-1).
Using the equation in step 3, a·concentration of exudates in the soil of 91.2 mg 13C·kg-1 (dry weight) was measured.
Acknowledgments
The method to measure root exudate content was developed in the Microbial Ecology laboratory jointly with Dr. L. Simon and published by Guyonnet et al. (2018a) Ecology and Evolution DOI: 10.1002/ece.3.4383. We thank the “Serre et chambres climatiques” platform (Université Lyon1, FR BioEnviS) for growing the plants, as well as the “Ecologie Isotopique” platform (Université Lyon 1, UMR 5023) for elemental and isotopic analysis. This work was supported by the French National Research Agency (ANR-18-CE32-0005, DIORE).
Competing interests
No competing interests to declare.
References
- Coplen, T. B., Brand, W. A., Gehre, M., Gröning, M., Meijer, H. A. J., Toman, B. and Verkouteren, R. M. (2006). New guidelines for δ13C measurements. Anal Chem 78(7): 2439-2441.
- Dunn, P. J. H. and Carter, J. F. (2018). Good practice guide for isotope ratio mass spectrometry. In: Dunn P. J. H. and Carter, J. F. (Eds.). 2nd Edition.
- Guyonnet, J. P., Cantarel, A. A. M., Simon, L. and Haichar, F. e. Z. (2018a). Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecol Evol 8(16): 8573-8581.
- Guyonnet, J. P., Guillemet, M., Dubost, A., Simon, L., Ortet, P., Barakat, M., Heulin, T., Achouak, W. and Haichar, F. E. Z. (2018b). Plant nutrient resource use strategies shape active rhizosphere microbiota through root exudation. Front Plant Sci 9: 1662-1662.
- Haichar, F. Z., Marol, C., Berge, O., Rangel-Castro, J. I., Prosser, J. I., Balesdent, J., Heulin, T. and Achouak, W. (2008). Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2(12): 1221-1230.
- Haichar, F. Z., Roncato, M. A. and Achouak, W. (2012). Stable isotope probing of bacterial community structure and gene expression in the rhizosphere of Arabidopsis thaliana. FEMS Microbiol Ecol 81(2): 291-302.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Simon, L. and Haichar, F. E. Z. (2019). Determination of Root Exudate Concentration in the Rhizosphere Using 13C Labeling. Bio-protocol 9(9): e3228. DOI: 10.21769/BioProtoc.3228.
Category
Plant Science > Plant biochemistry > Root exudation
Environmental science > Plant > Plant-microbe interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










