- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Silk Fibroin Nanoparticles and Enzyme-Entrapped Silk Fibroin Nanoparticles
Published: Vol 8, Iss 24, Dec 20, 2018 DOI: 10.21769/BioProtoc.3113 Views: 8799
Reviewed by: Bedabrata SahaMahfoudh Almusali Mohammed AbdulghaniAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Verification of N-Linked Glycosylation of Proteins Isolated from Plant or Mammalian Cell Lines Using PNGase Enzyme
Wuqiang Hong [...] Jiayang Li
Sep 20, 2025 1918 Views

The Generation of Tissue-Specific ECM Hydrogels From Melanoma and Associated Organs to Study Cancer Biology
Yuval Mogilevsky [...] Yuval Shaked
Feb 5, 2026 65 Views

Biochemical Reconstitution and FRAP Analysis of Membrane-Associated Condensates on Supported Lipid Bilayers
Longhui Zeng and Xiaolei Su
Feb 5, 2026 87 Views
Abstract
After silk fiber is degummed in boiling 0.2% Na2CO3 solution, the degummed fibroin fiber could be dissolved in highly concentrated neutral salts such as CaCl2. The partially degraded polypeptides of silk fibroin, commonly called as regenerated liquid silk, could be obtained via water dialysis. The silk fibroin nanoparticles (SFNs) or enzyme-entrapped SFNs are prepared rapidly from the liquid silk by using acetone. The globular particles with a range of 35-125 nm in diameter, are water-insoluble but well dispersed and stable in aqueous solution. The nanoparticles are potentially useful in biomaterials such as cosmetics, anti-UV skincare products, and surface improving materials, especially in enzyme/drug delivery system as vehicle. Here, a detailed protocol for SFNs and enzyme-entrapped SFNs preparation is described.
Keywords: Silk fibroinBackground
The development of silk fibroin as a biomaterial, especially biomedical tissue engineering materials, has been a hot topic in recent decades. Natural silk fiber from Bombyx mori has to undergo a series of processing steps to become a regenerated liquid silk fibroin. The liquid silk can be easily made into various forms of silk biomaterials, such as micro- or nano-particles, regenerated fibers, artificial skin, and porous matrix or 3D scaffolds, biomimetic nanofibrous scaffolds, a platform for transistors and various classes of photonic devices. The structure and properties of the final forms of silk biomaterials depend evidently on the molecular size of the regenerated liquid silk which is affected by a series of processing steps including degumming and fibroin fiber dissolution. Our investigation showed that boiling and degumming in a 0.1%-0.5% Na2CO3 solution used most frequently in the laboratory can not only cause a large amount of sericin degradation and hydrolysis but also leads to serious breakage of the silk fibroin peptide chain and its mechanical properties decreasing (Wang and Zhang, 2013). The low purity, high concentration, and improper storage conditions easily induce regenerated liquid silk gelling or coagulating and denaturing. The partially chain-broken polypeptides of silk fibroin can be converted into silk fibroin nanoparticles (SFNs) in acetone (Zhang et al., 2007). During this process the molecular structure of the regenerated liquid silk has changed rapidly from random-coil and a-helix form (Silk I) into anti-parallel β-sheet form (Silk II). The resulting crystalline silk nanoparticles may offer various possibilities for surface modification of industrial materials and enzyme/drug delivery system (Zhang et al., 2011).
Materials and Reagents
- Qualitative Filter Paper, 0.22 μm, Round, 15 cm (Sangon Biotech (Shanghai) Co., Ltd., catalog number: F503312)
- Dialysis membrane (Standard, RC, 5 KD, 18 mm Width) (BBI, catalog number: F128058)
- The cocoons of silkworm Bombyx mori, provided by Sericultural Institute, Soochow University, Suzhou, P. R. China
- Na2CO3 (Sangon Biotech (Shanghai) Co., Ltd., catalog number: A500840)
- CaCl2 (Sangon Biotech (Shanghai) Co., Ltd., catalog number: A501330)
- Ethanol (Sangon Biotech Shanghai Co., Ltd., catalog number: A500737)
- Acetone (Sigma-Aldrich, catalog number: 320110)
- Polyethylene glycol-2000 (Sangon Biotech (Shanghai) Co., Ltd., catalog number: A601785)
- 50 mmol/L phosphate buffer (pH 7.0) (see Recipes)
- Ajisawa's reagent (CaCl2/ethanol/H2O [1:2:8 molar ratio]) (see Recipes)
Equipment
- Glass Injector, 20 ml
- Glass beaker, 50 ml
- High-speed Refrigerated Centrifuge (Beckman Coulter, model: Avanti® J-30I)
- Scanning Electron Microscope (Hitachi, model: S-570)
- Ultra-sonic Instrument (Sonics Vibra cell) (Sonics & Materials, model: VCX 130 PB)
Procedure
Figure 1 shows a schematic of silk fibroin nanoparticles (SFNs) or enzyme-entrapped SFNs preparation using acetone.
Figure 1. Schematic of silk fibroin nanoparticles (SFNs) or enzyme-entrapped SFNs preparation using acetone
- Degum the clean cocoon shell twice in boiling 0.2% (w/v) Na2CO3 for 0.5 h each time.
- After the degumming treatment, wash the resulting silk materials repeatedly in deionized water and then air dry.
- Immerse the degummed silk fibers into 20-fold volume (w/v) of Ajisawa's reagent (CaCl2/ethanol/H2O, 1:2:8 molar ratio) (Ajisawa, 1998) and stir at 70 °C (water bath) for 4 h.
- Centrifuge the silk fibroin-salt solution at 7,000 x g for 10 min to remove impurities and insoluble substances.
- Dialyze the filtered solution or supernatant continuously for 48 h with running deionized water to remove CaCl2, smaller molecules and some impurities using a cellulose semi-permeable membrane (cutoff 5 kDa).
- Concentrate the aqueous solutions of regenerated silk fibroin to 1%-5% of silk fibroin solution at 4 °C by using PEG and finally store it at 4 °C for SFN preparation.
- If preparing enzyme-entrapped silk fibroin nanoparticles (SFNs), add a given amount of enzyme (liquid or powder) to the above silk fibroin solution and homogenize the mixture mildly via shaking by hand.
- Rapidly Inject the silk fibroin solution or a mixture with enzyme into acetone (acetone is more than 70% of the final mixture) by using a 20 ml injector at room temperature; while injection proceeded, rapidly shake the 50 ml beaker or container containing the protein/enzyme by hand.
- The milk-like silk protein particles or enzyme-entrapped silk protein particles form immediately and suspend in the mixture composed of water and acetone.
- These particles are water-insoluble and can precipitate slowly due to nanoparticle aggregating. Filtrate and collect the precipitates of these nanoparticles through 0.22 μm filter paper, or purify from the mixture by centrifugation at 108,000 x g for 0.5 h to separate the particles from the solvent.
- After subject these filtered retentates or centrifuged precipitates to ultrasonic treatment (Output Watts: 16) five times for 2-min each, the nanoparticles or enzyme-entrapped nanoparticles disperse evenly in 50 mM phosphate buffer or water and can be stored at 4 °C for a long time.
Data analysis
Our previous experiments showed that the solubilization of the degummed silk fibroin fiber in the CaCl2 ternary system at 70 °C for 4 h resulted in amide-bond breakages in the silk fibroin molecular chains. SDS-PAGE showed that the molecular masses and distributions of liquid silk fibroin are affected by the dissolving conditions (Zhang et al., 2007). In fact, the regenerated liquid silk is a mixture of gradually degraded polypeptides of silk fibroin, with a size range of about 10-70 kDa. When a given amount of silk fibroin solution without or with containing L-asparaginase is rapidly injected into an excess of acetone, a milky suspension of silk protein forms at once, and simultaneously L-asparaginase is tightly entrapped in the silk fibroin nanoparticles (SFNs). Scanning Electron Microscope (SEM) observations showed that the silk nanoparticles are globular granules whether it contains enzymes or no enzymes (Figure 2). All these granules are about 50-120 nm in diameter. 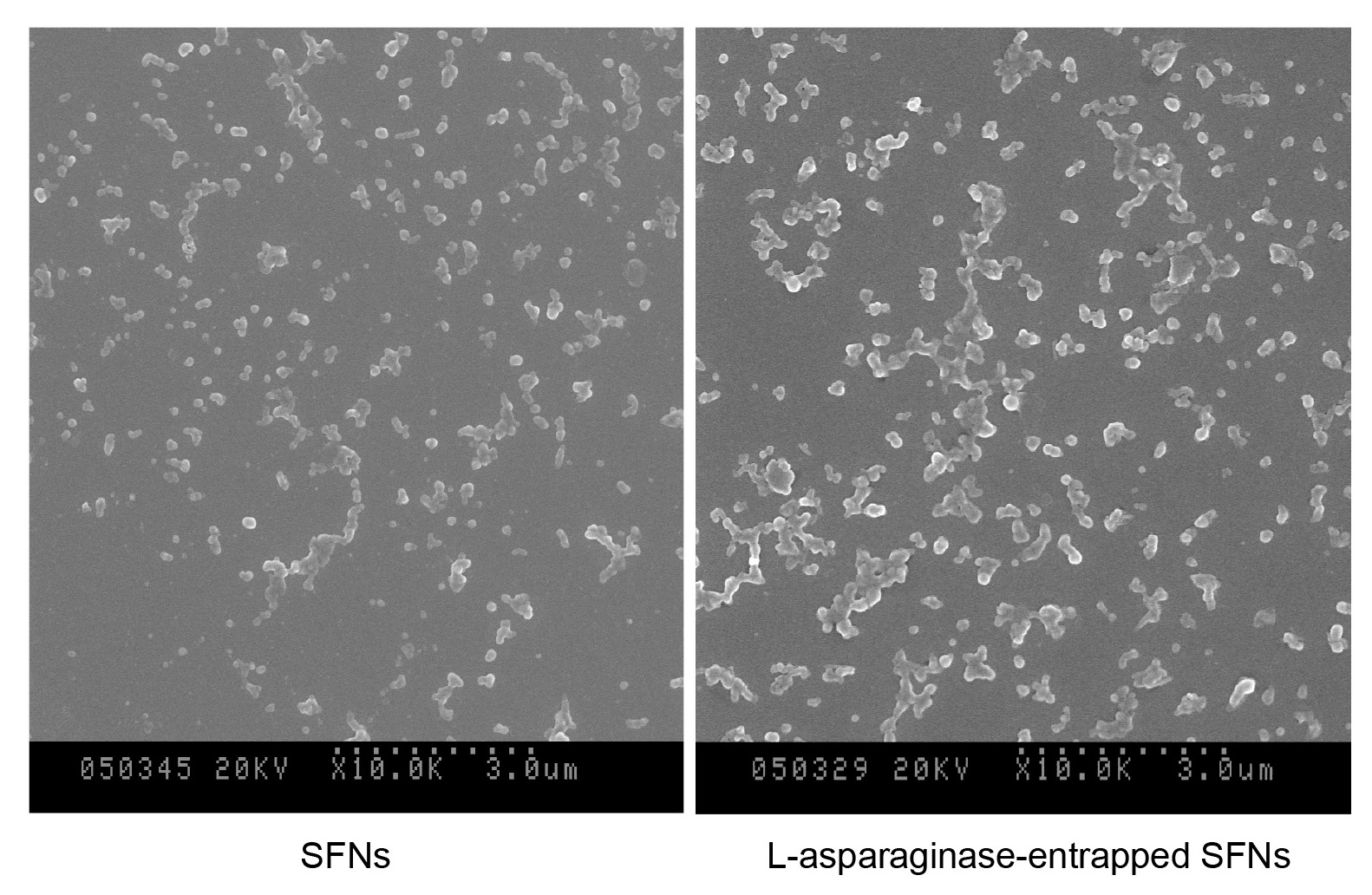
Figure 2. SEM images (104x) in the left (SFNs) and right (L-asparaginase-entrapped SFNs) by using a Hitachi S-570 Scanning Electron Microscope
Recipes
- 50 mmol/L phosphate buffer (pH 7.0)
Dissolve 7.800 g NaH2PO4•2H2O to 500 ml dH2O, and adjust the salt solution to pH 7.00 with 0.1 N NaOH solution, finally adjust the volume of the phosphate solution to 1,000 ml with dH2O - Ajisawa's reagent (CaCl2/ethanol/H2O [1:2:8 molar ratio])
Add 55.5 g CaCl2 to 72 ml dH2O, after the mixture solution cool to room temperature, add 57.5 ml ethanol the mixture. The total volume of the ternary solvent is about 165 ml
Acknowledgments
This work was supported by the earmarked fund for the China Agriculture Research System (CARS-18-ZJ0502) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, P. R. China. This modified protocol is based on our previously published work (Zhang et al., 2007 and 2011).
Competing interests
The author declares no conflicts of interest or competing interests.
References
- Ajisawa, A. (1998). Dissolution of silk fibroin with calcium chloride/ethanol aqueous solution. J Seric Sci Jpn 67(2): 91-94.
- Wang, H. Y. and Zhang, Y. Q. (2013). Effect of regeneration of liquid silk fibroin on its structure and characterization. Soft Matter 9(1): 138-145.
- Zhang, Y. Q., Shen, W. D., Xiang, R. L., Zhuge, L. J., Gao, W. J. and Wang, W. B. (2007). Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J Nanopart Res 9(5): 885-900.
- Zhang, Y. Q., Wang, Y. J., Wang, H. Y., Zhu, L. and Zhou, Z. Z. (2011). Highly efficient processing of silk fibroin nanoparticle-L-asparaginase bioconjugates and their characterization as a drug delivery system. Soft Matter 7(20): 9728-9736.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, Y. Q. (2018). Preparation of Silk Fibroin Nanoparticles and Enzyme-Entrapped Silk Fibroin Nanoparticles. Bio-protocol 8(24): e3113. DOI: 10.21769/BioProtoc.3113.
Category
Biochemistry > Protein > Modification
Biophysics > Bioengineering > Medical biomaterials
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









