- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Microplastic Extraction from Marine Vertebrate Digestive Tracts, Regurgitates and Scats: A Protocol for Researchers from All Experience Levels
Published: Vol 8, Iss 22, Nov 20, 2018 DOI: 10.21769/BioProtoc.3087 Views: 10875
Reviewed by: Sabine CastanoAnonymous reviewer(s)
Abstract
It is essential to provide a protocol for the separation and identification of microplastics in marine vertebrates (mammals, birds, turtles and fish) that is easy to follow and adaptable depending on research infrastructure. Digesting organic material is an effective way to analyze samples for microplastics. Presented here is an optimized protocol which uses potassium hydroxide (KOH) for processing samples of digestive tracts, scats and regurgitates. KOH is a cheap, effective and simple alkaline digestant that allows extraction of plastics from the sample matrix. Samples are first digested, then filtered before visual and chemical analysis of remaining particle. This allows size, shape, color and polymer of each particle to be ascertained. This protocol has been harmonized with other protocols for the collection of different samples (e.g., diet, parasites, other pathologies). The implementation of this protocol at different levels of economic and/or laboratory resources make information on microplastic incidence available to the entire research community.
Keywords: MicroplasticsBackground
Effectively monitoring plastic pollution in the environment has become a priority for scientists, non-governmental organizations (NGOs) and stakeholders. Standardized or harmonized protocols are required to allow comparisons between research groups from around the world (e.g., Taylor et al., 2016; Lusher et al., 2017; Unger et al., 2017). Microplastics and mesoplastics (plastics generally < 1 mm and < 5 mm respectively), as a form of marine litter, are now of major concern and have been included in international directives to monitor environmental health. Microplastics have been identified from surface waters to sediments, and from coastal areas to deep sea regions (Lusher, 2015); and, they might have physical and chemical effects on aquatic and terrestrial environments. Most worryingly, microplastics have been found in digestive tracts of aquatic organisms, including vertebrates (e.g., Tanaka and Takada, 2016; Karami et al., 2018). The presence of microplastics raises concern for cellular, individual level, food chain and ecosystem effects (Galloway et al., 2017).
Investigations on microplastics in marine vertebrate digestive tracts have increased over the last ten years. Methods include visual searching and manipulation of digestive tracts, digestion of organic material and sieving to separate contents (Lusher et al., 2017). Generally, these methods are focused on the collection of macro- and microplastics, and sometimes food remains (e.g., Fukuoka et al., 2016), however, additional material such as parasites or samples for histological examination and other pathologies are usually not considered. Currently utilized method studies do not consider a holistic view including other pathologies which might have an amplified effect on organisms. Thus, by building on microplastic research and utilizing other disciplines, we present a more simple and practical protocol that can be adapted depending on the research question being addressed and the resources available.
Utilizing a range of disciplines can deliver a thorough investigation on effects of microplastics as a pollutant. A standardized protocol to acquire samples for different disciplines (e.g., trophic ecology, parasitology, diseases) is necessary to establish a true understanding of the transfer and effects of this pollutant. This will contribute to comparative results within the scientific community whilst obtaining results from multidisciplinary approaches (e.g., Jepson et al., 2016).
The general approach to investigate macroplastics is focused either to extract microplastics directly, use density separation, or digest organic material and examining potential particles (Lusher et al., 2017). Chemical identification methods require costly and advanced instrumentation, with tedious protocols that are not commonly accessible to all researchers (e.g., focal plane array Fourier transform infrared spectrometry -FPA-FT-IR, for review see Löder and Gerdts, 2015).
Within current microplastic research, potassium hydroxide (KOH) is being pursued as the most effective technique for extracting microplastics from biotic samples (Rochman et al., 2015; Dehaut et al., 2016). It is cost efficient, utilizes accessible chemicals and requires a simple sampling procedure. What has been missing from previous research is the inclusion of other biological and ecological disciplines which can provide much more information on the health of organisms, thus leading to a better interpretation of results.
Some research groups collect dietary remains using a form of elutriation, or density separation (e.g., Bigg and Olesiuk, 1990). During this process, dietary contents are placed in a container with water overflooding and the material that sinks (hard remains such as otoliths, bones) are collected for dietary analysis; if the predator feeds on crustaceans, they will be collected from the surface. However, microplastics might be lost during this method. Extraction efficiencies of elutriation devices have been demonstrated to be inconsistent (Zobkov and Esiukova, 2017). In addition, dissection and visual inspection of digestive tracts contents are usually carried out under conditions in which airborne contamination may occur, especially on large samples (e.g., marine mammal digestive tracts). On the other hand, dissolving biotic material using chemicals is a more appropriate approach to obtaining microplastics from samples. There have been many different protocols developed to digest biotic material including: acid (e.g., HNO3, HClO4, CH2O2), alkaline (e.g., KOH and NaOH), oxidizing (e.g., H2O2), and enzymatic treatments (e.g., Proteinase K, Lipex and Savinase). Many of these protocols have been found to affect polymers, require expensive chemicals and have complex and time-consuming extraction (Lusher et al., 2017). Of these protocols, KOH, potassium hydroxide, has been identified as the most appropriate strategy because it is economically cost efficient, utilizes easily accessible chemicals, and requires a simple sampling procedure (e.g., Rochman et al., 2015; Dehaut et al., 2016; Kühn et al., 2017).
Here we present a protocol that has been developed for the extraction and identification of microplastics from digestive tracts, scats and regurgitates of marine vertebrates (marine mammals, seabirds, sea turtles and fish). The protocol has been optimized and established over the last five years to provide a feasible microplastic analysis to research teams which may not have access to more sophisticated techniques. Using this protocol researchers can acquire results that are comparable to more advanced research teams, while collecting other type of samples (e.g., diet, parasites, exudate) is feasible. The protocol proposed for microplastic extraction is not subject to the number of samples or sample size. Generally, the more samples available, the better interpretation of the results. However, access to samples from large vertebrates is not always easy or possible. This protocol will allow researchers from locations worldwide to use a flexible approach with different levels of complexity depending on their disciplines, facilities and available resources. Therefore, this protocol will provide a full understanding of microplastic presence in biota using a combination of different disciplines.
Materials and Reagents
Note: All materials and reagents can be adapted depending on laboratory resources. For example, specific forceps and glass equipment are not required, but some specific precautions may be performed. See Table 1 for options.
- Filter papers (e.g., glass microfibre filters, GF/D, 47 mm, pore size 0.47 microns, Whatman, catalog number: 1823-047)
- Aluminum foil lids cut to the required size or non-plastic lids
- Laboratory gloves (e.g., blue nitrile gloves, VWR, catalog number: 112-2373)
- Mesh (e.g., 200 µm monofilament Nylon fibre mesh)
- Filtered water (e.g., Milli-Q Filtered water, 0.22 μm membrane filter)
- Ethanol absolute > 99% (v/v) (VWR, catalog number: 20821.310E)
- Potassium hydroxide, KOH, 85.0%-100.5%, pellets (VWR, catalog number: 26668.296)
- Sodium chloride, NaCl, 99.5%-100.5%, pellets (VWR, catalog number: 27810.364)
- Sodium iodide, NaI, > 99.5% (VWR, catalog number: 27913.260)
- Sodium polytungstate solution, SPT 85% (v/v) (Sigma-Aldrich, catalog number: 80656)
Equipment
- 500 ml beakers (Corning, Pyrex®, catalog number: 1395-500)
- Magnetic stirrer (Sigma-Aldrich, Benchmark, catalog number: Z742550)
- Vacuum filtering flask assembly for 47 mm filters with funnel, filter folder (e.g., 2,000 ml, VWR, catalog number: 511-0264H and Fisher Scientific, KimbleTM, catalog number: K953771-0000)
- Vacuum pump (e.g., mini diaphragm vacuum pump, VP86, VWR, catalog number: 181-0065H)
- Stainless steel sieves: 1 mm, 0.5 mm, 0.25 mm, and smaller sieves when a lower size limit is required
- Cotton and white laboratory clothes
- Non-plastic dissection tools (including scissors, forceps and scalpel)
- Stereomicroscope with a camera (e.g., Leica Microsystems, model: MZ6)
- FT-IR instrumentation (or similar equipment where possible for chemical analysis)
- Fridge
- Incubator and shaker (e.g., New Brunswick Scientific, model: Innova 26R)
Procedure
- Sample collection
Here, we describe a step-by-step procedure for collecting samples from the field (Steps A1-A4, Figure 1). We understand that the methods used for collection can change depending on the samples targeted. The importance of contamination control steps is included as this information is required when collecting samples.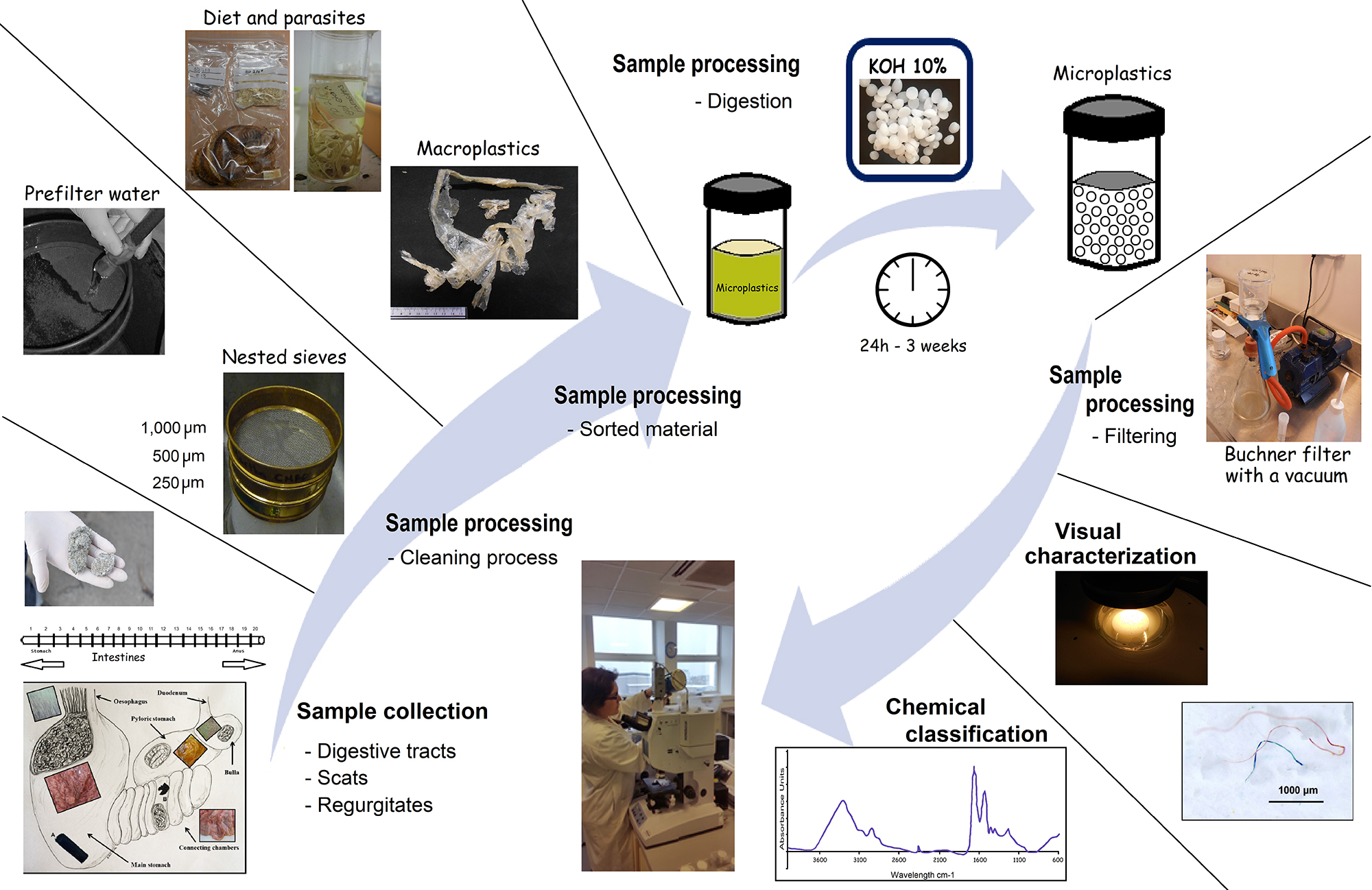
Figure 1. Procedural steps for extracting plastics and microplastics from vertebrate digestive tracts, scats and regurgitates- Collect digestive tracts following the standard protocols for animal necropsies (e.g., Kuiken and Garcia-Hartmann, 1991). Digestive tracts must be secured with a bridle or a cord to avoid loss of contents. If digestive tracts are not processed immediately, they should be stored in a durable container. It is recommended that when a plastic bag is used, that is made of a bright color. Therefore, in case of accidental contamination such plastic piece will be easy to identify.
- Scats and regurgitates are collected in transparent plastic zipped bags or transparent glass containers when possible.
- It is recommended that white gloves and white cotton laboratory coats/clothes be used during the collection of all samples. Any other color and polymeric material used must be noted for reference to take potential contamination into account when carrying out analysis.
- Samples should be processed fresh, but when this is not possible, samples can be frozen at -20 °C or lower until required.
- Reagent preparation
Caution: KOH is a caustic and irritant solvent. All researchers must wear laboratory gloves and eye protection at all times. If the solution touches skin, the affected area should be washed immediately with running water.- To make a 10% KOH solution, add 100 g KOH to 1,000 ml of water. Solution volume can be adjusted depending on the sample processing requirements. It is recommended to prepare the solution in a beaker or conical flask with a lid.
- Close the lid and gently mix until pellets are dissolved, this can be carried out by shaking by hand or using a magnetic stirrer depending on the volume being prepared. Once pellets have been dissolved, the solution will have turned colorless, leave for at least 15 min to cool.
- The final solution should be filtered using a Buchner filter or similar (Table 1) to ensure that there are no residual microplastics in the solution, either from the KOH pellets, water or from procedural contamination.
- Laboratory preparation
- The laboratory should be scrubbed before use to remove any sources of airborne contamination. There should be limited access to reduce external contamination.
- Researchers in the laboratory should wear white cotton laboratory coat and gloves. Any other color and polymeric material used must be noted for reference to take potential contamination into account when carrying out analysis.
- All equipment must be rinsed at least three times with pre-filtered water immediately before use. Glass or metal equipment is recommended, however, if unavoidable see Table 1. The water supply used for flushing the samples must be filtered using a sieve with a mesh size smaller than target microplastics. Any variation on the color of the equipment should be noted.
- Set up nested metal sieves in a sink connected to the pre-filtered water source. The sieve at the top will have the largest mesh size (e.g., 1 mm) and the bottom one the smaller one (e.g., 50 µm). Using consecutive sieves will allow removal of larger pieces of plastics, food remains and parasites. If researchers wish to target smaller plastics, they can add additional sieves. This allows for size differentiation and speeds up processing in later steps. The sieves should be covered with a lid when not in use to prevent contamination.
Table 1. Troubleshooting advice and alternative methodological approaches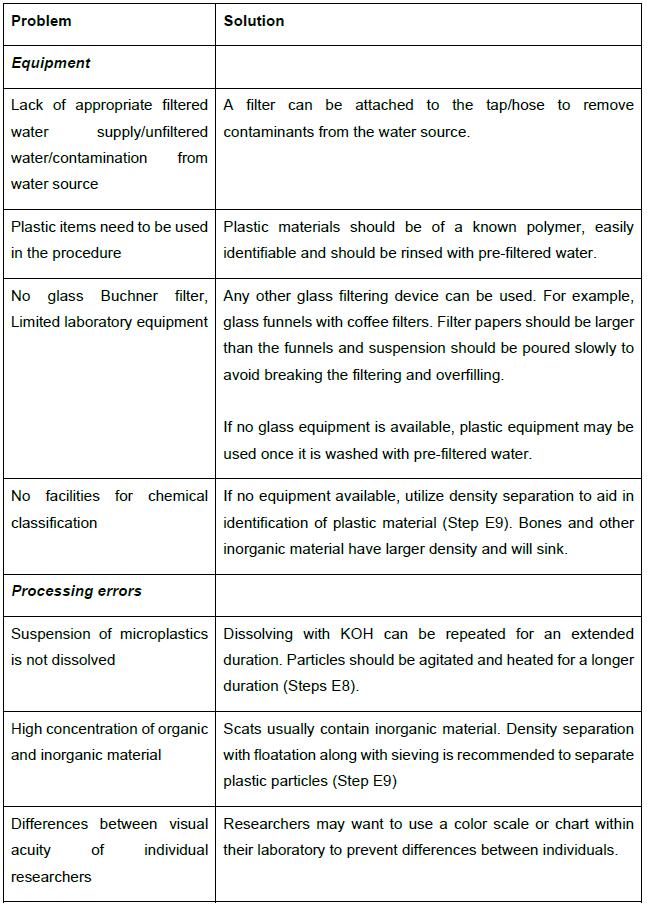
- Sample preparation
- If samples have been frozen, it is recommended that samples be defrosted slowly in a fridge (24-36 h), to avoid the fast decomposition of the tissues. It is also possible to defrost samples at room temperature in a clean laboratory.
Note: Depending on the sample collected there are different initial processing steps required. For digestive tract samples, proceed with Steps D2-D10, for regurgitate and scat samples proceed from Steps D11-13.
Digestive tracts- Digestive tracts must be rinsed externally before processing and removing the ties to remove external contamination.
Note: Ties are used to stop contents leaking. - Digestive tracts of marine organisms vary in length and number of compartments (esophagus, stomachs and intestines). Each compartment should be processed and rinsed independently (e.g., Hernandez-Milian et al., 2018).
- If different sections of fish digestive tracts cannot be differentiated, it can be analyzed without divisions.
- For large intestines (> 1 m, i.e., mammals), we recommend separating into smaller, equally sized pieces. The most appropriate number is 20 equal pieces. The division of this part of the digestive tracts will allow the researcher to investigate if microplastics tend to concentrate in any specific area as some parasites do (Lawlor et al., 1990), as well as make feasible the analysis of a large sample.
- Each compartment or intestine section must be opened using clean and sterilized scissors and forceps.
- A subsample for genetic analysis should be collected from the middle of the bulk of prey remains and stored frozen or in 70% pre-filtered ethanol.
- Transfer the remaining material to the nested sieve column avoiding touching the mucosal surface.
- Examination of the gastric and intestinal mucosal surface should be carried out with care to prevent damage to the surface by rubbing it with fingers or other material. If any pathology is detected, a sample of that area should be collected and stored with the fixed chemical required following the standard protocols.
- Compartments should be processed individually under the filtered water supply through nested sieves following Step E1 onwards.
- The container where scats and regurgitates are stored must be rinsed externally with pre-filtered water before processing. This will avoid procedural contamination.
- A subsample from the middle of the sample will be collected and frozen or stored in 70% ethanol for genetic analysis.
- The rest of the samples should be poured and processed in the nested sieves following Step E1 onwards. If the scat or the regurgitated sample is not fluid, they can be poured into a container with pre-filter water for few hours to hydrate before processing. Samples must always be covered with a lid to prevent procedural contamination.
- If samples have been frozen, it is recommended that samples be defrosted slowly in a fridge (24-36 h), to avoid the fast decomposition of the tissues. It is also possible to defrost samples at room temperature in a clean laboratory.
- Sample processing
- Rinse each sample or compartment into the nested sieves separately (Figure 1).
- Transfer the non-plastic material collected in both top sieves to containers with 70% ethanol for between 2 and 24 h to sterilize the material and prevent mold and odor.
- Store hard food remains (e.g., otoliths, bones, shells) dry in bags or containers, while cephalopod beaks, and other soft remains (e.g., crustaceans, worms, parasites) should be stored in 70% ethanol for further identification (Figure 1).
- Rinse the plastic material (> 1 mm) found in the larger sieve with pre-filtered water and store dry. Material kept in the smaller sieve will be transferred to a glass container with pre-filtered water to obtain a suspension for the microplastic sample (Figure 1).
- The suspension should be as concentrated as possible. This will reduce the volume of KOH solution required in the following step.
- Add 10% KOH solution to the suspension in a ratio of 3:1 (KOH:suspension) (Figure 1).
- Cover samples loosely with aluminum foil, or screw top lids to prevent contamination and evaporation.
- Incubate samples at 60 °C for 24 h with continuous agitation at 125 rpm; alternatively, heat can be forgone but the reaction will take longer (e.g., 3 weeks, Foekema et al., 2013).
- The solution will turn transparent/slight yellow in coloration when all biological material has been digested.
- Remove samples from the incubator and leave to cool before further processing.
- After cooling, filter solution under vacuum using equipment such as a glass Buchner filter with a microfiber filter (GF/D or alternative). Alternatively, if this equipment is unavailable, glass funnels with a microfiber filter covered during the filtration can be used.
Note: Filter papers must be checked under a microscope for contamination before use. - When large amounts of undigested organic material (e.g., bones) remain after filtering, density flotation can be used to separate undissolved organic material from low density plastics which will float. Great care is required when using density separation as there are some less dense dietary remains that can float, such as crustacean carapaces. NaCl is the recommended density separation solution (1.2 g cm-3). More costly solutions include NaI (1.8 g cm-3) and SPT (2.8 g cm-3).
- Another option to remove undigested organic matter involves rinsing the solution through a sieve once more before filtering; this reduces the likelihood of filter papers clogging.
- Visual characterization
Visual characterization is valuable when researchers are categorizing and sorting samples. Visual characterization of plastics must be used with other more robust identification techniques such as chemical analysis (e.g., Steps F4-F5, G1-G7), even if large plastics can be recognized. If more robust techniques are not available, Steps F4-F5 can aid researchers in reducing the likelihood of misidentification.- Use a stereomicroscope to investigate particles retained on the filter paper. It is recommended that a camera be attached to the microscope to allow researchers to record visual images of all particles.
- Visually inspect each filter paper for potential plastic microparticles.
- Carry out visual characterization following existing criteria (Lusher et al., 2014):
- Measured longest dimension (mm) and smallest aperture lengths.
- Record shape and color (Figure 1).
- Shape categories include: fiber, fragment and spherical (beads). Fragment can be further divided into films and foams depending on research objectives, but this division is not always necessary.
- Color is subjective and therefore not recommended as a stand-alone classification, but it does help researchers when categorizing samples.
- Particles observed need to be visually inspected for the following characteristics, otherwise they should be rejected or tested with other chemical techniques (Lusher et al., 2014):
- Homogeneous color.
- No natural structures (such as cells).
- Unnatural bending.
- Fibers should have a consistent thickness throughout length.
- There should be no fraying at ends of fibers.
- A hot needle can be used during visual characterization to aid in the presence of plastic particles, which in case of plastic will react to the heat through bending or melting. This method has been recommended by National Oceanographic and Atmospheric Administration (NOAA) and the European Union under the Marine Strategy Framework Directive (EU-MSFD) however the hot needle is not suitable for semi-synthetic fibers as they do not react. Therefore, some research groups are advised to take this step with caution.
- Chemical characterization
Note: Chemical characterization should only be carried out on clean and dry suspected plastic particles.- Chemical characterization should be carried out on a representative subsample. MSFD recommends 10% although as many particles as possible should be analyzed chemically to reduce identification errors.
- The specific instrumentation used for chemical classification will depend on the research facilities available. Here we describe FT-IR because this instrumentation is available at most of research laboratories (Figure 1).
- Trained personnel should conduct chemical analysis. Sufficient knowledge of polymer identification and spectra interpretation is required.
- Spectral analysis should follow the protocols available at individual laboratories to produce an output spectrum.
- All output spectra should be compared to a polymer library database.
- Only polymers which matched reference spectra with a high level of certainty (> 70% match) should be accepted as correct identification.
- Researchers with sufficient knowledge of polymers may also consider visual inspection of produced spectra that have a lower level of certainty (60%-70%). Caution should be taken for such interpretation. One example is water absorbance which can alter spectra and that have clear polymeric characteristics.
Data analysis
This protocol is a standard protocol for the collection of microplastics. Data analysis is subject to the specific user requirements of the protocol. It is recommended that researchers assess the number of particles in each identified compartment (stomach, intestine section or individual scat and/or regurgitate). Values obtained can be compared with other samples from the same research area or further afield. Specifically dividing the digestive tract and intestines allows researchers to compare microplastic levels between and within species (Lusher et al., 2018).
Acknowledgments
The authors obtained no external funding for the elaboration of this protocol, A.L.L. and G.H.M. acknowledge their previous funders which supported their earlier investigations. A.L.L. was funded by an Irish Research Council Postgraduate Scholarship and a GMIT 40th anniversary studentship between 2012-2015. G.H.M. was supported by a Beaufort Ecosystem Approach to Fisheries Management award, as part of the Irish Government’s National Development Plan (NDP) between 2013-2015. Both authors thank National Parks and Wildlife Service (NPWS) for reporting the strandings to the Irish Whale and Dolphin Group (IWDG).
Author Contributions: A.L.L. and G.H.M. conceived and designed the protocol; performed the experiments, analyzed the data; and wrote the paper equally.
This protocol is the culmination of adaptions from previous work by both the authors.
Competing interests
The authors declare no conflict of interest.
References
- Bigg, M. A. and Olesiuk, P. F. (1990). An enclosed elutriator for processing marine mammal scats. Mar Mammal Sci 6(4): 350-355.
- Dehaut, A., Cassone, A. L., Frere, L., Hermabessiere, L., Himber, C., Rinnert, E., Riviere, G., Lambert, C., Soudant, P., Huvet, A. and Duflos, G. (2016). Microplastics in seafood: benchmark protocol for their extraction and characterization. Environ Pollut 215: 223-233.
- Foekema, E. M., De Gruijter, C., Mergia, M. T., van Franeker, J. A., Murk, A. J. and Koelmans, A. A. (2013). Plastic in North sea fish. Environ Sci Technol 47(15): 8818-8824.
- Fukuoka, T., Yamane, M., Kinoshita, C., Narazaki, T., Marshall, G. J., Abernathy, K. J., Miyazaki, N. and Sato, K. (2016). The feeding habit of sea turtles influences their reaction to artificial marine debris. Sci Rep 6: 28015.
- Galloway, T. S., Cole, M. and Lewis, C. (2017). Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol 1(5): 116.
- Hernandez-Milian, G., Lusher, A., O’Brien, J., Fernandez, A., Berrow, S. and Rogan, E. (2018). New information on the Deep-diving True’s beaked whale (Mesoplodon mirus G. 1850), in Ireland with insights into foraging ecology on mesopelagic prey. Mar Pollut Bull 33(1): 1245-1254.
- Jepson, P. D., Deaville, R., Barber, J. L., Aguilar, A., Borrell, A., Murphy, S., Barry, J., Brownlow, A., Barnett, J., Berrow, S., Cunningham, A. A., Davison, N. J., Ten Doeschate, M., Esteban, R., Ferreira, M., Foote, A. D., Genov, T., Gimenez, J., Loveridge, J., Llavona, A., Martin, V., Maxwell, D. L., Papachlimitzou, A., Penrose, R., Perkins, M. W., Smith, B., de Stephanis, R., Tregenza, N., Verborgh, P., Fernandez, A. and Law, R. J. (2016). PCB pollution continues to impact populations of orcas and other dolphins in European waters. Sci Rep 6: 18573.
- Karami, A., Golieskardi, A., Choo, C. K., Larat, V., Karbalaei, S. and Salamatinia, B. (2018). Microplastic and mesoplastic contamination in canned sardines and sprats. Sci Total Environ 612: 1380-1386.
- Kühn, S., van Werven, B., van Oyen, A., Meijboom, A., Bravo Rebolledo, E. L. and van Franeker, J. A. (2017). The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar Pollut Bull 115(1-2): 86-90.
- Kuiken, T. and Garcia-Hartmann, M. (1991). Cetacean Pathology: Dissection Techniques and Tissue Sampling. Proceedings of the European Cetacean Society Workshop, Leiden, the Netherlands, 13-14 September, 1991. ECS Newsletter pp: 1-39.
- Lawlor, B. J., Read, A. F., Keymer, A. E., Parveen, G. and Crompton, D. W. T. (1990). Nonrandom mating in a parasitic worm: mate choice by males? Anim Behav 40: 870–876.
- Löder, M. G. J. and Gerdts, G. (2015). Methodology used for the detection and identification of microplastics–A critical appraisal. In: Bergmann, M., Gutow, L. and Klages, M. (Eds.). Marine Anthropogenic Litter (1st edition). Springer 201-227.
- Lusher A. (2015). Microplastics in the marine environment: distribution, interactions and effects. In: Bergmann, M., Gutow, L. and Klages, M. (Eds.). Marine Anthropogenic Litter (1st edition). Springer 245-307.
- Lusher, A. L., Burke, A., O'Connor, I. and Officer, R. (2014). Microplastic pollution in the Northeast Atlantic Ocean: validated and opportunistic sampling. Mar Pollut Bull 88(1-2): 325-333.
- Lusher, A. L., Hernandez-Milian, G., Berrow, S., Rogan, E. and O'Connor, I. (2018). Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of historical knowledge. Environ Pollut 232: 467-476.
- Lusher, A. L., Welden, N. A., Sobral, P. and Cole M. (2017). Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal Methods 9: 1346-1360.
- Rochman, C. M., Tahir, A., Williams, S. L., Baxa, D. V., Lam, R., Miller, J. T., Teh, F. C., Werorilangi, S. and Teh, S. J. (2015). Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep 5: 14340.
- Tanaka, K. and Takada, H. (2016). Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci Rep 6: 34351.
- Taylor, M. L., Gwinnett, C., Robinson, L. F. and Woodall, L. C. (2016). Plastic microfibre ingestion by deep-sea organisms. Sci Rep 6: 33997.
- Unger, B., Herr, H., Benke, H., Böhmert, M., Burkhardt-Holm, P., Dähne, M., Hillmann, M., Wolff-Schmidt, K., Wohlsein, P. and Siebert, U. (2017). Marine debris in harbour porpoises and seals from German waters. Mar Environ Res 130: 77-84.
- Zobkov, M. and Esiukova, E. (2017). Microplastics in Baltic bottom sediments: Quantification procedures and first results. Mar Pollut Bull 114(2): 724-732.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lusher, A. L. and Hernandez-Milian, G. (2018). Microplastic Extraction from Marine Vertebrate Digestive Tracts, Regurgitates and Scats: A Protocol for Researchers from All Experience Levels. Bio-protocol 8(22): e3087. DOI: 10.21769/BioProtoc.3087.
Category
Environmental science > Marine vertebrates > Microplastic
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










