- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice
Published: Vol 9, Iss 3, Feb 5, 2019 DOI: 10.21769/BioProtoc.3162 Views: 20182
Reviewed by: Oneil G. BhalalaEdel HennessyAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2606 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views
Abstract
The global population is aging and the prevalence of age-related diseases, such as Alzheimer's disease and vascular dementia is increasing. Understanding functional impairments and disease processes is of vital importance in order to develop effective therapeutics. Using the natural exploratory behavior of mice, the spontaneous alternation y-maze can assess short-term spatial working memory. The protocol for y-maze testing is straightforward and requires minimal resources, as well as animal training and output. Therefore, it can be broadly applied to study short-term memory in aged rodent models.
Keywords: Y-mazeBackground
The global population is aging, and in many countries the people over the age of 65 have surpassed the number of children (United Nations Department of Economic and Social Affairs, 2013). Since people are living longer, there is an increase in the prevalence of diseases associated with aging (Jaul and Barron, 2017), such as neurodegeneration (Brown et al., 2005). To understand the degree of impairment and to investigate therapeutic options scientists need tools, such as behavioral tests. Assessing behavioral outcomes facilitates this knowledge acquisition, which then gives rationale to study mechanisms and potential therapeutics.
In this protocol, we described how the spontaneous alternation y-maze can be used to assess short-term spatial working memory by using male aged methylenetetrahydrofolate reductase (MTHFR) heterozygote and wild-type mice as our model system. MTHFR is an enzyme involved in one-carbon metabolism, and deficiencies in MTHFR levels have been implicated in vascular diseases (Frosst et al., 1995; Devlin et al., 2004), such as stroke (Song et al., 2016) and dementia (Dam et al., 2017). The protocol for spontaneous alternation y-maze testing is straightforward and requires minimal resources, including animal training and output, therefore can be broadly applied to study short-term memory in aged rodent models. Additionally, the standard y-maze can be adapted to measure spatial and object recognition, as well as novelty exploration using the 2-trial y-maze.
Materials and Reagents
- Aged C57Bl/6 male mice (24-months-old) which model 56-69 year-old humans (Flurkey et al., 2007)
Notes:- Differences in the behavioral C57Bl/6 mice have been observed compared to other stains (Crabbe et al., 1999). Furthermore, sex differences in behavioral tests have also been widely described in the literature (Kopp et al., 2006).
- For testing on the spontaneous alternation y-maze, animals can be maintained on a diet of standard mouse chow and water ad-libitum. The mice were maintained on a regular light cycle.
- Surface disinfectant, a surface disinfectant to remove odors on the y-maze between animals (e.g., 70% ethanol)
Note: The literature should be consulted when selecting a disinfectant to determine which solution is ideal for mouse strain being tested (Campagna et al., 2016) and equipment being used.
Equipment
- Y-maze (Custom made or commercially purchased) (Stoelting, catalog number: 60180), the y-maze can be made of gray, white, or black plexiglass with the dimensions 39.5 x 8.5 x 13 cm for mice (Figure 1A)
Note: The plastic fiber makes it easy to disinfect between animals to remove any odors. The three arms of the maze are interconnected at an angle of 120°. Animals should be habituated to the room for at least 30 min prior to testing, without the y-maze being visible. - Video camera
Notes:- Animal behavior can be tracked using a video camera recording, real-time analysis, or automated tracking equipment such as AnyMaze (Stoelting, catalog number: 60000). For video camera recordings, no special frame rate is required.
- We video recorded all animals which facilitated scoring by two individuals blinded to experimental groups. The set-up should be above the y-maze and not interfere with the testing as shown in Figure 1B.
- Visual cues
Note: Paper visual cues can be custom made and hung on the walls of testing room. These cues should be uniform in color and examples are shown in Figure 1B.
Figure 1. Pictorial representations of spontaneous alternation y-maze apparatus (A) and testing set-up (B)
Software
- Animal tracking program, AnyMaze (Stoelting, catalog number: 60000)
- Statistical testing, GraphPad Prism (GraphPad Software)
Procedure
Note: Institutional animal ethics protocol approval is required for carrying the experiment described in this protocol.
- Habituate mice to handling
A minimum of one to two weeks prior to testing on the y-maze, experimenters should remove animals from cages and handle them (Crawley, 2007). Each week, the animals should be handled daily for 5 out of the 7 days. Each mouse should be handled for at least 5 min. - On the day of y-maze, habituate testing animals to the testing room 30 min prior to beginning behavioral testing (Rutkowsky et al., 2018). Animals should not be able to see the y-maze prior to testing.
- Conduct behavioral testing at the same time each day. We tested animals in the afternoon during their light cycle. It is important to remain consistent in testing time parameters.
- If possible, the lights in the testing room can be dimmed to increase exploration of the y-maze during testing. There should be indirect and homogenous illumination (150-200 Lux) in the center of the y-maze.
- Place each mouse, naive to the maze, at the same end of one arm and allow each mouse to move freely through the maze during an 8-min session (Sarter et al., 1988; Maurice et al., 1994; Holcomb et al., 1998; Li et al., 2010; Lee et al., 2017; de Sousa et al., 2018). Video record the whole process. Record the number of arm entries by two individuals blinded to the experimental groups. If animals are being tested over multiple days, then the orientation of the y-maze should be the same each day.
Notes:- If there are loud noises (e.g., construction) during testing, a white noise machine can be used. Animals should be habituated to the white noise prior to testing. In our study, we did not use any white noise during testing.
- Modifications can be made to the above testing protocol, these modifications are summarized in Table 1–Changes to testing time and paradigm (Mihalick et al., 2003; Võikar et al., 2004; Tchantchou et al., 2005; Barakat et al., 2018) as well as the number of trials per animal (Mihalick et al., 2003). Other modifications include object recognition (Romberg et al., 2013; Ibi et al., 2018) and the two-trial y-maze, which assess short-term memory (Dellu et al., 2000). and the two-trial y-maze, which assess short-term memory (Dellu et al., 2000). In the first trial, one arm was blocked, allowing the mouse to explore only two arms of the maze for 5 min. In the second trial, after a 60 min inter-trial interval, the block to the third arm was removed and the mouse was able to explore all three arms for a period of 5 min. The amount of time spent in the novel arm was calculated within the first minute.
- After each trial, the y-maze should be cleaned, disinfected, and dried prior to placing the next mouse. Alternatively, saw dust or bedding can be added to the y-maze and mixed between animals (Ma et al., 2007).
Table 1. Summary of changes that can be made to spontaneous alternation y-maze testing protocol and apparatus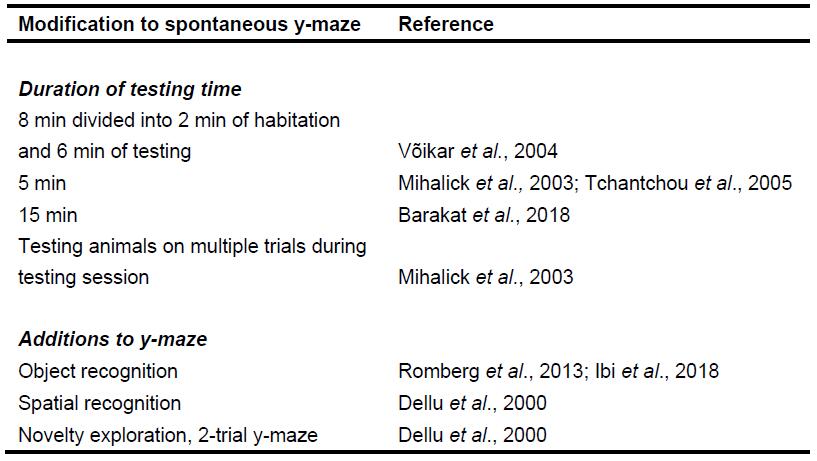
Data analysis
The three main outputs from the spontaneous alteration y-maze analysis are the number of alternations and entries, as well as percent alternations. The number of alternations can be calculated based on the sequence of arm entries. An alternation is defined as successive entries into 3 arms, on overlapping triplet sets (Figure 2). For example, entries into arms 1, 3, and 2 is considered an alternation. Whereas entries into arms 1, 2, and 1 would not be considered an alternation. Alternations can be used to measure short-term spatial memory in mice (Sarter et al., 1988). An arm entry is completed when the hind paws of the mouse had been completely placed in the arm (Ohno et al., 2004). The number of entries per arm is a measurement of activity and locomotion during the testing session and is also be used to calculate the percent alternations. The percentage of alternation can be calculated as the ratio of actual to alternations and it can be calculated by using the formula below:

Figure 2. Representative image depicting an alternation. Alternations are consecutive entries into each arm of the y-maze without any repeats. For each alternation, there are three steps. Steps of alternation outlined in (A) Step one entry into Arm 1; (B) Step two entry into Arm 3; and in (C) Step three entry into Arm 2.
Lack of motivation in older mice may be a problem. In that case water (Lyon et al., 2011) or food (Spence and Lippitt, 1946) motivated y-maze tests can be used. In terms of excluding mice, if a mouse continues to jump out of the maze or not move during testing, their behavior may make them an outlier. Experimenters should carefully review data before making the decision to remove mice. Therefore, we recommend that all trials be video recorded. We advise experimenter not to make premature conclusion concerning memory deficits until enough parametric research has been conducted and alternative explanations have been assessed, please refer to Table 2 for suggestions.
Table 2. Troubleshooting guide for spontaneous alternation y-maze task in rodents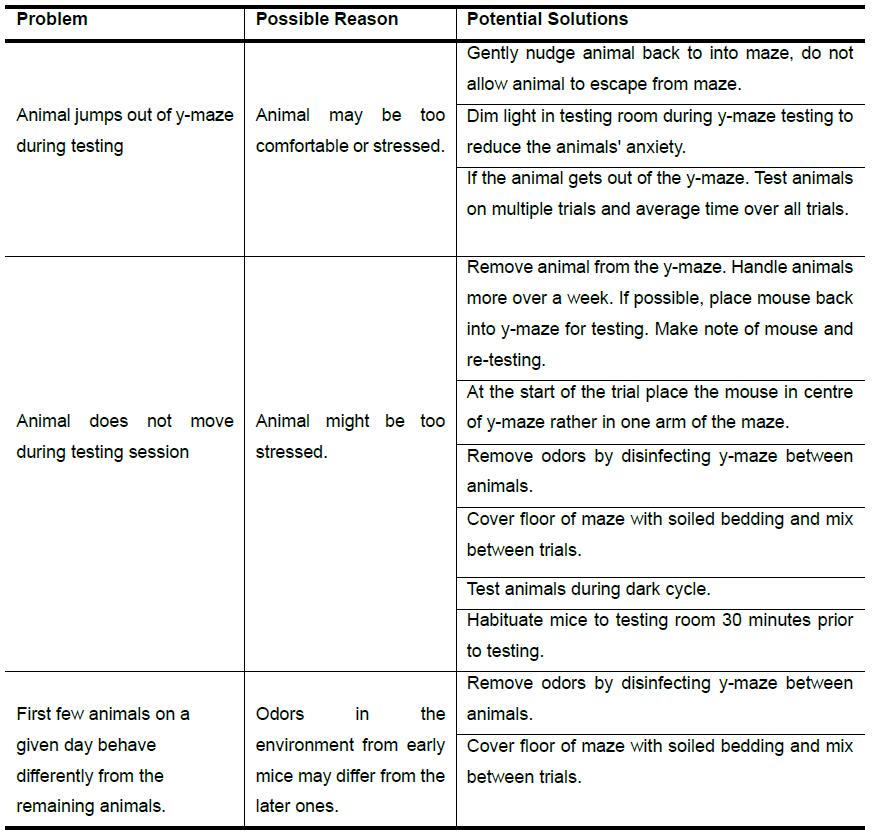
Statistical analysis of the data including the number of alternations and entries, as well as percent alternations can be conducted using GraphPad Prism 6.0 or any other statistical program. Depending on the number of groups, unpaired t-tests or analysis of variance (ANOVAs) can be used.
In Figure 3, we have provided a sample analysis of aged male C57Bl/6 mice deficient in methylenetetrahydrofolate reductase (MTHFR). All animals performed on behavioral task, there were no non-performers. We conducted statistical analysis using GraphPad Prism 6.0. Un-paired t-tests were used to compare genotype groups. In all analysis, a P < 0.05 was considered significant. All data are presented as mean ± standard error of the mean (SEM). Mthfr+/- mice made less alterations compared to wild-type controls [Figure 3A, t(17) = 2.6, P < 0.05]. It appeared that the Mthfr+/- mice made fewer total arm entries, but this was not statistically different [Figure 3B, t(17) = 1.1, P > 0.05]. The reduced number of arm entries is a marker for reduced activity. When the number of arm entries was factored into the alternations, there was still a reduction in the percent alternations of Mthfr+/- mice [Figure 3C, t(17) = 2.39, P ≤ 0.05].
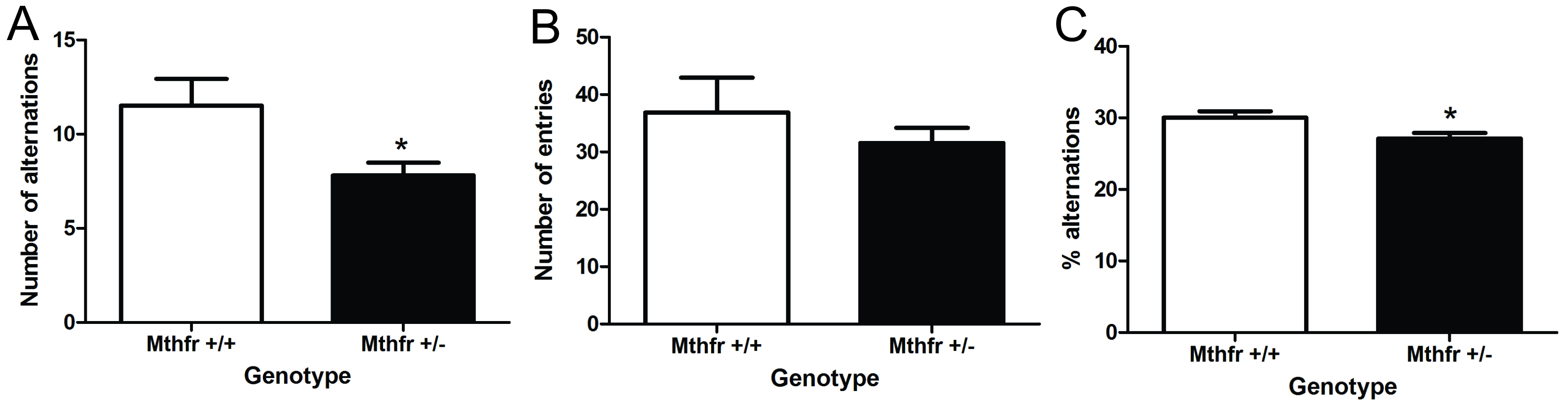
Figure 3. The impact of methylenetetrahydrofolate reductase (MTHFR)-deficiency on the spontaneous alternation y-maze in aged Mthfr+/+ andMthfr+/- male mice. The number of alternations (A), entries (B), and percent alternations (C). Mean ± SEM of 8-9 mice per group. * indicates P < 0.05, unpaired t-test.
Conclusion: Using this spontaneous alternation y-maze we were able to measure short-term spatial memory in aged animals. In mice, spatial recognition memory can also be assessed by the T-maze, radial arm maze and Barnes maze tasks, but all of these tasks require extensive training compared to the spontaneous y-maze (Crawley, 2007). Furthermore, dietary restriction is required for the T-maze and radial arm maze, this may be challenging for aged mice. The spontaneous y-maze measures spatial recognition memory at a rudimentary level using the natural exploratory behavior of rodents. This type of memory is consistently evaluated in aging studies and is therefore a valuable test. The y-maze does not require significant output resources or training of animals, so assessing aged animals is not difficult. With the increase in the aging population (United Nations Department of Economic and Social Affairs, 2013) and prevalence of age-related diseases (Bach et al., 2011) there is an urgent need to understand disease processes associated to aging and therapeutic development. The spontaneous alternation y-maze provides researchers with data on memory as well as exploratory activity of animals in a short time frame. Furthermore, the standard y-maze is flexible and can be adapted to measure spatial and object recognition, as well as novelty exploration using the 2-trial y-maze.
Acknowledgments
This research was supported by Fonds de la recherché en santé Québec, Council of Ontario Universities Postdoctoral Women’s Health Scholars Fellowship and Natural Sciences and Engineering Research Council of Canada fellowship (N.M.J.).
Competing interests
No conflicts.
Ethics
Mice were bred and maintained in the Department of Neuroscience, Faculty of Science, Carleton University. All experiments performed in this study were in accordance with the Canadian Council on Animal Care (CCAC). Animals were maintained on a diet of standard mouse chow and water ad-libitum.
References
- Bach, J. P., Ziegler, U., Deuschl, G., Dodel, R. and Doblhammer-Reiter, G. (2011). Projected numbers of people with movement disorders in the years 2030 and 2050. Mov Disord 26(12): 2286-2290.
- Barakat, R., Lin, P. C., Park, C. J., Best-Popescu, C., Bakry, H. H., Abosalem, M. E., Abdelaleem, N. M., Flaws, J. A. and Ko, C. (2018). Corrigendum to "Prenatal exposure to DEHP induces neuronal degeneration and neurobehavioral abnormalities in adult male mice". Toxicol Sci 164(2): 645.
- Brown, R. C., Lockwood, A. H. and Sonawane, B. R. (2005). Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect 113(9): 1250-1256.
- Campagna, M. V., Faure-Kumar, E., Treger, J. A., Cushman, J. D., Grogan, T. R., Kasahara, N. and Lawson, G. W. (2016). Factors in the selection of surface disinfectants for use in a laboratory animal setting. J Am Assoc Lab Anim Sci 55(2): 175-188.
- Crabbe, J. C., Wahlsten, D. and Dudek, B. C. (1999). Genetics of mouse behavior: interactions with laboratory environment. Science 284(5420): 1670-1672.
- Crawley, J. N. (2007). What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. 2nd Edition. New Jersey: John Wiley & Sons.
- Dam, K., Fuchtemeier, M., Farr, T. D., Boehm-Sturm, P., Foddis, M., Dirnagl, U., Malysheva, O., Caudill, M. A. and Jadavji, N. M. (2017). Increased homocysteine levels impair reference memory and reduce cortical levels of acetylcholine in a mouse model of vascular cognitive impairment. Behav Brain Res 321: 201-208.
- de Sousa, L. P., de Almeida, R. F., Ribeiro-Gomes, F. L., de Moura Carvalho, L. J., TM, E. S., de Souza, D. O. G. and Daniel-Ribeiro, C. T. (2018). Long-term effect of uncomplicated Plasmodium berghei ANKA malaria on memory and anxiety-like behaviour in C57BL/6 mice. Parasit Vectors 11(1): 191.
- Dellu, F., Contarino, A., Simon, H., Koob, G. F. and Gold, L. H. (2000). Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol Learn Mem 73(1): 31-48.
- Devlin, A. M., Arning, E., Bottiglieri, T., Faraci, F. M., Rozen, R. and Lentz, S. R. (2004). Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood 103(7): 2624-2629.
- Flurkey, K., Currer, J. and Harrison, D. (2007). The Mouse in Aging Research. In: The mouse in biomedical research. 2nd Edition. In: Fox, J., Davisson, M., Quimbly, F., Barthold, S., Newcomer, C. and Smith, A. (Eds.). pp 637-672. Burlington, MA: American College of Laboratory Animal Medicine (Elsevier).
- Frosst, P., Blom, H. J., Milos, R., Goyette, P., Sheppard, C. A., Matthews, R. G., Boers, G. J., den Heijer, M., Kluijtmans, L. A., van den Heuvel, L. P. and et al. (1995). A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10(1): 111-113.
- Holcomb, L., Gordon, M. N., McGowan, E., Yu, X., Benkovic, S., Jantzen, P., Wright, K., Saad, I., Mueller, R., Morgan, D., Sanders, S., Zehr, C., O'Campo, K., Hardy, J., Prada, C. M., Eckman, C., Younkin, S., Hsiao, K. and Duff, K. (1998). Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4(1): 97-100.
- Ibi, D., Suzuki, F. and Hiramatsu, M. (2018). Effect of AceK (acesulfame potassium) on brain function under dietary restriction in mice. Physiol Behav 188: 291-297.
- Jaul, E. and Barron, J. (2017). Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health 5: 335.
- Kopp, C., Ressel, V., Wigger, E. and Tobler, I. (2006). Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behav Brain Res 167(1): 165-174.
- Lee, G. Y., Lee, C., Park, G. H. and Jang, J. H. (2017). Amelioration of scopolamine-induced learning and memory impairment by α-pinene in C57BL/6 mice. Evid Based Complement Alternat Med 2017: 4926815.
- Li, B., Arime, Y., Hall, F. S., Uhl, G. R. and Sora, I. (2010). Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice. Eur J Pharmacol 628(1-3): 104-107.
- Lyon, L., Burnet, P. W., Kew, J. N., Corti, C., Rawlins, J. N., Lane, T., De Filippis, B., Harrison, P. J. and Bannerman, D. M. (2011). Fractionation of spatial memory in GRM2/3 (mGlu2/mGlu3) double knockout mice reveals a role for group II metabotropic glutamate receptors at the interface between arousal and cognition. Neuropsychopharmacology 36(13): 2616-2628.
- Ma, M. X., Chen. Y. M., He, J., Zeng, T. and Wang, J. H. (2007). Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience 147(4):1059-1065.
- Maurice, T., Hiramatsu, M., Itoh, J., Kameyama, T., Hasegawa, T. and Nabeshima, T. (1994). Behavioral evidence for a modulating role of sigma ligands in memory processes. I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res 647(1): 44-56.
- Mihalick, S. M., Ortiz, D., Kumar, R., Rogers, E. and Shea, T. B. (2003). Folate and vitamin E deficiency impair cognitive performance in mice subjected to oxidative stress: differential impact on normal mice and mice lacking apolipoprotein E. Neuromolecular Med 4(3): 197-202.
- Ohno, M., Sametsky, E. A., Younkin, L. H., Oakley, H., Younkin, S. G., Citron, M., Vassar, R., Disterhoft, J. F. (2004). BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron 41(1): 27-33.
- Romberg, C., Yang, S., Melani, R., Andrews, M. R., Horner, A. E., Spillantini, M. G., Bussey, T. J., Fawcett, J. W., Pizzorusso, T. and Saksida, L. M. (2013). Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci 33(16): 7057-7065.
- Rutkowsky, J. M., Lee, L. L., Puchowicz, M., Golub, M. S., Befroy, D. E., Wilson, D. W., Anderson, S., Cline, G., Bini, J., Borkowski, K., Knotts, T. A., Rutledge, J. C. and Mouse Metabolic Phenotyping Center Imaging Working, G. (2018). Reduced cognitive function, increased blood-brain-barrier transport and inflammatory responses, and altered brain metabolites in LDLr -/-and C57BL/6 mice fed a western diet. PLoS One 13(2): e0191909.
- Sarter, M., Bodewitz, G. and Stephens, D. N. (1988). Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist β-carbolines. Psychopharmacology (Berl) 94(4): 491-495.
- Song, Y., Li, B., Wang, C., Wang, P., Gao, X. and Liu, G. (2016). Association between 5,10-methylenetetrahydrofolate reductase C677T gene polymorphism and risk of ischemic stroke: A Meta-analysis. J Stroke Cerebrovasc Dis 25(3): 679-687.
- Spence K. W. and Lippitt R. (1946). An experimental test of the sign-gestalt theory of trial and error learning. J Exp Psychol 36(6):491-502.
- Tchantchou, F., Chan, A., Kifle, L., Ortiz, D. and Shea, T. B. (2005). Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J Alzheimers Dis 8(3): 283-287.
- United Nations Department of Economic and Social Affairs. (2013). World population ageing 2013.
- Võikar, V., Vasar, E. and Rauvala, H. (2004). Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav 3(1): 27-38.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Prieur, E. A. and Jadavji, N. M. (2019). Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice. Bio-protocol 9(3): e3162. DOI: 10.21769/BioProtoc.3162.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link