- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Rice Ragged Stunt Virus Propagation and Infection on Rice Plants
Published: Vol 8, Iss 20, Oct 20, 2018 DOI: 10.21769/BioProtoc.3060 Views: 6495
Reviewed by: Feng LiSonali ChaturvediAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
Jade Jansen [...] Neeltje A. Kootstra
Jul 20, 2025 2457 Views

Assembly and Mutagenesis of Human Coronavirus OC43 Genomes in Yeast via Transformation-Associated Recombination
Brett A. Duguay and Craig McCormick
Aug 20, 2025 3033 Views
Abstract
Virus inoculation is a basic experimental procedure to evaluate the resistance of a rice variety or a transgenic material upon virus infection. We recently demonstrated that Rice Ragged Stunt Virus (RRSV), an oryzavirus that is transmitted by brown planthopper (BPH), can suppress jasmonic acid-mediated antiviral defense through the induction of microRNA319 and facilitate virus infection in rice. To verify this, we performed virus inoculation experiments on wild-type rice plants and miR319-TCP21-associated transgenic rice plants through a modified group inoculation method. Here, we presented the detailed procedure of RRSV propagation and infection process on rice plants.
Keywords: Rice ragged stunt virusBackground
Studying mechanism of viral pathogenesis and screening virus-resistant rice varieties have been doing to overcome rice virus diseases and keep food security. In this field, virus inoculation is the essential and reliable method to evaluate the resistance of a transgenic material or a rice variety. Rice Ragged Stunt Virus (RRSV) can transmit rice ragged stunt disease by brown planthopper in a persistent propagative manner in many Asian countries (Ling et al., 1978; Hibino, 1979). Two classical methods, including single seedling inoculation and group inoculation, had been applied to conduct virus inoculation experiments (Zhang et al., 2013). By modifying traditional group inoculation method, we provided a convenient approach which closely resembles the natural infection condition.
Materials and Reagents
- Pipette tips (RNase free 1 ml, 0.2 ml and 0.02 ml, Corning, Axygen®, catalog numbers: T-1000 , T-200 , TF-20 )
- 1.5 ml clear Microtubes (Corning, Axygen®, catalog number: MCT-150-C )
- Clip-cage (Home-made, 40 x 30 x 35 cm, Figure 1A)
- Artificial suction-implement (Home-made, Figure 1B)
- Ceramic mortar (external diameter = 120 mm)
- PVDF membrane (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88585 )
- Filter paper (Bio-Rad Laboratories, catalog number: 1703967 )
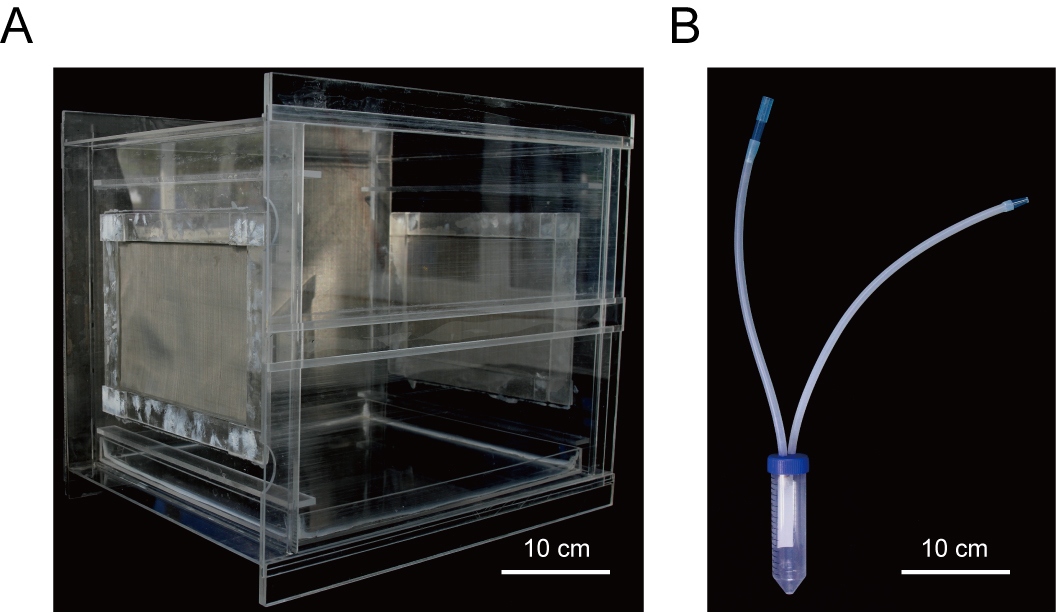
Figure 1. Home-made clip-cage and artificial suction-implement used in this study. A. Clip-cage used for raising insect vectors and virus transmission. B. An artificial suction-implement used for transferring insect vectors. - X-ray film
- RRSV-infected rice plants (at vegetative growth phase)
- Host plants (Oryza sativa L. spp. japonica var. Nippobare) (all the transgenic lines are Nippobare background)
- Insect vector (Nilaparvate lugens Stal) (collected from a field population in Fuzhou, Fujian Province, China, and cultured at 25 °C with a photoperiod of 16 h/8 h [light/dark] in a tissue culture frame); the insect vectors will mate during cultivation naturally.
- Primers
For β-Actin gene:
5'-CAGCCACACTGTCCCCATCTA-3’
5'-AGCAAGGTCGAGACGAAGGA-3’
For RRSV CP:
5'-GAGCAAACTTGAGGCGTA-3’
5'-AAGCTACCGTGTAGGTGGCG-3’ - Plant RNA Kit (Omega Bio-Tek, catalog number: R6827 )
- DNase I (Omega Bio-Tek, catalog number: E1091 )
- ReverTra Ace qPCR RT Kit (TOYOBO, catalog number: FSQ-301 )
- THUNDERBIRD qPCR Mix (TOYOBO, catalog number: QPS-201 )
- Liquid nitrogen
- Agarose
- A homemade monoclonal antibody against RRSV-encoded CP
- ProteinFind Anti-β-Tubulin Mouse Monoclonal Antibody (TransGen Biotech, catalog number: HC201-02 )
- ProteinFind Goat Anti-Mouse IgG(H+L), HRP Conjugate (TransGen Biotech, catalog number: HS201-01 )
- Chemiluminescent HRP substrate (Merck, catalog number: WBKLS0100 )
- Prestained marker
- Ethanol (ALADDIN, catalog number: E111963 )
- Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 75746-1KG )
- Tris-HCl (Sigma-Aldrich, catalog number: V900312 )
- β-mercaptoethanol (Sigma-Aldrich, catalog number: 97622 )
- Glycerinum (Sigma-Aldrich, catalog number: G5516 )
- NaCl (Sigma-Aldrich, catalog number: S7653 )
- KCl (Sigma-Aldrich, catalog number: P9333 )
- KH2PO4 (Sigma-Aldrich, catalog number: P0662 )
- Na2HPO4•12H2O (Sinopharm Chemical Reagent, CAS number: 10039-32-4)
- Skim milk powder (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: LP0031B )
- 30% acrylamide solution
- (NH4)2S2O8 (Sigma-Aldrich, catalog number: A3678-25G )
- TEMED (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 17919 )
- Tween 20 (Xilong Scientific, CAS number: 9005-64-5)
- Glycine (Solarbio, catalog number: G8200 )
- Methanol (Sinopharm Chemical Reagent, catalog number: 10014108 )
- 10% PAGE gel (see Recipes)
- Plant total protein extraction buffer (see Recipes)
- 10x PBS buffer (see Recipes)
- PBS-T (see Recipes)
- Blocking solution (see Recipes)
- Transferring buffer (see Recipes)
- Monoclonal anti-CP antibody solution (see Recipes)
Equipment
- Eppendorf pipettes suite (1 ml, 0.2 ml, 0.02 ml, 0.01 ml and 0.0025 ml)
- Ceramic mortar
- Centrifuge (Eppendorf, models: 5424 R and 5427 R )
- Vortex (Kylin-Bell Lab Instruments, model: VORTEX-5 )
- PCR amplifier (Bio-Rad Laboratories, model: T100 )
- Fluorescence quantitative PCR detection system (Bio-Rad Laboratories, model: CFX ConnectTM )
- Gel Imaging System (Bio-Rad Laboratories, model: GelDocTM XR+ )
- Basic electrophoresis apparatus (Bio-Rad Laboratories, model: PowerPacTM Basic )
- Mini-Protean Tetra (Bio-Rad Laboratories, model: Mini-PROTEAN Tetra Cell )
- Semi-dry transfer slot (Bio-Rad Laboratories, model: Trans-Blot SD )
- Ultra-micro UV spectrophotometer (Thermo Fisher Scientific, model: NanoDropTM 2000 )
- Developing machine (Carestream Health, model: 102 )
- Decolorizing orbital shaker (Kylin-Bell Lab instruments, catalog number: TS-1000 )
- Black box
- Film Cassette (Guangdong YueHua Medical Instrument Factory, model: AX-II )
Software
- SPSS for Windows version 11.5 (IBM, Armonk, NY, USA)
- Gel imaging software (Bio-Rad Laboratories, model: GelDocTM XR+ )
- Bio-Rad qPCR software (Bio-Rad Laboratories, model: CFX ConnectTM )
Procedure
- Detection of RRSV-diseased rice plants through RT-PCR and Western blots
The source of RRSV-diseased rice plants, exhibiting typical symptoms including stuntedness, excess tillering, and leaves that are dark green and/or have serrated edges or twisted tips (Zhang et al., 2016), are collected from the field (Fuzhou City, Fujian Province). The presence of RRSV is confirmed by RT-PCR and Western blot.- RT-PCR
- Extract total RNA from 0.1 g leaves with disease symptoms using a Plant RNA kit (OMEGA Bio-Tek Inc.) following the manufacturer’s instructions.
- Reverse transcribe 1 μg total RNA by using the ReverTra Ace qPCR RT Kit (TOYOBO), following the manufacturer’s manuals.
- Then amplify the RRSV CP gene with the primers: 5’-GAGCAAACTTGAGGCGTA-3’, and 5’-AAGCTACCGTGTAGGTGGCG-3’.
- Run PCR on the Bio-Rad CFX ConnectTM PCR System under the cycling conditions of 95 °C for 5 min, followed by 25 cycles of 95 °C for 20 sec, 55 °C for 30 sec and 72 °C for 5 sec.
- Using rice β-Actin gene amplified with the primers: 5’-CAGCCACACTGTCCCCATCTA-3’, and 5’-AGCAAGGTCGAGACGAAGGA-3’ as a control.
- Perform Agarose gel electrophoresis and then visualize the amplification products of RRSV CP gene using Gel Imaging System was used to.
- Western blots
Total protein is extracted from 0.1 g RRSV-infected leaves, which were grounded into powder with liquid nitrogen in ceramic mortar and then transferred into a 1.5 ml pre-cooled clear Microtubes, followed by adding 100 μl plant protein extraction buffer. Make the sample mix evenly by using a vortex. Then centrifuge at 6,010 x g 4 °C for 10 min. For RRSV detection, 20 µl of the prepared total protein is used to conduct polyacrylamide gel electrophoresis and Western blot. The detailed steps are as follows:- Load 20 µl of the prepared total protein in each well of the PAGE gel, along with prestained marker, and run the gel for 90 min at 120 V.
- Prepare a PVDF membrane which is slightly bigger than the size of the gel. Prepare 2 pieces of filter paper which is as big as PVDF membrane. Place the PVDF membrane, gel and filter papers in 1x transferring buffer for 10 min. Prepare the transfer sandwich (filter paper-PVDF membrane-gel-filter paper) and place it in a Semi-dry transfer slot with 3.0-5.5 mA/cm2 for 30 min.
- Block the membrane in 5% skimmed milk in PBS-T (see Recipes) at room temperature (RT) for 0.5-1 h.
- Wash the membrane with PBS-T three times for 5 min each time.
- Incubate the membrane in the monoclonal anti-CP antibody solution (see Recipes) by using a decolorizing orbital shaker at RT for 2 h or at 4 °C overnight.
- Wash the membrane with PBS-T three times for 10 min each time.
- Incubate in the HRP-conjugated antibody solution by using Decolorizing Orbital Shaker at RT for 1 h. Wash the membrane with PBS-T five times for 5 min each time.
- Use the Chemiluminescent HRP substrate to blot according to the manufacturer’s instruction.
- Put a single piece of x-ray film into a film cassette in a black box.
- Use the developing machine to automatically develop, fix, wash, and dry exposed film.
- RT-PCR
- Vector virus-acquisition
- Wash RRSV-infected rice plants confirmed as described in Procedure A with water and plant them in a square plastic box, then put the box into a clip-cage (Figure 2).
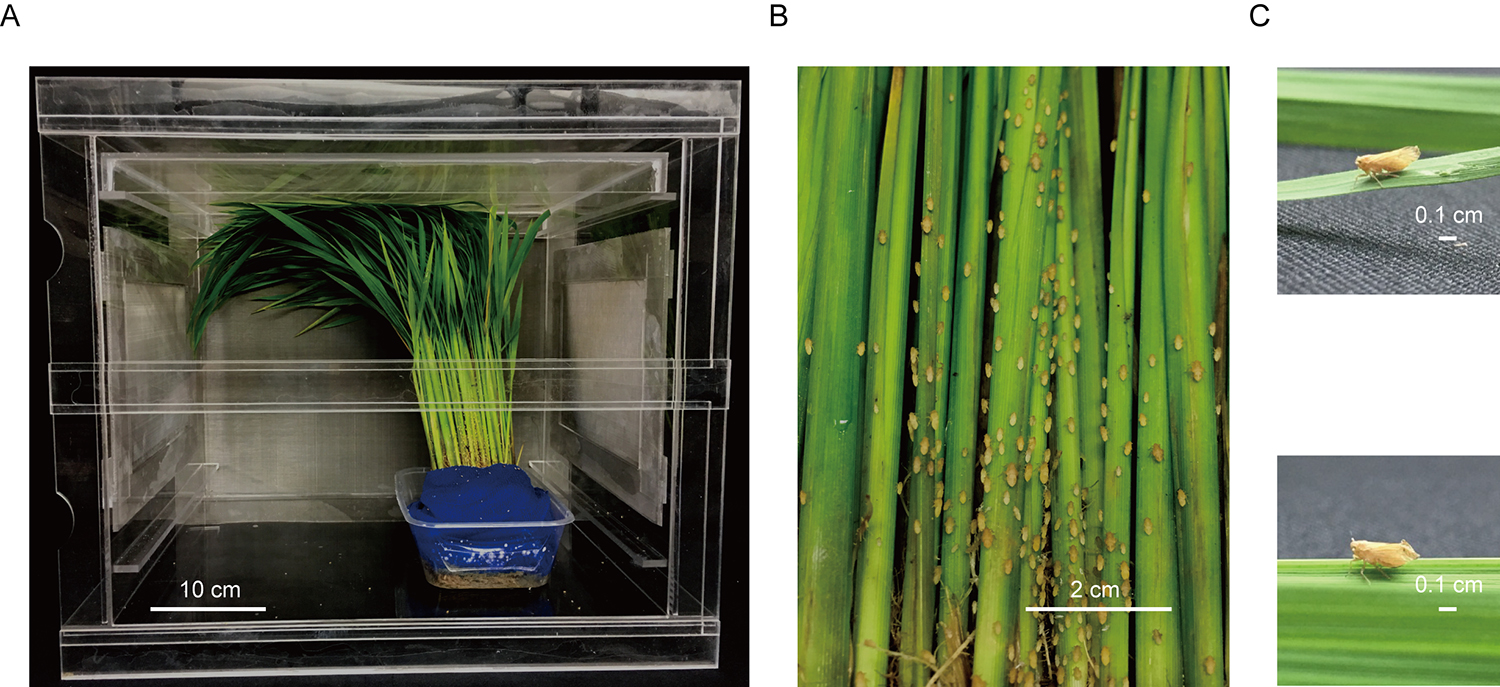
Figure 2. The instar nymphs acquire virus on RRSV-infected rice plants. A. An overview of the feeding virus process of insect vector in RRSV-infected rice plants in the clip-cage. B. The insect vector on the diseased rice plants. C. The morphology of female BPH before (upside) and after (bottom) mating process. - To prepare viruliferous BPH population with high virus-carrying rate and overcome the limited lifespan of BPH, we allow 20 female BPH adults finished in mating to be fed on RRSV-diseased rice plants, and 3-5 days later, they lay eggs on RRSV-infected rice plants. About 7 days later, nymphs began to hatch, feed on infected rice and acquire virus as early as they can.
- About 12 days later, after nymphs hatching, all the nymphs go through a 9-day latent period (Jia et al., 2012), and then they can be used for virus transmission.
- Simultaneously, we put 20 female BPH adults finished in mating to be fed on healthy rice plants to generate non-viruliferous BPH population as control vector.
- Wash RRSV-infected rice plants confirmed as described in Procedure A with water and plant them in a square plastic box, then put the box into a clip-cage (Figure 2).
- Preparation of rice seedlings for virus transmission
- Add 150-200 ml of fine soil to clean square plastic boxes and moisten the soil with 100 ml water added along the box wall.
- Scatter about 100 pre-germinated seeds on the soil and then sprinkle with a thin layer of soil. Put the square plastic boxes into a clip-cage (Figures 3A and 3B) and place on the tissue culture frame in a growth room at 25 °C with a photoperiod of 16 h/8 h (light/dark) with intensity 5,000-7,000 Lux until the rice seedlings have grown to 2 leaf stage, when they can be used for virus transmission.
- Virus transmission
- Allow the nymphs to feed at test seedlings for 3 days on the tissue culture frame in the growth room.
- Suck up viruliferous and non-viruliferous BPH nymphs by using an artificial suction-implement to test rice seedlings at three-leaf stage. The ratio of the number of nymphs and the number of seedlings is 2:1, owing to the virus carrying rate of BPH nymphs is approximately 70% (Zhang et al., 2013).
- Transplanting of RRSV-inoculated rice seedlings
Once rice seedlings inoculated with viruliferous and non-viruliferous BPH nymphs, remove the vector thoroughly from the test seedlings and then grow the rice seedlings in the greenhouse for symptom observation. As shown in Figure 3C, usually sixteen rice seedlings are transplanted into one basin evenly (Figure 3C).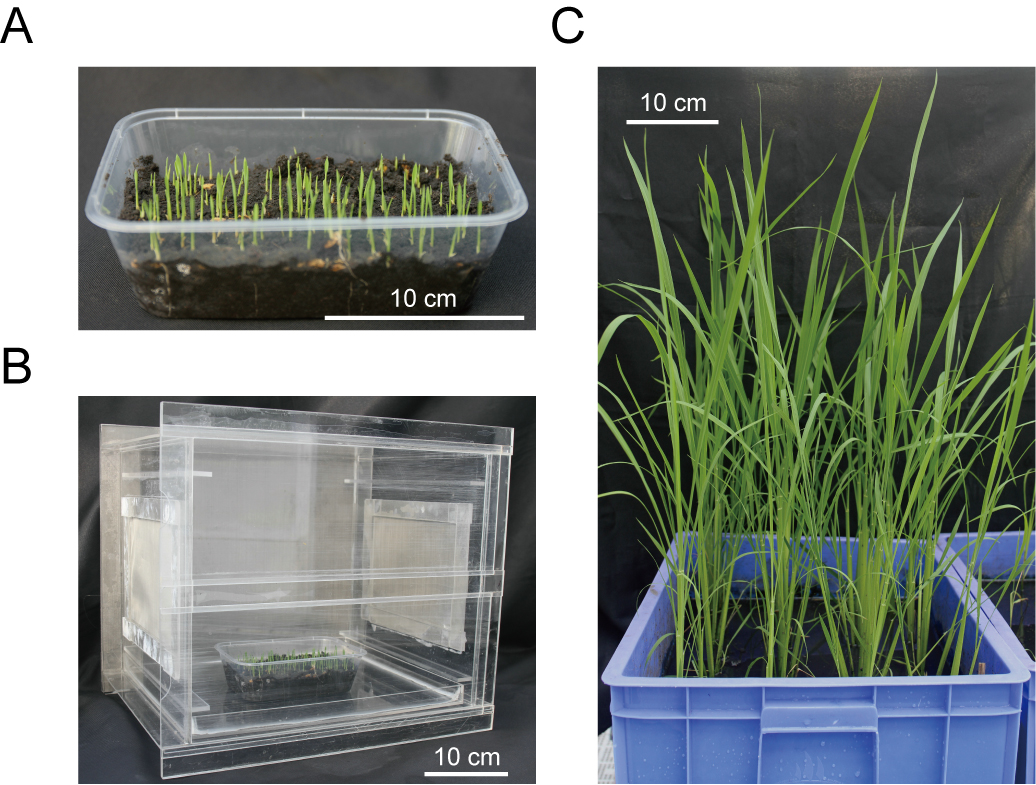
Figure 3. Preparation of test rice seedlings and transplanting of rice plants that finished virus transmission. A. Preparation of test rice seedlings in a clean square plastic box containing 150-200 ml of humid fine soil. B. The rice seedlings grown in a clip-cage to avoid vector feeding. C. Virus-inoculated rice seedlings transplanted into a basin grown in the greenhouse.
Data analysis
- RT-PCR and Western blot identify the RRSV-infected source plants
RRSV-infected source plants are identified by RT-PCR with 236 bp PCR products of gene RRSV CP (Figures 4A and 4B). The expression of RRSV CP is further verified by Western blots (Figure 4C) with β-Tubulin as a loading control. These results indicate that these plants used for virus inoculation were infected by RRSV.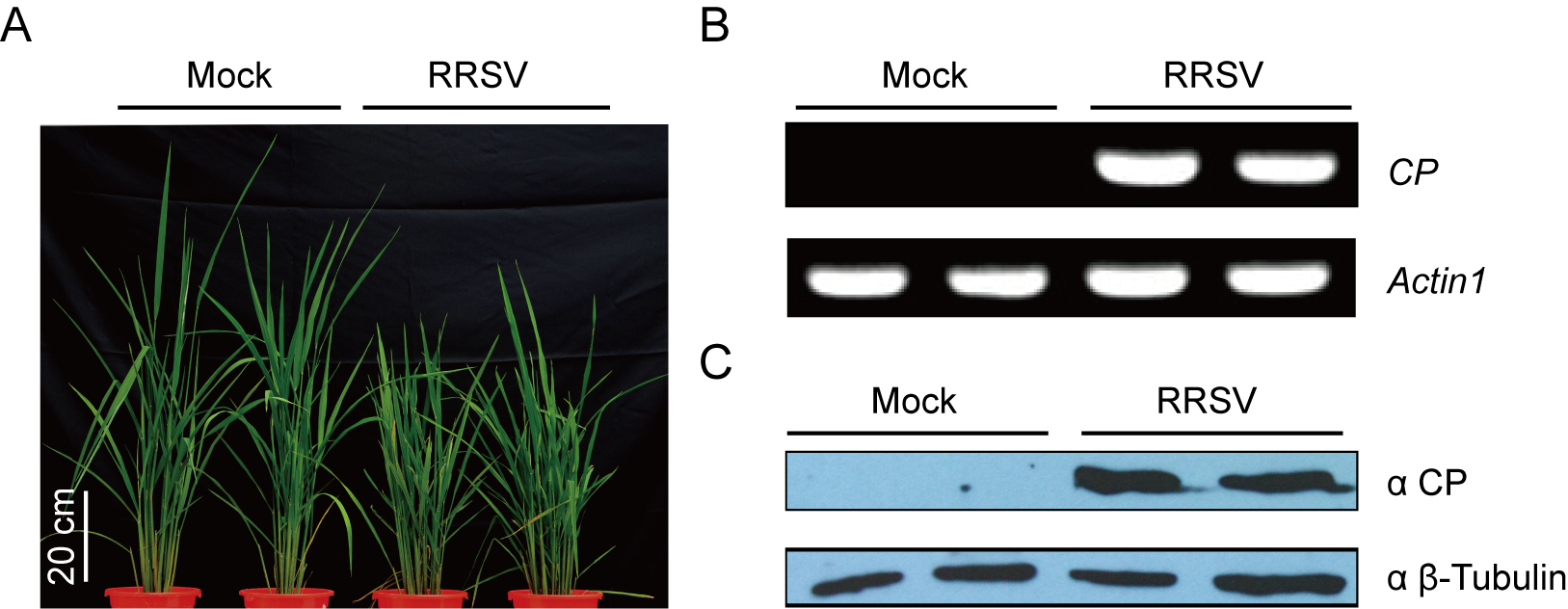
Figure 4. RRSV-diseased rice plants were identified by RT-PCR and Western blots. A. RRSV-diseased rice plants with typical symptoms including stuntedness, increased tillering number and leaves that are dark green and/or have serrated edges or twisted tips. B. RT-PCR detection of RRSV CP gene for identification of RRSV-infected source plants. C. Identification of RRSV-infected source plants by Western blots. Rice actin1 and β-Tubulin were conducted as loading controls. - Observation of disease symptoms
The disease symptoms of test rice plants emerge from 20 days after inoculation. The differences of disease symptoms of test rice samples can be observed from plant height and tillering number. We usually take pictures of each rice sample at 45 days after inoculation. - Incidence statistics
The number of RRSV-diseased rice plants is counted at 28 days after inoculation. Virus inoculation is repeated three times. The RRSV-infected rice plants are identified by RT-PCR (Figure 5A). The incidence of all the test samples is compared by column diagram (Figure 5B) and significant difference is analyzed by Student's t-test. A P-value of ≤ 0.05 was considered statistically significant.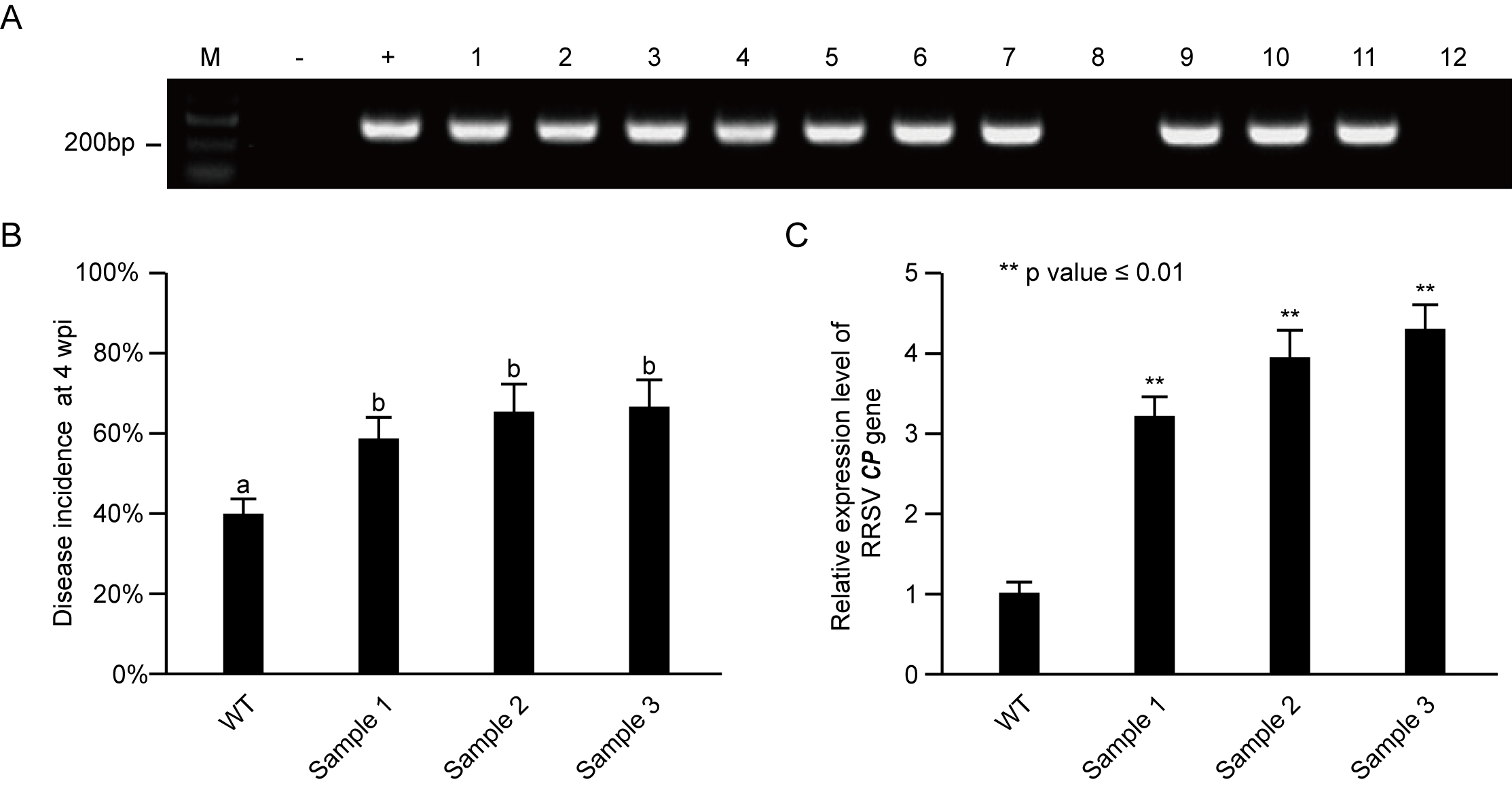
Figure 5. Incidence statistics of RRSV-infected samples and comparison of virus content in different samples. A. RRSV-diseased rice plants were detected by RT-PCR. M, DNA marker; -, negative control detected from cDNA of healthy rice plants; +, positively control, this band was amplified from a plasmid containing RRSV CP gene. B. The disease incidence of RRSV-inoculated rice plants at 4 weeks post inoculation. C. RT-qPCR was performed to indicate the relative expression level of RRSV CP gene in different RRSV-inoculated rice plants. - Make a comparison of virus content by qRT-PCR
The severity degree of disease symptoms is always positively correlated with the virus accumulation in a virus-infected rice plant. We compare the different virus content by detecting RRSV CP gene relative expression level through qRT-PCR (Figure 5C).
To integrally describe the whole procedure of virus inoculation assay, a process diagram is illustrated in Figure 6.
Figure 6. An overview of RRSV propagation and infection on rice plants
Notes
- Finish virus inoculation before viruliferous insect vector spawning.
- Rice seedlings with two leaves are suitable for virus inoculation.
- All the diluted antibody solution can be stored at 4 °C for no longer than 1 day.
Recipes
- 10% PAGE gel (5 ml)
1.9 ml ddH2O
1.7 ml 30% acrylamide solution
1.3 ml 1.5 M Tris-HCl (pH = 8.8)
0.05 ml 10% (NH4)2S2O8
0.002 ml TEMED
Prepare 10% (NH4)2S2O8 solution by adding 100 mg (NH4)2S2O8 into 1 ml deionized water - Plant total protein extraction buffer
0.25 M Tris-HCl (pH = 6.8)
8% β-mercaptoethanol
20% Glycerinum
8% SDS - 10x PBS buffer
16 g NaCl
4 g KCl
4 g KH2PO4
6 g Na2HPO4•12H2O
Dissolve in 900 ml of distilled water, make the final volume to 1,000 ml (pH = 7.5) - PBS-T
10x phosphate-buffered saline (PBS) diluted by 1:9 volume ratio, added 0.1% Tween 20 - Blocking solution
5% (w/v) skimmed milk powder in PBS-T - Transferring buffer
48 mM Tris
39 mM glycine
20% methanol
0.04% SDS - Monoclonal anti-CP antibody solution
Add 5 μl monoclonal anti-CP antibodies into 10 ml blocking solution
Acknowledgments
This work was supported by grants to C. Zhang and J. G. Wu from the National Natural Science Foundation of China (Nos. 31722045; 31772128; 31701757 and 31201491); the National Basic Research Program 973 (2014CB138400); and the Natural Science Foundation of Fujian Province of China, Outstanding Young Scientific Research Plan and Excellent Talent Plan in the New Century of Fujian Province (JA3091 and 2014J06011). We also appreciate the previous work related to virus propagation and infection on rice plants conducted by others.
Competing interests
There are no any conflicts of interest or competing interests.
References
- Hibino, H. (1979). Rice ragged stunt, a new virus disease occurring in tropical Asia. Rev Plant Prot Res 12: 98-110.
- Jia, D., Guo, N., Chen, H., Akita, F., Xie, L., Omura, T. and Wei, T. (2012). Assembly of the viroplasm by viral non-structural protein Pns10 is essential for persistent infection of rice ragged stunt virus in its insect vector. J Gen Virol 93(Pt 10): 2299-2309.
- Ling, K. C., Tiongco, E. R., and Aguiero, V. M. (1978). Rice ragged stunt, a new virus disease. Plant Dis Rep 62(8): 701-705.
- Zhang, C., Ding, Z., Wu, K., Yang, L., Li, Y., Yang, Z., Shi, S., Liu, X., Zhao, S., Yang, Z., Wang, Y., Zheng, L., Wei, J., Du, Z., Zhang, A., Miao, H., Li, Y., Wu, Z. and Wu, J. (2016). Suppression of jasmonic acid-mediated defense by viral-inducible microRNA319 facilitates virus infection in rice. Mol Plant 9(9): 1302-1314.
- Zhang, S. B., Song, G. W., Yang, L., Wu, Z. J. and Xie, L. H. (2013). Determination of Rice ragged stunt virus and vector transmission characteristics. J Fujian Agric For Uni (in Chinese).
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, C., Shi, C., Chen, D. and Wu, J. (2018). Rice Ragged Stunt Virus Propagation and Infection on Rice Plants. Bio-protocol 8(20): e3060. DOI: 10.21769/BioProtoc.3060.
Category
Microbiology > Microbe-host interactions > Virus
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











