- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Cellular Uptake and Endocytic Pathways
(*contributed equally to this work) Published: Vol 9, Iss 4, Feb 20, 2019 DOI: 10.21769/BioProtoc.3169 Views: 11575
Reviewed by: Chiara AmbrogioMauro Sbroggio'Muhammad Aslam

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Methods to Quantify the Dynamic Recycling of Plasma Membrane Channels
Rawad Hodeify and Khaled Machaca
Sep 5, 2023 1676 Views

In vitro Assessment of Efferocytic Capacity of Human Macrophages Using Flow Cytometry
Ana C.G. Salina [...] Larissa D. Cunha
Dec 20, 2023 5155 Views

Quantitative Measurement of Plasma Membrane Protein Internalisation and Recycling in Heterogenous Cellular Samples by Flow Cytometry
Hui Jing Lim and Hamish E. G. McWilliam
May 5, 2024 2243 Views
Abstract
Efficiency of drug and gene delivery via nonviral vehicles is contingent on proper cellular uptake and intracellular release. Further, various cargos, such as nucleases for gene editing or inhibitors for endosomal receptors, require transport to specific compartments of the cell. Hence, characterization of cellular uptake and endocytic pathways is crucial for the optimization of any nanoparticle-mediated intracellular delivery system. Previous work on endocytic pathways looks at the effect of various pathway inhibitors on the uptake efficiency of nanoparticles carrying fluorescently-labeled cargo. While this helps attribute particle uptake to specific pathways like caveolae-mediated or clathrin-mediated endocytosis, this does not provide a holistic picture of the delivery process. Here, we provide a general protocol that combines systematic studies of inhibitor effects on efficiency with quantification of particle-induced cell membrane permeability. By applying this methodology to a nucleic acid delivery system, for example a helical polypeptide-based nanoparticle for plasmid and guide RNA delivery, we gain understanding of the endocytic mechanisms and cell uptake for intelligent design of intracellular delivery.
Keywords: EndocytosisBackground
While nonviral delivery has made significant progress over the past decade, from the initial inert nanoparticle design to biofunctional and ligand-targeting nanocarriers with improved delivery efficiency, these novel delivery vehicle systems still lack viral delivery’s high transduction efficiency. Because of this inadequacy, a large portion of research has been dedicated to determining the limiting factors restricting nonviral delivery through mapping the cellular uptake and intracellular trafficking of delivery vehicles. Further, in the field of nucleic acid therapeutics, where the success of nanoparticle delivery is measured through a secondary mechanism such as gene-cleavage detection or quantitative PCR, developing an analytical platform to resolve and quantify vehicle uptake, intracellular trafficking and endosomal escape is crucial. Previous work applies fluorescently-tagged nanoparticles, fluorescent protein gene expression and flow cytometry to explore uptake of the delivery vehicle as a whole (Dausend et al., 2008). More recently, researchers investigated cellular trafficking by employing image-based analysis for colocalization of fluorescently-tagged vehicles with lysotracker and application of uptake pathway inhibitors (Gilleron et al., 2013). This protocol builds on this breadth of research, to provide a robust method (Figure 1) to characterize the specific uptake and trafficking characteristics of any nucleic acid therapeutic in a nonviral delivery system (Wang et al., 2018). By the characterization of the intracellular transport of the therapeutic load, regardless of nanoparticle size, charge, material, and target cell type, the field can begin to pinpoint the weaknesses in nonviral genetic delivery and improve upon them (Iversen et al., 2011). As a broader spectrum of cell types and delivery vehicles are interrogated using this protocol, various genetic and alternative therapeutic cargos can be delivered through intelligently designed vehicles for optimized on-target effects (Douglas et al., 2008).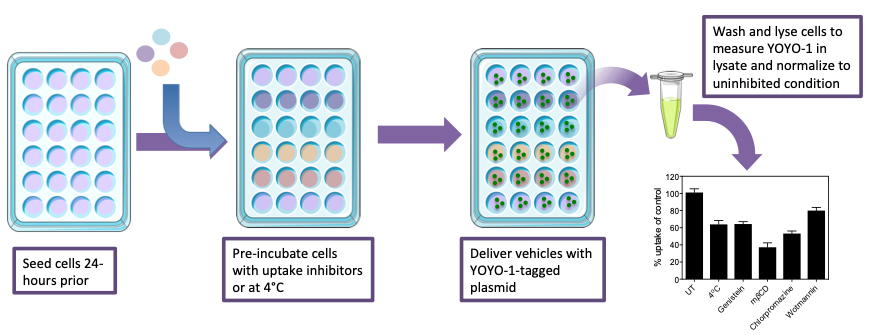
Figure 1. Schematic showing the process for determination of the contribution of each endocytosis pathway to particle uptake. Beginning from the seeding of cells, to the treatment with inhibitors, followed by delivery vehicle application and then analysis of the lysate.
Materials and Reagents
- Pipette tips
- Microscope slides
- Cover slips (0.17 to 0.25 mm thickness)
- 24-well cell culture plates
- 1.5 ml tubes
- FITC-labeled in vitro transcribed single guide RNA, sgRNA (synthetic can also be used)
- pSPCas9 (Addgene, px165)
- Opti-MEM reduced serum medium (Thermo Fisher, Gibco, catalog number: 31985062)
- Dulbecco’s modified Eagle medium, DMEM (Thermo Fisher, Gibco, catalog number: 11960077)
- Fetal bovine serum (Thermo Fisher, Gibco, catalog number: 16000044)
- Penicillin-streptomycin (Thermo Fisher, Gibco, catalog number: 15140148)
- Phosphate buffered saline, or PBS (Fisher Scientific, Corning, catalog number: 21040CV)
- Delivery vehicle such as a helical nanoparticle (HNP) formulated from poly(γ-4-((2-(piperidin-1-yl)ethyl)aminomethyl) benzyl-L-glutamate (PPABLG) (see Recipes) or a lipid nanoparticle like Lipofectamine 3000 (Thermo Fisher, catalog number: 300001)
- Heparin (Stemcell Technologies, catalog number: 07980)
- RadioImmunoPrecipitation Assay (RIPA) lysis buffer (Thermo Fisher, Pierce, catalog number: 89900)
- BCA protein assay (Thermo Fisher, Pierce, catalog number: 23225)
- Paraformaldehyde (Thermo Fisher, ACROS, catalog number: 28906)
- DAPI (Thermo Fisher, Invitrogen, catalog number: D1306)
- Lysotracker Red (Thermo Fisher, Invitrogen, catalog number: L12492)
- Mounting medium (Sigma-Aldrich, Fluoromount, catalog number: F4680)
- Trypsin-EDTA 0.25% (Thermo Fisher, Gibco, catalog number: 25200056)
- Genistein (Sigma-Aldrich, Sigma, catalog number: G6649)
- Chlorpromazine hydrochloride (Sigma-Aldrich, Sigma, catalog number: C8138)
- Wortmannin (Sigma-Aldrich, Sigma, catalog number: W1628)
- Methyl-β-cyclodextrin or mβCD (Sigma-Aldrich, Sigma, catalog number: C4555)
- FITC (Thermo Fisher, Invitrogen, catalog number: F2181)
- Tris buffer (Sigma-Aldrich, Sigma, catalog number: 21685)
- YOYO-1 Iodide (Thermo Fisher, Invitrogen, catalog number: Y3601)
- HiScribe T7 High Yield RNA Synthesis Kit (New England Biolabs, HiScribe, catalog number: E2040S)
- Fluorescein-12-UTP (Sigma-Aldrich, Roche, catalog number: 11427857910)
- γ-(4-vinylbenzyl)-L-glutamate N-carboxyanhydride (VB-L-Glu-NCA)
- Anhydrous dimethyl formamide (DMF)
- Hexamethyldisilazane
- Nitrobenzene
- Hexane
- Ether
- Chloroform
- O3 gas
- Methanol
- 1-(2-aminoethyl) piperidine
- Borane-pyridine complex
- Cell culture medium (see Recipes)
- 4% paraformaldehyde (see Recipes)
Equipment
- Pipettes
- 37 °C, 5% CO2 incubator
- Flow Cytometer (BD Biosciences, BD FACSCalibur, catalog number: 342975)
- Microcentrifuge
- Confocal laser scanning microscope with 40x objective (Zeiss, model: LSM 700)
- 4 °C fridge
- Vortexer
- Glovebox
- Laminar flow hood
- Fluostar Optima (BMG, Fluostar Optima, catalog number: 0413B0001J) or any fluorescence plate reader with excitation wavelength of 485 nm and an emission wavelength of 530 nm
Software
- Computer running Flowjo (Flowjo LLC, https://www.flowjo.com/) or any flow cytometry data analysis software
- Computer running BMG Optima Windows (BMG, https://www.bmglabtech.com/discontinued-microplate-readers/) or any other fluorescence plate reader software
Procedure
- For determination of cell uptake of particles using YOYO-1 labeled plasmid
- Prepare YOYO-1 labeled plasmid by taking pSPCas9 (Addgene, px165) at 1 mg/ml in molecular biology grade water and adding 20 μM YOYO-1, such that there is 1 dye molecule per 50 bp of DNA. For example, for a 1 ml total volume, add 1 mg plasmid and 25.4 μg YOYO-1.
- Prepare cells 24 h before uptake studies, by seeding 5 x 104 cells/well, for example, HEK293T, per well in a 24-well plate.
- Load YOYO-1 labeled plasmid into delivery vehicle (e.g., combine 30:1 PPABLG to plasmid by adding 30 μg of PPABLG to 1 μg of plasmid and let sit on ice for 15 min).
- Add particles from Step A3 to each well with a total volume of 500 μl of 1 μg sgRNAs/ml OptiMEM.
- Remove culture medium from the 24-well plate, replace with 500 μl OptiMEM and delivery vehicle containing fluorescently-tagged nucleic acid. Incubate for 4 h.
- Wash the cells three times with 500 μl/well cold PBS containing heparin (20 U/ml) each to remove externally-associated fluorescently-tagged nucleic acid.
- Apply 500 μl of RIPA lysis buffer and collect lysate.
- Use fluorescence spectrophotometer at an excitation wavelength of 485 nm and an emission wavelength of 530 nm to get specific fluorescence intensity of each lysate. Use a standard curve of labeled plasmid to correlate fluorescence intensity of lysate to ng of plasmid.
- Perform BCA assay to determine the total protein concentration of lysate.
- Cellular uptake level is then presented as ng YOYO-1 labeled plasmid per mg protein.
- For determination of cell uptake of particles using FITC-labeled sgRNA
- Prepare FITC-labeled in vitro transcribed sgRNA by using the HiScribe T7 High Yield RNA Synthesis Kit and subbing Fluorescein-12-UTP for the un-tagged UTP at a specific ratio. To calculate the ratio of Fluorescein-12-UTP to UTP, calculate the number of Uracils in the sgRNA sequence and, based off of 2 fluorescein per sgRNA strand, add Fluorescein-12-UTP to UTP at the ratio of 2 to (total number of UTP) -2.
Example: With an RNA strand sequence GGCTGATGAGGCCGCACATG, which includes three UTPs, we would want to add Fluorescein-12-UTP to UTP in a 2:1 ratio. Therefore, with a 7.5 mM final concentration of UTP in the reaction mix, we would want 5 mM to be Fluorescein-12-UTP and 2.5 mM UTP. - Prepare cells 24 h before uptake studies, by seeding 2.5 x 104 cells/cm2, for example, HEK293T, per well in a 24-well plate.
- Load FITC-labeled sgRNA into delivery vehicle (e.g., combine 30:1 PPABLG to sgRNA and let sit on ice for 15 min).
- Add particles from Step B3 to well with total volume of 500 μl/well, 1 μg sgRNAs/ml OptiMEM.
- Remove culture medium from 24-well plate, replace with 500 μl OptiMEM and delivery vehicle containing fluorescently-tagged nucleic acid. Incubate for 4 h.
- Wash the cells with cold PBS containing heparin (20 U/ml) three times.
- Trypsinize each well into a cell suspension (about 200 μl DMEM per well) and then subject to flow cytometric analysis using BD FACSCalibur flow cytometer. Quantify uptake using FITC positivity and intensity.
- Prepare FITC-labeled in vitro transcribed sgRNA by using the HiScribe T7 High Yield RNA Synthesis Kit and subbing Fluorescein-12-UTP for the un-tagged UTP at a specific ratio. To calculate the ratio of Fluorescein-12-UTP to UTP, calculate the number of Uracils in the sgRNA sequence and, based off of 2 fluorescein per sgRNA strand, add Fluorescein-12-UTP to UTP at the ratio of 2 to (total number of UTP) -2.
- For determination of intracellular distribution of genetic cargo
- Prepare YOYO-1 labeled plasmid by taking pSPCas9 (Addgene, px165) at 1 mg/ml in molecular biology grade water and adding 20 μM YOYO-1, such that there is 1 dye molecule per 50 bp of DNA.
- Seed cells on coverslips in a 24-well plate at a density of 5 x 104 cells/well and culture for 24 h.
- Proceed with transfection as in Steps A3-A5.
- Wash cells 3 times with cold PBS containing heparin (20 U/ml).
- Add 500 μl/well of 4% paraformaldehyde in PBS (see Recipes) and incubate at room temperature for 15 min to fix.
- Stain nuclei with DAPI and endosomes/lysosomes with Lysotracker Red according to the standard protocol provided by Thermo Fisher.
- Mount slides using glass slide and Fluoromount Aqueous Mounting Medium.
- Observe via confocal laser scanning microscopy with a 40x objective. Do observation immediately after lysotracker labeling as bleaching and loss of label occurs within hours.
- Via fluorescent images, note the colocalization of Lysotracker and YOYO-1 labeled plasmid to determine if the plasmid is in the endosome or cytosol.
Note: Cohesive YOYO-1 fluorescence in cell body implies endosomal escape, while pointed colocalization with Lysotracker implies endosomal entrapment.
- For determination of energy-dependent contribution to cell uptake
Note: Repeat Procedure A but at 4 °C.- Prepare YOYO-1 labeled plasmid by taking pSPCas9 (Addgene, px165) at 1 mg/ml in molecular biology grade water and adding 20 μM YOYO-1, such that there is 1 dye molecule per 50 bp of DNA.
- Prepare cells 24 h before uptake studies, by seeding 5 x 104 cells/well, for example HEK293T, per well in a 24-well plate.
- Pre-incubate cells at 4 °C for 30 min before transfection.
- Load YOYO-1 labeled plasmid into delivery vehicle (e.g., combine 30:1 PPABLG to plasmid and let sit on ice for 15 min).
- Add particles from Step D4 to each well with a total volume of 500 μl 1 μg sgRNAs/ml OptiMEM.
- Remove culture medium from the 24-well plate, replace with 500 μl of precooled OptiMEM and delivery vehicle containing fluorescently-tagged nucleic acid. Incubate at 4 °C for 2 h.
- Wash the cells with cold PBS containing heparin (20 U/ml) three times.
- Apply 500 μl of RIPA lysis buffer and collect lysate.
- Use fluorescence spectrophotometer at an excitation wavelength of 485 nm and an emission wavelength of 530 nm to get specific fluorescence intensity of each lysate.
- Perform BCA assay to determine the total protein concentration of lysate.
- Cellular uptake level is then presented as ng YOYO-1 labeled plasmid per mg protein and can be compared to Procedure A as control. Use a standard curve of labeled plasmid to correlate fluorescence intensity of lysate to ng of plasmid.
- For determination of contribution to cell uptake for caveolae-mediated endocytosis (a), clathrin-independent endocytosis (b), clathrin-mediated endocytosis (c), and macropinocytosis (d)
- Prepare YOYO-1 labeled plasmid by taking pSPCas9 (Addgene, px165) at 1 mg/ml in molecular biology grade water and adding 20 μM YOYO-1, such that there is 1 dye molecule per 50 bp of DNA.
- Prepare cells 24 h before uptake studies, by seeding 5 x 104 cells/well, for example, HEK293T, per well in a 24-well plate.
- Pre-incubate cells with a specific uptake mechanism inhibitor mixed with complete culture medium for 30 min at 37 °C.
Note: Each inhibitor of interest should be applied alone to determine the specific significance of each pathway.- 100 μg/ml genistein
- 5 mM mβCD
- 10 μg/ml chlorpromazine
- 10 μg/ml wortmannin
- Load YOYO-1 labeled plasmid into delivery vehicle (e.g., combine 30:1 PPABLG to plasmid and let sit on ice for 15 min).
- Add particles from Step E4 to each well with a total volume of 500 μl 1 μg sgRNAs/ml OptiMEM.
- Remove culture medium from the 24-well plate, replace with 500 μl OptiMEM and delivery vehicle containing fluorescently-tagged nucleic acid. Incubate for 2 h.
- Wash the cells with cold PBS containing heparin (20 U/ml) three times.
- Apply 500 μl of RIPA lysis buffer and collect lysate.
- Use fluorescence spectrophotometer at an excitation wavelength of 485 nm and an emission wavelength of 530 nm to get specific fluorescence intensity of each lysate.
- Perform BCA assay to determine the total protein concentration of lysate.
- Cellular uptake level is then presented as ng YOYO-1 labeled plasmid per mg protein. Use a standard curve of labeled plasmid to correlate fluorescence intensity of lysate to ng of plasmid.
- For determination of membrane permeability
- Prepare cells 24 h before uptake studies, by seeding 5 x 104 cells/well, for example HEK293T, per well in a 24-well plate.
- Prepare FITC-tris by incubating FITC in Tris buffer for an hour before usage or storage at -20 °C. Add 50 μl of FITC (1 mg/ml) for 1 ml of Tris buffer.
- Pre-incubate cells with transfecting agent by adding particles (PPABLG) at a concentration of 30 μg/ml in OptiMEM and adding to cells at 500 μl/well.
- Then add FITC-tris at 1 μg/well.
- Incubate at 37 °C for 2 h.
- Wash the cells with cold PBS containing heparin (20 U/ml) three times.
- Apply 500 μl of RIPA lysis buffer and collect lysate.
- Use fluorescence spectrophotometer at an excitation wavelength of 488 nm and an emission wavelength of 530 nm to get specific fluorescence intensity of each lysate.
- Perform BCA assay to determine the total protein concentration of lysate.
- Cellular uptake level is then presented as ng FITC per mg protein. Use a standard curve of FITC-tris to correlate fluorescence intensity of lysate to FITC uptake.
Data analysis
Total cell uptake of the particle was initially determined via Procedure A and was represented as ng YOYO-1 labeled plasmid per mg protein. Following this initial uptake study, Procedures C-H provided uptake results as ng YOYO-1 labeled plasmid per mg protein; this could be divided by the results of Procedure A, to give the percentage uptake after inhibition, as seen in Figure 2. Further, this could be subtracted from 100% to approximate percent inhibition.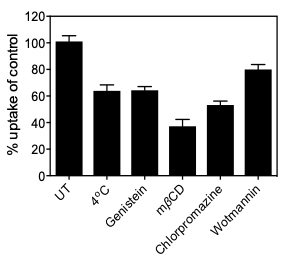
Figure 2. Comparison of uptake for various treatments. Use uptake from initial endocytosis experiments as the reference for normalization of the inhibitor uptake measurements.
For example: A standard curve can be determined as below: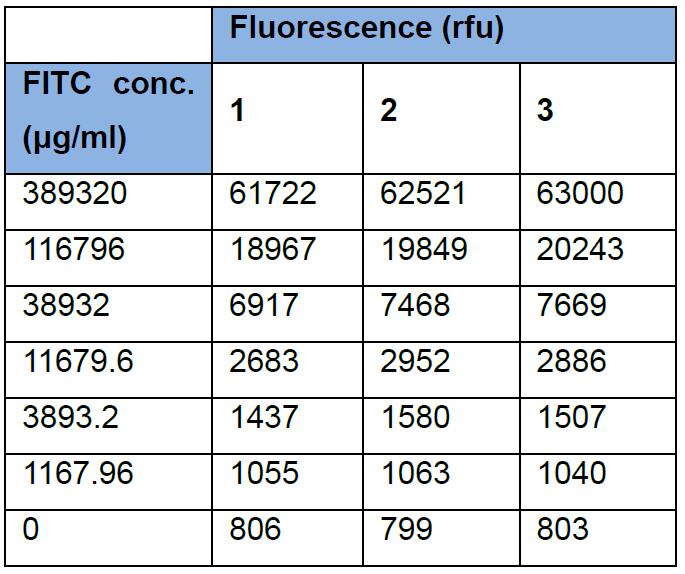
Concentration can then be calculated (Y) from fitting the data to a standard curve, as above, and supplying X from fluorescence readings of the lysate. Below, we provide an example calculation, where b is the slope derived from fitting the data to a linear curve of concentration versus fluorescence and a is the Y-intercept.
X = b * Y + a
X = 61800Y + 1100
Y = 1/(61800) * (X-1100)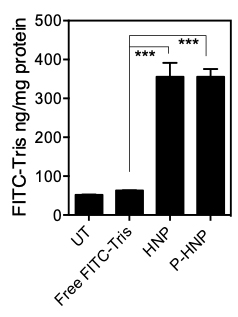
Figure 3. Cell membrane permeability. Use the amount of FITC-Tris in lysate to correlate to membrane permeability. The more FITC-Tris able to cross into the cell, the more permeability the nanoparticle (or any cell treatment) induced in the cell membrane. Here UT refers to the untreated group, while Free FITC-Tris is an untreated group exposed to the FITC-Tris; HNP and P-HNP are helical nanoparticles and PEGylated helical nanoparticles respectively.
Then we can calculate the percent uptake and inhibition as in Figure 3, with the following fluorescence readings from Procedures A, C, D, as in the first column below: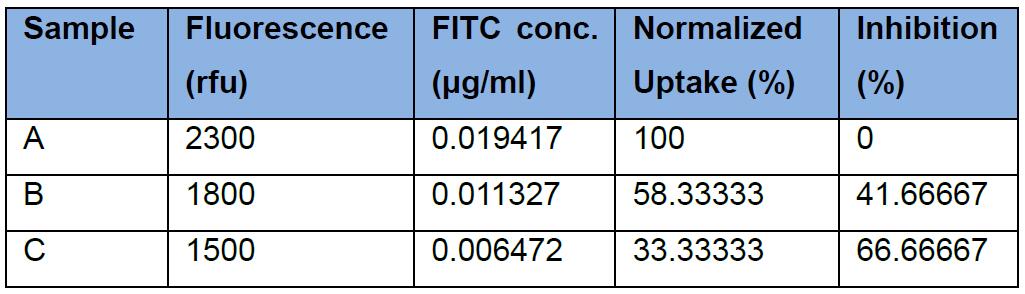
Recipes
- Cell culture medium
500 ml of Dulbecco’s modified Eagle’s medium
5 ml of Penicillin-streptomycin (10,000 U/ml)
50 ml of fetal bovine serum - HNPs composed of PPABLG (delivery vehicles)
Note: PPABLG is just a polymer that can be used to formulate helical nanoparticles with genetic cargo and is only an example of a delivery platform that you can use to observe the intracellular trafficking and uptake in cells. Some simpler vehicles that can be directly purchased are materials like Silica Oxide nanoparticles or Lipofectamine.- In a glovebox, first add γ-(4-vinylbenzyl)-L-glutamate N-carboxyanhydride (VB-L-Glu-NCA) and dissolve in anhydrous dimethyl formamide (DMF), then add a DMF solution of hexamethyldisilazane (M/I = 200) and nitrobenzene
- The polymerization is carried out at room temperature until the conversion of NCA reached > 99% (monitored by Fourier Transform Infrared Spectroscopy). Purify the polypeptide precursor, poly(γ-(4-vinyl)benzyl-L-glutamate) (PVBLG), by precipitation in hexane:ether (1:1, v/v)
- Then dissolve PVBLG in chloroform, and the side-chain vinyl groups are oxidized into aldehydo groups by bubbling O3 gas into the solution at -78 °C
- Purify the resulting polypeptide, poly(γ-(4-aldehydo)benzyl-L-glutamate) (PABLG) by precipitation in methanol, and analyze by 1 H NMR to confirm the conversion of side-chain vinyl groups
- Then Dissolve PABLG in DMF, into which 1-(2-aminoethyl) piperidine and borane-pyridine complex are added sequentially to react with the side-chain aldehyde groups of PABLG
- Purify the final product PPABLG by dialysis against DI water and then lyophilize to yield white powder
- 4% paraformaldehyde
25 ml of paraformaldehyde
75 ml of PBS
Acknowledgments
The research was supported by the NIH (Grants nos. HL109442, AI096305, GM110494, and UH3 TR000505).
Competing interests
The authors declare no conflicts of interest or competing interests.
References
- Dausend, J., Musyanovych, A., Dass, M., Walther, P., Schrezenmeier, H., Landfester, K. and Mailander, V. (2008). Uptake mechanism of oppositely charged fluorescent nanoparticles in HeLa cells. Macromol Biosci 8(12): 1135-1143.
- Douglas, K. L., Piccirillo, C. A. and Tabrizian, M. (2008). Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur J Pharm Biopharm 68(3): 676-687.
- Gilleron, J., Querbes, W., Zeigerer, A., Borodovsky, A., Marsico, G., Schubert, U., Manygoats, K., Seifert, S., Andree, C., Stoter, M., Epstein-Barash, H., Zhang, L., Koteliansky, V., Fitzgerald, K., Fava, E., Bickle, M., Kalaidzidis, Y., Akinc, A., Maier, M. and Zerial, M. (2013). Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol 31(7): 638-646.
- Iversen, T. G., Skotland, T. and Sandvig, K. (2011). Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today 6(2):176-85.
- Wang, H. X., Song, Z., Lao, Y. H., Xu, X., Gong, J., Cheng, D., Chakraborty, S., Park, J. S., Li, M., Huang, D., Yin, L., Cheng, J. and Leong, K. W. (2018). Nonviral gene editing via CRISPR/Cas9 delivery by membrane-disruptive and endosomolytic helical polypeptide. Proc Natl Acad Sci U S A 115(19): 4903-4908.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gong, J., Wang, H. and Leong, K. W. (2019). Determination of Cellular Uptake and Endocytic Pathways. Bio-protocol 9(4): e3169. DOI: 10.21769/BioProtoc.3169.
Category
Cell Biology > Cell-based analysis > Endocytosis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








