- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Insulin Tolerance Test under Anaesthesia to Measure Tissue-specific Insulin-stimulated Glucose Disposal
(*contributed equally to this work) Published: Vol 9, Iss 2, Jan 20, 2019 DOI: 10.21769/BioProtoc.3146 Views: 8314
Reviewed by: Gal HaimovichSara JohnsonAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2540 Views

Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2277 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 481 Views
Abstract
Insulin resistance is a pathophysiological state defined by impaired responses to insulin and is a risk factor for several metabolic diseases, most notably type 2 diabetes. Insulin resistance occurs in insulin target tissues including liver, adipose and skeletal muscle. Methods such as insulin tolerance tests and hyperinsulinaemic-euglycaemic clamps permit assessment of insulin responses in specific tissues and allow the study of the progression and causes of insulin resistance. Here we detail a protocol for assessing insulin action in adipose and muscle tissues in anesthetized mice administered with insulin intravenously.
Keywords: InsulinBackground
Surrogate measures of whole-body insulin sensitivity such as homeostatic model assessment for insulin resistance (HOMA-IR) often do not reflect insulin-stimulated glucose uptake in peripheral tissues of the mouse (Lee et al., 2008; Mather, 2009). Ex vivo adipose tissue explants or isolated skeletal muscles preparations (Burchfield et al., 2018; Fazakerley et al., 2018b) can allow direct measurement of insulin responses in specific tissues if they are amenable to these procedures, but do not preserve the organism environment where finely articulated delivery of glucose and insulin via the vasculature, uptake into cells and intracellular metabolism may all contribute to the overall rate of tissue glucose uptake (Wasserman et al., 2011). Commonly employed methods to assess whole-body insulin action in mice include intraperitoneal (IP) or intravenous insulin tolerance tests (ITTs) and, the gold standard, hyperinsulinaemic-euglycaemic clamp (Ayala et al., 2010; Brandon et al., 2016). These techniques can assess whole-body insulin action by measuring either changes in blood glucose (ITT) or changes in the amount of glucose required to maintain glycemia (glucose infusion rate; hyperinsulinaemic-euglycaemic clamp) and can be adapted to measure tissue-specific insulin responses. For example, glucose tracers such as radiolabeled 2-deoxyglucose (2-DOG), which is often used as a surrogate for glucose in assessing glucose uptake as it is not metabolized through glycolysis and is ‘trapped’ in the cells following uptake, can be introduced to permit assessment of glucose uptake into specific tissues during these tests. Note that 2-DOG is only trapped in tissues that do not possess significant glucose-6-phosphatase activity, and so 2-DOG tracer is therefore not useful for assessing glucose disposal in the liver.
Both ITTs and hyperinsulinaemic-euglycaemic clamps can be performed in conscious or anesthetized mice. Certain anesthetics affect whole-body glucose metabolism and should therefore be avoided when assessing insulin action or glucose homeostasis [e.g., isoflurane (Pomplun et al., 2004; Tanaka et al., 2009; Windelov et al., 2016; Hoyer et al., 2018)]. However, anesthetics that minimally affect glucose metabolism, such as pentobarbitone (Guarino et al., 2013), may actually be advantageous for studying insulin-stimulated glucose uptake. This is because insulin-stimulated 2-DOG/glucose uptake in conscious mice may be affected by movement (e.g., due to mechanical- and/or exercise- stimulated glucose uptake) and/or stress responses [e.g., catecholamines (Cooney et al., 1985; Furler et al., 1991) and glucocorticoids (Pasieka and Rafacho, 2016)].
Here, we describe a protocol for a terminal intravenous ITT performed under pentobarbitone-induced anesthesia to assess insulin-stimulated glucose uptake into tissues of interest. The protocol can be performed with minimal delays between mice, making it amenable to assessing insulin action in large cohorts of animals. Intravenous administration of a bolus of insulin/tracer rapidly delivers insulin to tissues, minimizing the time between the start of the assay and initiation of insulin responses within tissues. Also, rapid equilibration of the 2-DOG tracer with the total blood glucose pool ensures that the tracer is immediately available for uptake into tissues. Alternative methods of insulin/tracer delivery (i.e., intraperitoneal injection, oral gavage) may result in delays or inconsistent insulin/tracer uptake into the central circulation. For example, in the case of IP injection, there may be a considerable delay before insulin reaches the circulation. These time lags may differ between mice or between injections, increasing experimental variability. Rapid and consistent tissue uptake is also particularly important when performing time-series experiments in live animals. Indeed, hepatic portal-vein administration of insulin has enabled the assessment of the time-resolved effects of insulin on the liver phosphoproteome (Humphrey et al., 2015). For oral gavage, 2-DOG may exhibit markedly different kinetics of appearance in the circulation compared to glucose since the sodium-dependent glucose transporters, which play a key role in oral glucose absorption, exhibit a strong preference for glucose (Bissonnette et al., 1996), and this may limit tracer availability to tissues during the assay.
In this protocol, mice are anesthetized with pentobarbitone and saline or insulin and radiolabeled 2-DOG tracer administered via the hepatic portal vein. Insulin action in muscle and adipose tissues of interest can be assessed via Western blotting for insulin signaling intermediates or through measuring radiolabeled 2-DOG tracer accumulation as an index of glucose uptake.
Materials and Reagents
- Pipette tips (e.g., Axygen, catalog numbers: T-1000-C, T-200-C)
- Cotton bud (e.g., McFarlane Medical Equipment, catalog number: 18049DE)
- 1.5 ml microfuge tubes (Axygen, catalog number: MCT-150-C)
- 2.0 ml microfuge tubes (Eppendorf, catalog number: 0030120.094)
- 5 ml polystyrene tubes (SARSTEDT, catalog number: 60.9921.531)
- 0.5 ml Ultra-Fine Insulin Syringes (Becton Dickinson, catalog number: 230-45094)
- Masking tape (e.g., Lyreco, catalog number: 737.665)
- 96-well plates (Corning, catalog number: CLS2595)
- Carbon Steel, size 22 scalpel blades (Livingstone, catalog number: SBLDCL22)
- Chromatography columns (Bio-Rad, catalog number: 731-1550)
- 6 ml pony vial scintillation tubes (PerkinElmer, catalog number: 6000292)
- Adult mice (> 8 weeks old)
Note: Ensure experiments using mice are carried with the approval of local ethics committee. Mice may be subjected to different dietary/exercise regimes or be knockout/transgenic lines for genes of interest. For knockout/transgenic lines we recommend using littermate controls. - Double-distilled water (ddH2O)
- Bicinchoninic acid assay (BCA) protein assay kit (Thermo Fisher Scientific, catalog number: 23225)
- Antibodies
- Anti-phospho-Thr308 Akt antibody (Cell Signaling Technology, catalog number: 9275)
- Anti-phospho-Ser473 Akt antibody (Cell Signaling Technology, catalog number: 4051)
- Anti-Akt antibody (Cell Signaling Technology, catalog number: 4685)
- Pentobarbitone (Lethabarb, Virbac, Australia)
Note: Pentobarbitone may be a regulated substance. Ensure that use follows local regulations. - Human insulin (Actrapid, Novo Nordisk)
- 0.9% Sodium Chloride Injection BP (Pfizer, catalog number: 158594)
- [3H]2-DOG (PerkinElmer, catalog number: NET328001MC)
- Accu-Chek performa Glucose Strips (Accu-Check II; Roche Diagnostics, catalog number: 06454038)
- Liquid nitrogen
- AG1-X8 resin (Bio-Rad, catalog number: 140-1443)
- Ulitma Gold XR Scintillation fluid (PerkinElmer, catalog number: 6013119)
- Barium hydroxide (Sigma, catalog number: 433373)
- Dry ice
- 2.75% zinc sulfate solution (see Recipes)
Zinc sulfate (ZnSO4) (Sigma, catalog number: 307491) - Phosphorylated [3H]2-DOG elution buffer (see Recipes)
- Trifluoroacetic acid (Sigma, catalog number: 302031)
- NaCl (Sigma, catalog number: S7653)
- Western blot lysis buffer (see Recipes)
- HEPES (Sigma, catalog number: 54457)
- Sucrose (Sigma, catalog number: S8501)
- EDTA (Sigma, catalog number: 03620)
- SDS (Sigma, catalog number: 71729)
- Protease inhibitors (Roche, cOmpleteTM, catalog number: 11697498001)
- Sodium pyrophosphate (Sigma, catalog number: 71501)
- Sodium orthovanadate (Sigma, catalog number: S6508)
- Sodium fluoride (Sigma, catalog number: S7920)
Equipment
- Scalpel or surgical scissors (e.g., World Precision Instruments, catalog number: 501259-G)
- Pipettes (P20, P200, P1000) (e.g., Finnpipette F1 Pipettes, Thermo Fisher Scientific, catalog numbers: 4641050N, 4641080N, 4641100N)
- Echo-magnetic resonance imaging (MRI) scanner (Echo Medical Systems LLC, model: EchoMRI-4 in 1TM for Live Animals,) to assess mouse lean mass for insulin and pentobarbitone dosing
- Heat pads (Able Scientific, catalog number: ASCHP-RP)
- Blood glucometer (Roche Diagnostics, model: Accu-Check II)
- Mortar and pestle for tissue preparation (e.g., Maxwell & Williams, catalog number: AA1891)
- Ultrasonic tip-probe sonicator/homogenizer (Bendelin, model: Sonopuls)
- Refrigerated centrifuge (Thermo Fisher Scientific, model: Heraeus Fresco 21)
- Vortex (Ratek, model: VM1)
- Scintillation Counter (Beckman Coulter, model:LS6500)
Software
- Microsoft Excel or GraphPad Prism
Procedure
- Hepatic portal vein cannulation
- Determine mouse lean mass by ECHO-MRI according to the manufacturer’s instructions 24-48 h prior to procedure.
- Remove food at least 2 h prior to starting experiment, depending on study requirements.
- Prepare a 96-well plate containing 75 μl 2.75% ZnSO4 per well for blood collection during the procedure. Prepare enough wells for collection of blood at 6 time points per mouse. Keep plate on ice throughout the procedure.
- Inject mice with 80 mg/kg lean mass (determined by ECHO-MRI) pentobarbitone intraperitoneally. Mice with increased adiposity may require more pentobarbitone, up to 80 mg/kg body weight. See Videos 1 and 2.Video 1. Intraperitoneal injection of pentobarbitone (This video was made at The University of Sydney. Procedures were approved by The University of Sydney Animal Ethics Committee under project # 2017/1274)Video 2. Intraperitoneal injection of pentobarbitone (close up) (This video was made at The University of Sydney. Procedures were approved by The University of Sydney Animal Ethics Committee under project # 2017/1274)
- Assess the quality of anesthesia using the toe-pinch reflex once the animal appears to be fully unconscious. When the mouse does not respond (approximately 15-20 min after injection), place animal on back (dorsal recumbency) on a heat pad (~30 °C) and secure the limbs to the surface using tape.
Note: In the event that a mouse still has a toe-pinch reflex 20 min post-anesthetic, administer up to 20% more pentobarbitone and test for toe-pinch reflex again after 10 min. Euthanize mouse if still responsive. - Cut the tip (1 mm) off the tail with a scalpel blade. Take a baseline blood glucose measurement from a drop of blood taken from the tail using a glucometer. Take 5 μl blood from the tail for scintillation counting to act as a background sample. Add this 5 μl blood to one well of a 96-well plate containing 75 μl 2.75% ZnSO4.
- Make a 3 cm incision through the skin and peritoneum using a scalpel or surgical scissors across the midline and perpendicular up towards the rib cage to open the abdominal cavity. Avoid puncturing the diaphragm. See Video 3.
- Using a blunt sterile implement (e.g., cotton bud moistened with saline), carefully move the intestines to the right and the liver right and left medial lobes upward toward the ribcage to expose the hepatic portal vein and inferior vena cava (Figure 1A). See Video 3.Video 3. Surgery to access the hepatic portal vein (This video was made at The University of Sydney. Procedures were approved by The University of Sydney Animal Ethics Committee under project # 2017/1274)
- Using a 25-29 G insulin syringe, inject a bolus of saline (for non-insulin-stimulated glucose uptake) or insulin (for insulin-stimulated glucose uptake; 1 U/kg lean body mass) containing 5-10 μCi [3H]2-DOG tracer into the hepatic portal vein (Figures 1A and 1B) or inferior vena cava if preferred. See Video 4 and Figure 1.
Note: Ensure that use of radioactive material is performed following local guidelines and regulations, including appropriate use of protective equipment, safe work practices, cleaning of workspaces after experiments and waste disposal.Video 4. Administration of saline to the hepatic portal vein. (This video was made at The University of Sydney. Procedures were approved by The University of Sydney Animal Ethics Committee under project # 2017/1274) - The needle can either:
- Be rested to the side and left in the vein (Figure 1B).
- Be removed and a clamp applied to minimize bleeding.
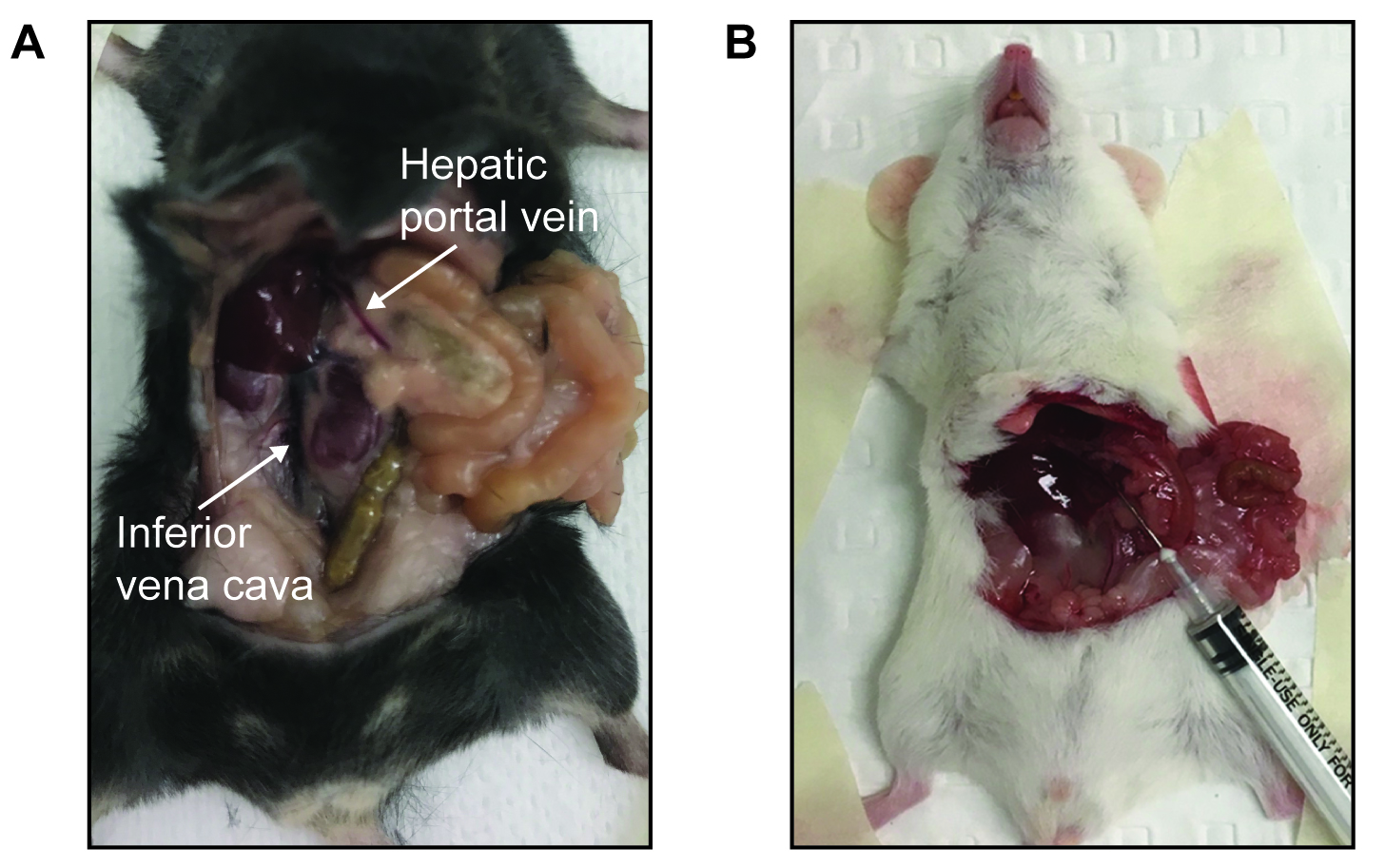
Figure 1. Hepatic portal vein cannulation in anesthetized mice. A. Location of the hepatic portal vein or inferior vena cava in mice. B. Picture of a Balb/c mouse during experiment where saline/insulin and tracer was injected via the hepatic portal vein and needle left in for the duration of the study.
- Measure blood glucose (from tail) and collect 5 μl blood (and add to ZnSO4 as in Step A6) per mouse to determine blood radioactivity after 2, 5, 10, 15, 20, and 30 min.
- After 30 min, terminate the mouse by cervical dislocation and excise tissues of interest (e.g., quadriceps muscle, tibialis anterior muscle, gastrocnemius muscle, epididymal adipose tissue, inguinal adipose tissue, heart). Tissues should be snapped frozen in liquid nitrogen and stored at -80 °C until further analysis. Tissues can be weighed before or after freezing.
Note: Mice should be culled at a time where blood tracer counts are still decreasing and have not plateaued. We typically perform 30 min ITTs.
- Western blot preparation
- Weigh out 50 mg epididymal white adipose tissue or muscle on dry ice, keeping the tissue frozen.
- Add 250 μl of Western Blot Lysis Buffer to frozen tissue and immediately homogenize by sonication (90% power, 3 x 10 s, allowing sample to cool between pulses).
- Centrifuge at 13,000 x g for 10 min at 12 °C.
- Remove the supernatant for muscle tissue, or carefully remove the infranatant for adipose tissue, being careful not to disturb the lipid layer.
- Determine protein concentration using BCA or similar protein assay and prepare samples for Western blot as per standard protocol using phospho-specific antibodies to signaling intermediates of interest (e.g., phospho-and total Akt) (Figure 2).
Note: Care should be taken to ensure that waste from SDS-PAGE is disposed of according to local regulations since samples contain radioactivity.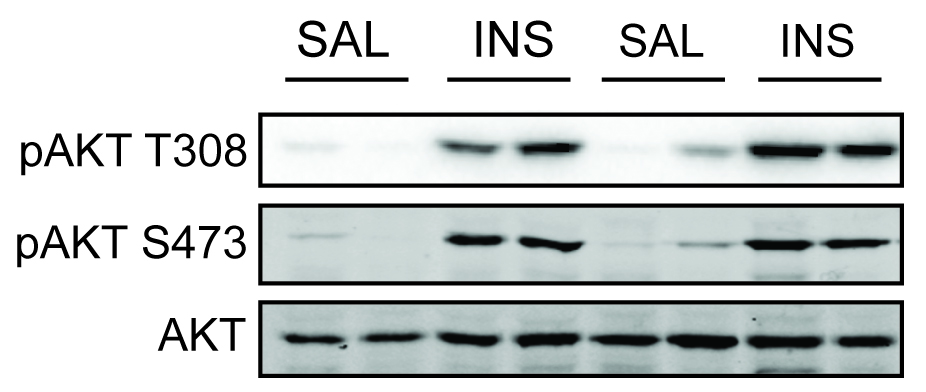
Figure 2. Assessment of insulin signaling in adipose tissue. Adipose tissue was excised from mice administered with saline or insulin. Phosphorylation of Akt at activating sites (Thr308 and Ser473) was assessed by Western blotting with phospho-specific antibodies. Insulin increased Akt phosphorylation at both sites.
- Tracer disappearance and uptake
- To measure tracer disappearance from the blood, add 25 μl of a saturated Ba(OH)2 solution (in ddH2O) to wells containing blood samples in ZnSO4 (Step A5 above, final volume 105 μl). This deproteinizes the samples. Centrifuge the 96-well plate at 1,000 x g for 5 min to pellet precipitated protein and transfer 50 μl of the cleared sample to a scintillation vial and add 3 ml of scintillant. Count 3H DPM in each sample using a liquid scintillation counter.
- To measure tracer uptake into a tissue of interest, powder tissue in liquid nitrogen using a mortar and pestle and then weigh an aliquot of powder for analysis. Smaller tissues such as the soleus muscle can be homogenized according to Step C3 and do not require powdering.
- Homogenize ~40 mg tissue (as little as 10 mg soleus and EDL muscle can be used) in 1 ml ddH2O in a 1.5 ml tube by sonication (90% power, 3 x 10 s, allowing sample to cool between pulses) and centrifuge at 13,000 x g for 15 min. Collect supernatant (~800 μl) and transfer to a new 2.0 ml tube. Bring up volume to 2 ml with ddH2O.
- Prepare the phospho-2-DOG affinity elution columns by adding 1 ml AG1-X8 resin diluted in ddH2O (70% volume resin) to a 0.8 x 4 cm chromatography column using a wide-bore pipette tip (e.g., P1000 tip cut 5 mm from the tip using a scalpel blade to increase the aperture).
- Place 5 ml tubes below the columns and add 1 ml of the supernatant to the columns followed by three 1 ml washes with ddH2O.
- Place new scintillation vials below the columns and add 1 ml of the elution buffer (see Recipes below) to columns followed by another 1 ml of elution buffer.
- Add 3 ml scintillation fluid (PerkinElmer) to the scintillation vials, vortex thoroughly, and measure 3H DPM in each sample using a liquid scintillation counter to quantify [3H]2-DOG-6-P.
Data analysis
The aim of data analysis is to normalize for the amount of tracer delivered to each mouse and available for uptake into tissues, and the amount of tissue analyzed. Methods for normalizing such data have been discussed extensively elsewhere (Sokoloff et al., 1977; Goodner et al., 1980; Hom et al., 1984; Cooney et al., 1985; Kraegen et al., 1985).
We describe two methods below: 1) the data are normalized to tracer availability in the blood to calculate the amount of tracer taken into the tissue of interest as a proportion of the amount of tracer available to the tissue, and 2) approximate an index of glucose uptake into tissues by using DPM and blood glucose to calculate a specific activity (DPM/mol glucose). In each case, data can be expressed per unit weight and/or protein/DNA content of the analyzed tissue.
Note: The methods described below use an area under the curve (AUC) calculation to normalize for tracer availability to tissues. This method works best if mice are culled when blood tracer counts are decreasing and have not plateaued. We typically perform 30 min ITTs.
- To calculate tracer 2-DOG clearance into specific tissues as a proportion of total 2-DOG available to the tissue:
This calculation assumes that the 2-DOG tracer measured by a tail bleed is indicative of tracer available in the interstitial space for uptake into the tissue.
First, extrapolate DPM per 5 μl to 1 ml to yield DPM/ml. Since the tracer will disappear from the blood by an exponential decay, calculate the AUC by fitting the DPM/ml at measured time points to a single exponential function, and integrate this function over the experimental period. This estimates the change in blood DPM/ml throughout the experiment (DPM/ml•min). This AUC value provides a normalization factor that takes into account differences in tracer availability between mice.
Tissue DPMs can be normalized using this AUC value to calculate the proportion of available tracer taken up into the tissue. This can be further normalized to the weight of tissue analyzed (g). The final units are: 2-DOG clearance (ml/min/g). Data normalized using this method are presented in Figures 3A and 3B. These data show insulin-stimulated 2-DOG clearance into muscle and adipose tissues, but not into brain (Figure 3A) and that feeding mice a diet high in fat and sucrose leads to impaired insulin-stimulated 2-DOG clearance into epididymal adipose tissue and quadriceps muscle (Figure 3B). - To obtain a tissue-specific index of glucose uptake:
The blood glucose concentration during this assay is non-steady-state. This calculation aims to take into account differences in blood glucose over the course of the experiment and approximate glucose uptake into tissue. This calculation assumes that there is no discrimination between 2-DOG and glucose at the glucose transporter and therefore the rate of [3H]2-DOG accumulation in tissue is equivalent to the rate of glucose uptake into tissue (Ferre et al., 1985). Since the kinetics of 2-DOG and glucose uptake may differ, we advise referring to 2-DOG accumulation as a “glucose uptake index”.
First calculate the AUC for blood DPM (as above by exponential curve fitting; DPM/ml•min) and blood glucose during the ITT (by the trapezoidal method; μmol/ml•min). The average specific activity of 2-DOG in the blood can be calculated by dividing the blood DPM AUC by blood glucose AUC (DPM/μmol). Tissue DPM can then be converted to an index for the rate of glucose uptake by dividing by the average specific activity, and further normalized to the weight of tissue analyzed (g) and expressed per min or h. The final units are: Glucose uptake index (μmol/g/h). Data normalized using this method are presented in Figure 3B. These data show that feeding mice a high fat high sucrose diet for 14 d lowers the insulin-stimulated glucose uptake index in epididymal adipose tissue and quadriceps muscle (Figure 3C).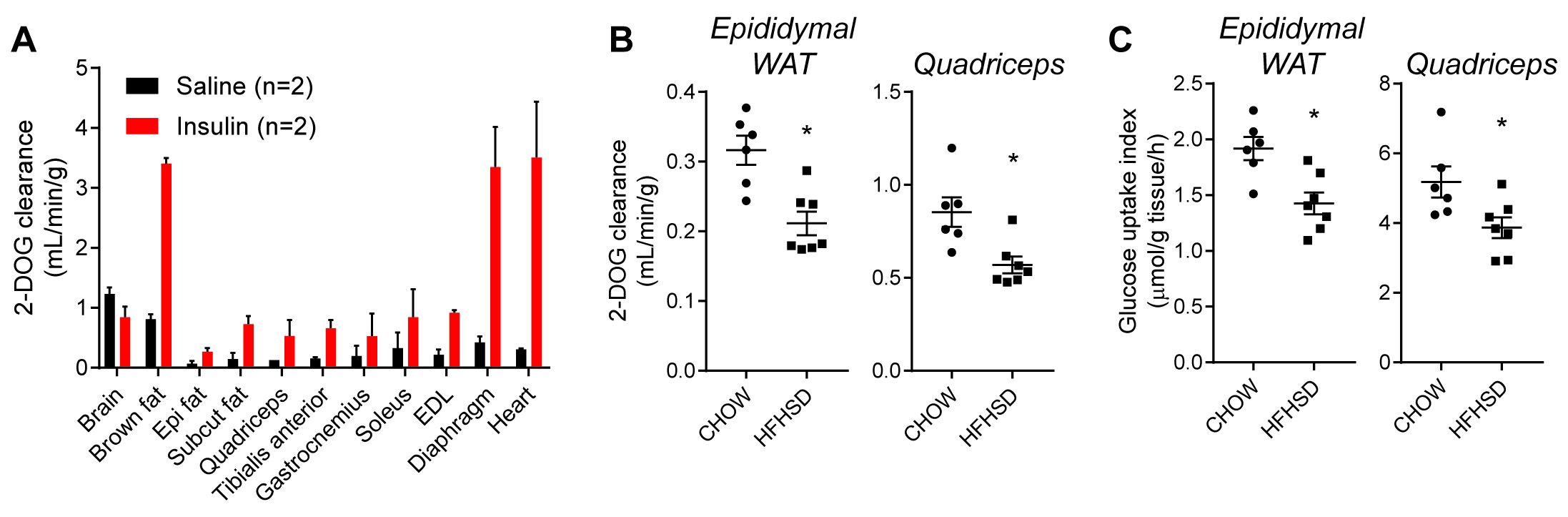
Figure 3. 2-DOG uptake into tissues. A. 2-DOG clearance into indicated tissues was assessed in C57Bl/6J mice following saline or insulin administration (Epi; Epididymal, Subcut; subcutaneous/inguinal, EDL; Extensor digitorum longus). Adipose and muscle tissues exhibited insulin-responsive 2-DOG clearance. B and C. Insulin-stimulated 2-DOG clearance (B) or glucose uptake index (C) for epididymal white adipose tissue (WAT) (left inset) and quadriceps muscle (right inset) in C57Bl/6J mice fed a chow diet or high fat high sucrose diet (HFHSD). *P < 0.05 versus mice fed a chow diet, Student’s t-test, n = 6-7. Data in B and C were recalculated from Fazakerley et al. (2018a).
Recipes
- 2.75% zinc sulfate solution
100 ml of 2.75% zinc sulfate contains 4.44 g zinc sulfate in ddH2O - Phosphorylated [3H]2-DOG elution buffer
Low pH, high salt solution (e.g., 1% Trifluoroacetic acid, 2 M NaCl)
100 ml of elution buffer contains 1 ml 100% Trifluoracetic acid and 11.69 g NaCl in ddH2O - Western blot lysis buffer
10 mM HEPES, pH 7.4
250 mM sucrose
1 mM EDTA
2% SDS
Protease and phosphatase inhibitors (1 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 10 mM sodium fluoride)
We advise making 10x stocks of HEPES pH 7.4, sucrose, EDTA and SDS.
10 ml of lysis buffer contains, 23.8 mg HEPES, 855.6 mg sucrose, 2.9 mg EDTA, 576.8 mg SDS, 1x Roche cOmpleteTM protease inhibitors, 1 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 10 mM sodium fluoride. Adjust the solution pH to 7.4
Acknowledgments
This protocol is a modified version of the protocol reported in Fazakerley et al., 2018a. This work was supported by National Health and Medical Research Council of Australia (NHMRC) Project Grants 1061122 and 1086850 (to D. E. J.). D.E.J. is a National Health and Medical Research Council of Australia Senior Principal Research Fellow. The contents of the published material are solely the responsibility of the individual authors and do not reflect the views of NHMRC. We thank Dr. James Krycer and Dr. Lake-Ee Quek for helpful discussion.
Competing interests
The authors have no competing interests to declare.
Ethics
All experiments were carried out with the approval of the University of Sydney Animal Ethics Committee, following guidelines issued by the National Health and Medical Research Council of Australia.
References
- Ayala, J. E., Samuel, V. T., Morton, G. J., Obici, S., Croniger, C. M., Shulman, G. I., Wasserman, D. H. and McGuinness, O. P. (2010). Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3(9-10): 525-534.
- Bissonnette, P., Gagne, H., Coady, M. J., Benabdallah, K., Lapointe, J. Y. and Berteloot, A. (1996). Kinetic separation and characterization of three sugar transport modes in Caco-2 cells. Am J Physiol 270(5 Pt 1): G833-843.
- Brandon, A. E., Stuart, E., Leslie, S. J., Hoehn, K. L., James, D. E., Kraegen, E. W., Turner, N. and Cooney, G. J. (2016). Minimal impact of age and housing temperature on the metabolic phenotype of Acc2-/- mice. J Endocrinol 228(3): 127-134.
- Burchfield, J. G., Kebede, M. A., Meoli, C. C., Stockli, J., Whitworth, P. T., Wright, A. L., Hoffman, N. J., Minard, A. Y., Ma, X., Krycer, J. R., Nelson, M. E., Tan, S. X., Yau, B., Thomas, K. C., Wee, N. K. Y., Khor, E. C., Enriquez, R. F., Vissel, B., Biden, T. J., Baldock, P. A., Hoehn, K. L., Cantley, J., Cooney, G. J., James, D. E. and Fazakerley, D. J. (2018). High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J Biol Chem 293(15): 5731-5745.
- Cooney, G. J., Caterson, I. D. and Newsholme, E. A. (1985). The effect of insulin and noradrenaline on the uptake of 2-[1-14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett 188(2): 257-261.
- Fazakerley, D. J., Chaudhuri, R., Yang, P., Maghzal, G. J., Thomas, K. C., Krycer, J. R., Humphrey, S. J., Parker, B. L., Fisher-Wellman, K. H., Meoli, C. C., Hoffman, N. J., Diskin, C., Burchfield, J. G., Cowley, M. J., Kaplan, W., Modrusan, Z., Kolumam, G., Yang, J. Y., Chen, D. L., Samocha-Bonet, D., Greenfield, J. R., Hoehn, K. L., Stocker, R. and James, D. E. (2018a). Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife 7: e32111.
- Fazakerley, D. J., Minard, A. Y., Krycer, J. R., Thomas, K. C., Stockli, J., Harney, D. J., Burchfield, J. G., Maghzal, G. J., Caldwell, S. T., Hartley, R. C., Stocker, R., Murphy, M. P. and James, D. E. (2018b). Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J Biol Chem 293(19): 7315-7328.
- Ferre, P., Leturque, A., Burnol, A. F., Penicaud, L. and Girard, J. (1985). A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J 228(1): 103-110.
- Furler, S. M., Jenkins, A. B., Storlien, L. H. and Kraegen, E. W. (1991). In vivo location of the rate-limiting step of hexose uptake in muscle and brain tissue of rats. Am J Physiol 261(3 Pt 1): E337-347.
- Goodner, C. J., Hom, F. G. and Berrie, M. A. (1980). Investigation of the effect of insulin upon regional brain glucose metabolism in the rat in vivo. Endocrinology 107(6): 1827-1832.
- Guarino, M. P., Santos, A. I., Mota-Carmo, M. and Costa, P. F. (2013). Effects of anaesthesia on insulin sensitivity and metabolic parameters in Wistar rats. In Vivo 27(1): 127-132.
- Hom, F. G., Goodner, C. J. and Berrie, M. A. (1984). A [3H]2-deoxyglucose method for comparing rates of glucose metabolism and insulin responses among rat tissues in vivo. Validation of the model and the absence of an insulin effect on brain. Diabetes 33(2): 141-152.
- Hoyer, K. F., Nielsen, T. S., Risis, S., Treebak, J. T. and Jessen, N. (2018). Sevoflurane impairs insulin secretion and tissue-specific glucose uptake in vivo. Basic Clin Pharmacol Toxicol. doi: 10.1111/bcpt.13087.
- Humphrey, S. J., Azimifar, S. B. and Mann, M. (2015). High-throughput phosphoproteomics reveals in vivo insulin signaling dynamics. Nat Biotechnol 33(9): 990-995.
- Kraegen, E. W., James, D. E., Jenkins, A. B. and Chisholm, D. J. (1985). Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol 248(3 Pt 1): E353-362.
- Lee, S., Muniyappa, R., Yan, X., Chen, H., Yue, L. Q., Hong, E. G., Kim, J. K., and Quon, M. J. (2008). Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 294: (2) E261-270.
- Mather, K. (2009). Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab 296(2): E398-399.
- Pasieka, A. M. and Rafacho, A. (2016). Impact of glucocorticoid excess on glucose tolerance: clinical and preclinical evidence. Metabolites 6(3): E24.
- Pomplun, D., Mohlig, M., Spranger, J., Pfeiffer, A. F. and Ristow, M. (2004). Elevation of blood glucose following anaesthetic treatment in C57BL/6 mice. Horm Metab Res 36(1): 67-69.
- Sokoloff, L., Reivich, M., Kennedy, C., Des Rosiers, M. H., Patlak, C. S., Pettigrew, K. D., Sakurada, O. and Shinohara, M. (1977). The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28(5): 897-916.
- Tanaka, K., Kawano, T., Tomino, T., Kawano, H., Okada, T., Oshita, S., Takahashi, A. and Nakaya, Y. (2009). Mechanisms of impaired glucose tolerance and insulin secretion during isoflurane anesthesia. Anesthesiology 111(5): 1044-1051.
- Wasserman, D. H., Kang, L., Ayala, J. E., Fueger, P. T. and Lee-Young, R. S. (2011). The physiological regulation of glucose flux into muscle in vivo. J Exp Biol 214(Pt 2): 254-262.
- Windelov, J. A., Pedersen, J., and Holst, J. J. (2016). Use of anesthesia dramatically alters the oral glucose tolerance and insulin secretion in C57Bl/6 mice. Physiol Rep 4(11): e1282.
Article Information
Copyright
Fazakerley et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Fazakerley, D. J., Fritzen, A. M., Nelson, M. E., Thorius, I. H., Cooke, K. C., Humphrey, S. J., Cooney, G. J. and James, D. E. (2019). Insulin Tolerance Test under Anaesthesia to Measure Tissue-specific Insulin-stimulated Glucose Disposal. Bio-protocol 9(2): e3146. DOI: 10.21769/BioProtoc.3146.
- Fazakerley, D. J., Chaudhuri, R., Yang, P., Maghzal, G. J., Thomas, K. C., Krycer, J. R., Humphrey, S. J., Parker, B. L., Fisher-Wellman, K. H., Meoli, C. C., Hoffman, N. J., Diskin, C., Burchfield, J. G., Cowley, M. J., Kaplan, W., Modrusan, Z., Kolumam, G., Yang, J. Y., Chen, D. L., Samocha-Bonet, D., Greenfield, J. R., Hoehn, K. L., Stocker, R. and James, D. E. (2018a). Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife 7: e32111.
Category
Biochemistry > Protein > Activity
Cell Biology > Tissue analysis > Physiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











