- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Modified Barnes Maze for Juvenile Rats
Published: Vol 8, Iss 22, Nov 20, 2018 DOI: 10.21769/BioProtoc.3084 Views: 6512
Reviewed by: Karen N KrukowskiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2606 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views
Abstract
To better understand neural codes for spatial navigation and to address cognitive development questions, the neurobiology of postnatal hippocampal development is receiving increased attention. While the Morris Water Maze is the gold standard for assessing hippocampal integrity, potential confounds can be difficult to control for in juvenile rodents (around three weeks of age, when spatial navigation ability first emerges) and this wet maze is not amenable to electrophysiological recording. A dry maze alternative with minimal training, like the Barnes Maze adapted for juvenile rats, can help overcome these obstacles. This paper describes in detail the experimental procedure and data analyses for the adapted Barnes maze for juvenile animals.
Keywords: Barnes mazeBackground
The Barnes maze (Barnes, 1979) is a widely acknowledged dry alternative to the Morris Water Maze (MWM) (Morris, 1984). The Barnes maze uses bright light as a motivating factor to cause animals to locate a darkened box hidden beneath one of several escape holes using spatial cues. It has recently been shown that a Barnes maze adapted for juvenile rats can be used to assess the maturation of spatial learning and memory, as spatial navigation ability first emerges around the end of the third postnatal week (McHail et al., 2018). Unlike the MWM, the Barnes maze requires less time away from the dam and does not involve swimming and temperature stressors that can differentially impact juvenile animals (Albani et al., 2014). Also, the Barnes maze is more compatible with in vivo electrophysiological recording, enabling a greater signal to noise ratio (McHail et al., 2018).
The Barnes maze adapted for juvenile rats consists of a round white platform that is smaller than the adult maze with eight evenly spaced holes around the perimeter, a reduced number of perimeter holes compared to the adult apparatus. Distal visual cues (extra-maze contextual cues) are placed around the maze and used by the animal to determine the spatial location of an escape box hidden underneath one of the perimeter holes. These spatial cues are enlarged to allow better visualization for the younger animals (McHail et al., 2018).
Behavioral testing consists of four days: an exploration trial on Day 1, 5 blocks of training trials followed by a spatial learning probe on Days 2 and 3, and a final spatial memory probe approximately 24 h later (24 HP). The exploration trial on Day 1 allows the animal to freely roam the platform in dim lighting (no escape box) and permits the experimenter to map place cells in animals having tetrodes implanted. Soy-based infant formula droplets are scattered on the maze to encourage exploration. Training on Days 2 and 3 are conducted under bright lighting with the escape box positioned under the same escape hole for all training trials. Each block contains four trials where the animal has a maximum of 60 s to locate the goal starting from four different start locations that are randomly ordered within a block. Training blocks are separated by 15-30 min. Following the final block on each training day, a probe is conducted wherein the escape box is removed and the animal is observed for 60 s. Following the immediate probe on Day 3, the escape box is returned to conduct a final refresher block. The 24 HP is performed on Day 4 to test long-term spatial reference memory.
Animals are tested for the length of time it takes them to locate the escape box (escape latency), the distance traveled to reach the escape box (path length), and the maximum and mean movement speed during this path. These measures are repeated excluding trials for which the animal did not locate the goal (non-escape trials). The probe trials examine recall by calculating the amount of time spent near the escape box location (dwell time), distance from the goal at every second (similar to Gallagher et al., 1993), and nose pokes before and after the first goal nose poke, in addition to the measures calculated for training trials (escape latency, path length, and movement speed).
Materials and Reagents
- Nontoxic permanent markers, primary colors
- Scoop
- Expanded polystyrene foam (Purchased from The Home Depot, Cellofoam®, catalog number: 150705)
- 50 ml Falcon tube (Thermo Fisher Scientific, Falcon® 50 ml Conical Centrifuge Tubes, catalog number: 14-432-22)
- Paper towels (Kimberly-Clark Professional*, Scott® C-Fold Towel, catalog number: 06041)
- 1 ml syringe or Pasteur pipette (Thermo Fisher Scientific, BD Lo-DoseTM U-100 Insulin Syringes, catalog number: 14-826-79)
- Long Evans Rats (Original breeders purchased from Charles River Laboratories and bred at an AAALAC accredited animal facility, 17 to 25 days of age)
- 500 ml 70% ethanol solution in an LDPE spray or squirt bottle (4-liter ethanol [Anhydrous] purchased from Thermo Fisher Scientific, catalog number: A405P-4)
- Soy-based infant formula (22 oz purchased from Walmart, EnfamilTM ProSobee Soy Infant Formula, catalog number: 2667028)
- Distilled water (150 ml to mix with ethanol and ~40 ml to mix with soy formula)
- Examination gloves (Thermo Fisher Scientific, FisherbrandTM Powder-Free Nitrile Exam Gloves, catalog number: 19-130-1597C)
Equipment
- Brightly lit testing room (3.6 x 5.25 m with a biosafety hood, overhead fluorescent lamps and two standing floor lamps with adjustable brightness on the highest setting) (Figure 1A)
- Barnes maze apparatus (Figure 1B)
- Circular platform (wood coated with clear resin, 1.2 m in diameter) with eight equally spaced holes (10 cm in diameter) along perimeter 5 cm from platform edge (Resin purchased at The Home Depot, Rust-Oleum Parks®, Gloss Super Glaze Interior Finish and Preservative, model: 241352)
- PVC platform stand elevated 0.75 m off the ground with wooden base (Figure 1C)
- PVC escape box, 9.19 x 3.00 in (Madesmart®, catalog number: 59973) (Figure 1D)
- Rotating bar attached under platform to hold the escape box and allow the goal location to be altered per litter (Figure 1E)
- Foam or bubble wrap on the floor below the maze to cushion potential falls (Figure 1F)
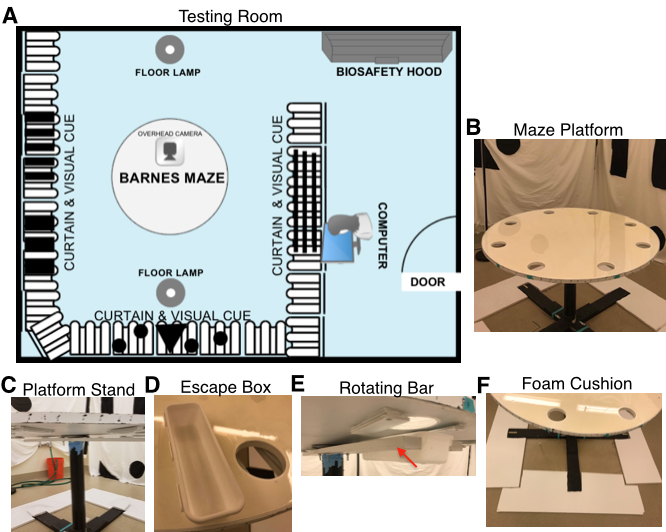
Figure 1. Experimental setup for Barnes maze adapted for juvenile animals. A. Arrangement of behavioral testing room and analysis room. B-F. Images of adapted Barnes maze platform (B), PVC platform stand with wooden base (C), PVC escape box (D), rotating bar for escape box (E), foam cushion for potential falls (F). - White curtains with distinct patterns made of large geometric shapes to create four walls of visual cues around the maze, as well as keep the experimenter hidden during testing (Figure 2)
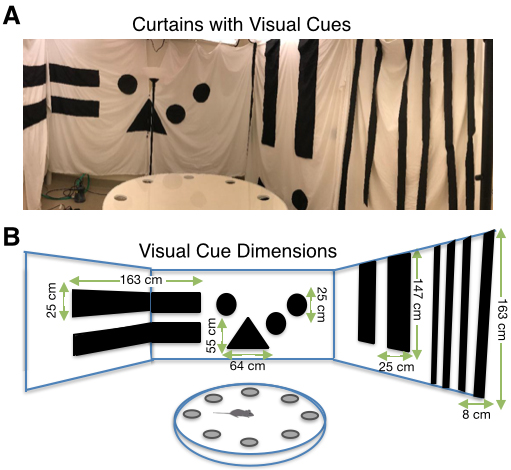
Figure 2. Visual Cues for Barnes maze adapted for juvenile animals. A. White curtains with enlarged visual cues. B. Dimensions of visual cues from right to left: horizontal rectangle (163 x 25 cm), triangle (base = 55 cm, height = 64 cm), circles (diameter = 25 cm), vertical rectangle (25 x 147 cm), stripes (8 x 163 cm). - Overhead camera (1/3" CCD, 30 frames/s, Panasonic, model: WV-CP280-N)
- PC computer
- Stopwatch
Software
- Video software (ULead Video Studio, Corel)
- TopScan Version 2.00, CleverSys
- Microsoft Excel
Procedure
See Figure 3A for the outline of the whole procedure.
- Prior to testing
Assign each of the 8 escape holes a letter from A-H going clockwise. Assign an escape box to one of the maze holes and attach the escape box to the rotating arm positioned under this hole. Keep this position consistent for the remainder of training (Days 2-3). Assign four drop-off locations (start location 1-4) by dividing the maze equally into four quadrants (Figure 3B). - Exploration in dim lighting (Day 1)
- Place home cage in biosafety hood in the testing room for 15 min prior to maze exposure for habituation. With a sharpie, mark each animal a different color on the tail.
- Set room lights to lowest intensity (~25 lux) and keep visual cues unaltered throughout all testing Days 1-4. Place bubble wrap or foam on the floor around the circumference of the maze to cushion any falls. There is no escape box for Day 1 exploration.
- Open ULead video software and focus camera for dim lighting. Ensure the maze is centered in the picture and evenly spaced from the edges of the room when viewed with the camera.
- Clean the maze with 70% ethanol and allow 60 s to dry.
- Add one scoop (approximately 8.8 g) of soy infant formula powder to a 50 ml Falcon tube and fill with distilled water. Shake vigorously until formula has fully been dissolved.
- Fill one syringe or pipette with ~1 ml mixed soy infant formula and scatter dime-sized droplets fairly equally around the entire face of the maze.
- Gently carry the animal to the platform and place it in the center of the maze apparatus. Allow the animal to explore for 15 min. Record the time between the releases of the animal and when the 15 min of exploration is up. If the animal falls, quickly place it back in the center of the maze and watch its movement to detect any injury.
Note: If falls are frequent, a raised border (~1 cm high) made of a serpentine automotive belt or similar material can be installed around the maze edge. Similar inserts inside of maze holes can also be used. - Clean the maze with 70% ethanol and replace mixed soy infant formula droplets for the next animal. Wipe gloved hands with 70% ethanol in between handling different animals for this and all subsequent steps.
- Training (Days 2-3) (Figure 3E) (Video 1)Video 1. Training trial. (All procedures were performed in accordance with the regulations stated in the Guide for Care and Use of Laboratory Animals by the National Research Council and approved by the George Mason University Institutional Animal Care and Use Committee.)
- Set room lights to their highest intensity (~525 lux).
- Place the home cage in the biosafety cabinet and allow animals 15 min in the home cage for habituation prior to testing the first animal. Clean the escape box and maze with 70% ethanol.
- Training consists of 5 blocks, with each block containing 4 trials (a 6th ‘refresher’ block will be administered after an immediate probe only on Day 3). The trials allow the animal 60 s to locate the escape box. The order of the four start locations varies randomly for each block, and remains consistent for all animals tested in the litter. Hold a stopwatch in one hand and place animal at the designated quadrant facing the center of the maze with its rear paws in line with the escape holes (Figure 3C). Immediately after placing the animal in the maze, move out of sight behind the curtain and observe the animal’s behavior on the video display. Record the time between the releases of the animal and when all four paws enter the escape box (Figure 3D). If the animal does not locate the escape box within 60 s, hold the scruff on the upper back of the animal and gently guide its front paws to the escape hole. Clean maze apparatus and escape box with 70% ethanol prior to each block. When the last animal has completed block 1, repeat with the first animal for block 2. For a single animal, the start of one block and the next block should be within 15 to 30 min. After completion of the 5 training blocks, begin the immediate probe.
- Immediate probe (Days 2-3)
- The immediate probe is used to test the animal’s recall of the goal location after training.
- The immediate probe is a 60 s probe directly following the five blocks of training trials. After five blocks of training have been completed, remove the escape box from the platform. Assign a start location for the probe at one of the four start locations opposite to the escape box location (Figure 3B). For instance, if the escape box was positioned under hole ‘G’ during the training trials, the opposite is start location 2 (S2). Therefore, S2 would be assigned as the start location for the immediate probe. The same probe start location is used for all subsequent probe trials and remains consistent within each litter. Begin the probe by placing the animal at the designated start location and allow 60 s for the animal to explore the maze.
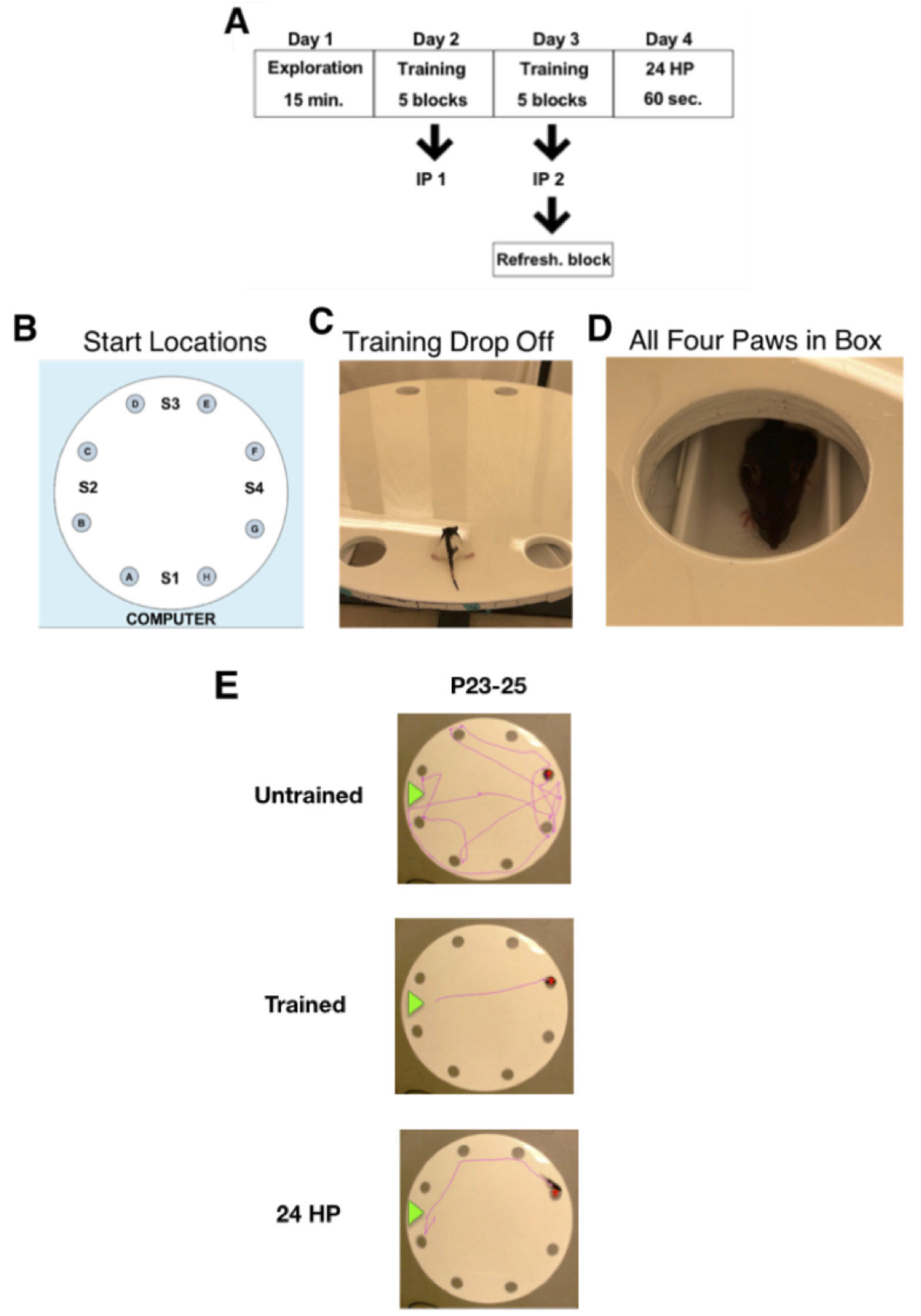
Figure 3. Barnes maze procedure. A. Layout of experimental timeline. B. Maze platform with labeled start locations (S1-S4) and escape holes (A-H) going clockwise. C. Image of drop off at start location. D. Image of animal with all four paws inside the escape box, indicating the end of analysis. E. Trace images of a juvenile (P23-25) animal at the beginning of training Day 2 (untrained), end of training Day 3 (trained), and the 24-Hour Probe. The green arrow displays the release location, and the red star marks the goal location.
- Refresher block (Day 3)
Following the Day 3 immediate probe, a 6th block (4 trials with an escape box exactly like blocks 1-5) is administered to reinforce learning. This is to ensure the animal is still motivated to find the escape location during the 24 HP on Day 4. - 24-Hour probe (Day 4) (Figure 3E)
- The 24-h probe is similar to the immediate probes but begins approximately 24 h after the start of the final training period and is used to assess long-term spatial memory.
- Turn lights to their highest intensity with no escape box present and allow the animal 60 s to explore the maze.
Data analysis
Dwell time, velocity, path length and distance to goal are analyzed using TopScan.
- Create an Arena: this defines the informational region
- Open TopScan Version 2.00.
- Open trial: On the top left, click on the video camera icon and select a training video file. One training session is needed to create the arena. Arenas can be reused if maze position is unaltered.
- If the circular maze has been distorted, select the eye icon to reveal the ‘Image Distortion Correction’ box. Next to ‘Morphing Option:’ select ‘Scale Adjust H/V:’ and type the first number as the scale in the north and south direction of the platform and the second number as the east and west (for example: 50/45) and hit ‘Apply’. The maze should no longer be oval (Figure S1A).
- Under ‘Arena’ on the top menu, select ‘Enter arena design mode’. A toolbar with different shapes will appear.
- On the toolbar, use the single ‘point’ and ‘center based circle’ icon to create zones:
- Outer circle: select the ‘circle’ design tool and create a circle from the center of the maze that covers the entire circular platform. Once done, select ‘Finish zone operation’.
Note: The Outer and Inner circle will be discarded later on as they are templates to create the Octants.
On the right hand ‘Arena Design’ box, under ‘zones’ there will be a new zone named ‘1 CircleCtr’ that is color-coded to match the arena. Every new zone will appear similarly in the order in which it was created (Figure S1B). - Inner circle: create a smaller circle within the Outer circle that nearly reaches the escape holes.
Note: The Inner circle will be discarded later on.
Select ‘Finish Zone Operation’ (Figure S1C). - Octants: right-click the Outer circle and select ‘split circle’ to equally divide it into eight sections. Rotate the octants so that the escape holes are centered. Repeat this step using the Inner circle and rotate the inner octants to line up with outer octants. Select ‘Finish Split Circle’. The octants will be labeled ‘PieCtr’ under ‘Zones’ (Figure S1D).
- In the right hand ‘Arena Design’ box under ‘zones’, select the first two circles made (Outer and Inner circle named ‘1 CircleCtr’ and ‘2 CircleCtr’) and delete (Figure S1E).
- Escape Hole Center: Click on the ‘point’ design tool. With the right clicker, place a point at the center of the goal escape hole, then select “Set Point Position’ (a green point will appear). Select ‘Finish Point Operation’ when done (point will turn red). This will allow measurement of the distance from the escape hole for the Gallagher analysis (Figure S1F).
- In the ‘Arena Design’ box, select the ‘Calibrate’ button. Right-click on one edge of the maze platform and select ‘First Calibration Point’. Redo this step on the exact opposite side of the maze edge and select ‘Second Calibration Point’. Enter the calibration distance as the diameter of the maze (1,200 mm). Right-click and select ‘Finish Calibration’ when done. This will allow the computer to estimate distance on the maze (Figure S1G).
- Define Zones: Select ‘new’ in the ‘Arena Design’ box. An ‘Area Definition’ box will pop up. Check the zones and name them as shown:
- Name the Arena according to litter and Select ‘Finish’ when done.
1)ALL: Check every zone (Figure S1H).2)PERI: This defines the perimeter from the Inner circle and Outer circle. Check every Outer circle octant (PieCtr).3)CENTER: This defines the area within the perimeter. Select all the octants that make up the Inner circle of the maze.4)(A-H): Individually check the two ‘PieCtr’ that make a single octant slice on the maze (this includes a part of the Perimeter and Center) and label according to the assigned hole letter A-H located in that octant (Figure S1I). - Outer circle: select the ‘circle’ design tool and create a circle from the center of the maze that covers the entire circular platform. Once done, select ‘Finish zone operation’.
- Set Background: defines where the animal is traced.
- Fast-forward the video until the animal is alone in the maze at the beginning of the trial.
- In the top menu under ‘Background’, select ‘Generate Background’.
- Run background generator until animal disappears.
- Stop and select ‘Save Background’ under ‘Background’ in the top menu.
Note: Background may be reused if maze is unaltered and software is able to properly trace the animal without background noise. - Alternatively, a single frame of an empty maze taken from the same video used for analysis can be used as a background ('Set Frame to Background Image') option.
- Set Arena: Check ‘Arena’ on the left and select arena from file.
- Select ‘Event’. In the ‘Event Rule Settings’ click the ‘Stop Rules’ tab and select ‘Duration’ under ‘Available Rule List’. Type ‘60 sec’ in the ‘After Analysis Duration >’ box. This will stop analysis from running past 60 s (Figure S2A).
- Press the play button on the top left corner, then stop immediately after the video displays the experimenter releasing the animal and the hand is completely out of the maze.
Note: To prevent interference of experimenter's hand, clothing, or shadow with video tracking, instruct experimenters to quickly withdraw hand after releasing the animal and take a step backward away from the maze. - Check ‘Analyze’ (located above the Area and Background box), then click fast forward and allow the video to run until the animal reaches the escape box. For probe trials, allow 60 s of analysis.
- Click on ‘Export’ under ‘Result’ on the left. Select ‘OK’ and save text files.
- Exporting Bin File to Excel
- Click on ‘Proc’.
- In ‘Proc’, select ‘Move Distance’ and click ‘OK’.
- Select ‘Velocity’ and click ‘OK’.
- Select ‘Dis to Point’, ‘Add’ the escape hole point created in arena mode ‘1’ and select ‘OK’ (Figure S2B).
- Change ‘Bin Size’ to 1. This analyzes the distance to the point every 1 s.
- Select ‘Export Data’.
- The Topscan output is used to quantify escape latency, path length, and maximum and mean velocity measurements. For probe trials, these measurements are calculated for the path to first goal nose poke (by finding when distance to goal center first drops < 10 cm, followed by visual confirmation on the video) as well as the whole 60 s trial. The % dwell time per octant is also taken directly from the Topscan output. A relatively shorter escape latency and path length to goal as well as a higher % dwell time per octant are all indicators of superior maze performance. For a more detailed description of how these measurements might relate to spatial navigation strategy and hippocampal integrity, see McHail et al. (2018).
- Nose pokes are manually scored by counting the number of times an animal pokes its nose inside of a hole as viewed by overhead video. If the animal pokes its nose in a hole, withdraws it, and then pokes its nose in the same hole again, this counts as two nose pokes. Separate nose poke tallies are recorded before and after the first goal nose poke. The number of nose pokes prior to the first goal poke can be used to compute an error score, that might also be weighted based on the distance of these nose pokes from the goal. An example of this scoring system applied to a developmental study can be found in McHail et al. (2018).
- Maze performance differences between rats older and younger than three weeks of age are reported in McHail et al. (2018).
Notes
- All cues are extra-maze visual cues. To accommodate juvenile eyesight, visual cues should be larger in size relative to the visual cues used to test adult animals.
- Rodents can track their own and other animals’ odor trails (Wallace et al., 2002). To control for confounding olfactory cues (Lavenex and Schenck, 1997), it is suggested to rotate the maze between blocks, while keeping the goal location fixed relative to the visual cues. The maze apparatus and escape box should be wiped with 70% ethanol prior to each block (Rosenfeld and Ferguson, 2014).
- It is recommended to use background white noise, such as from an overhead ventilation system, to mask potential auditory cues.
- It is recommended to test juvenile animals of the same age and cohort since performance can vary greatly at this stage of development (McHail et al., 2018). While sex differences were reported in adult rats in the Morris Water Maze (Roof and Stein, 1999), no sex differences were observed in the pre-pubertal rat performance on the Barnes maze (Ojeda and Urbanski, 1994; McHail et al., 2018).
Acknowledgments
This protocol was used in our previous study to analyze spatial navigation in juvenile rats (P17-P25) (McHail et al., 2018). The authors thank Richard H. Ogoe, Diyi Chen, Amir Behsudi and Celeste Chong for their technical support. This work was supported by the National Institutes of Health (NIH) National Institute on Aging (NIA) through Grant 1R15AG045820-01A1 and the Krasnow Institute for Advanced Study at George Mason University.
Competing interests
The authors declare that there is no conflict of interest.
Ethics
All procedures were performed in accordance with the regulations stated in the Guide for Care and Use of Laboratory Animals by the National Research Council and approved by the George Mason University Institutional Animal Care and Use Committee.
References
- Albani, S. H., McHail, D. G. and Dumas, T. C. (2014). Developmental studies of the hippocampus and hippocampal-dependent behaviors: insights from interdisciplinary studies and tips for new investigators. Neurosci Biobehav Rev 43: 183-190.
- Barnes, C. A. (1979). Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93(1): 74-104.
- Gallagher, M., Burwell, R. and Burchinal, M. (1993). Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107(4): 618-626.
- Lavenex, P. and Schenk, F. (1997). Olfactory cues potentiate learning of distant visuospatial information. Neurobiol Learn Mem 68(2): 140-153.
- McHail, D. G., Valibeigi, N. and Dumas, T. C. (2018). A Barnes maze for juvenile rats delineates the emergence of spatial navigation ability. Learn Mem 25(3): 138-146.
- Morris, R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1): 47-60.
- Ojeda, S. R., and Urbanski, H. F., (1994). Puberty in the rat. In The Physiology of Reproduction pp. 363-409.
- Rosenfeld, C. S. and Ferguson, S. A. (2014). Barnes maze testing strategies with small and large rodent models. J Vis Exp (84): e51194.
- Wallace, D. G., Gorny, B. and Whishaw, I. Q. (2002). Rats can track odors, other rats, and themselves: implications for the study of spatial behavior. Behav Brain Res 131(1-2): 185-192.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Valibeigi, N., McHail, D. G., Kimball, C. L. and Dumas, T. C. (2018). A Modified Barnes Maze for Juvenile Rats. Bio-protocol 8(22): e3084. DOI: 10.21769/BioProtoc.3084.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Neuroscience > Development > Spatial navigation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link