- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pneumatic Method to Measure Plant Xylem Embolism
(*contributed equally to this work) Published: Vol 8, Iss 20, Oct 20, 2018 DOI: 10.21769/BioProtoc.3059 Views: 7348
Reviewed by: Amey RedkarChristine ScoffoniDheeraj Singh Rathore

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Evaluating Arabidopsis Primary Root Growth in Response to Osmotic Stress Using an In Vitro Osmotic Gradient Experimental System
Selene Píriz-Pezzutto [...] Mariana Sotelo-Silveira
Jul 20, 2025 1911 Views

Synchronizing Germination Rates Across Plant Species for Fabricated Ecosystems EcoFAB 2.0
Romane S. F. Charbeaux [...] Michelle Watt
Dec 5, 2025 1471 Views

Quantification of Protochlorophyllide (Pchlide) Content in Arabidopsis Seedlings Using a High-Performance Liquid Chromatography (HPLC) System
Fan Zhang [...] Liangsheng Wang
Jan 5, 2026 562 Views
Abstract
Embolism, the formation of air bubbles in the plant water transport system, has a major impact on plant water relations. Embolism formation in the water transport system of plants disrupts plant water transport capacity, impairing plant functioning and triggering plant mortality. Measuring embolism with traditional hydraulic methods is both time-consuming and requires large amounts of plant material. While the stem hydraulic methods measure loss of xylem hydraulic conductance due to embolism formation, the pneumatic method directly quantifies the amount of emboli inside the xylem as changes in xylem air content. The pneumatic method is an easy and fast (8+ embolism curves per day) method to measure plant embolism requiring minimal plant material. Here, we provide detailed descriptions and recent technical improvements on the pneumatic method.
Keywords: Embolism resistanceBackground
Plant xylem embolizes due to the entry of air into the xylem vessels under drought conditions. Resistance to embolism formation is one of the most important plant traits strongly determining species distribution, mortality and evolution (Choat et al., 2012; Rowland et al., 2015; Larter et al., 2017) and has been recently suggested as a key trait to model plant function and predict plant responses to global changes (Sperry and Love, 2015; Brodribb, 2017). Most methods used to estimate embolism resistance measures the hydraulic conductivity of embolized branch segments and relate it to the hydraulic conductance of the branch segment without embolism (Sperry et al., 1988; Melcher et al., 2012). These methods are usually time-consuming and prone to several artifacts (Wheeler et al., 2013; Trifilò et al., 2014; Beikircher and Mayr, 2016).
The pneumatic method has been recently proposed as an alternative method to estimate embolism resistance from a different point of view, not from the water flow perspective but from the direct consequence of embolism-air presence in the xylem (Pereira et al., 2016). As plant embolizes, air spaces inside the xylem increase. Embolism thus changes the pneumatic properties of branch segments. In this method, a vacuum is applied to a cut branch and the air flowing outside the branch is measured as an estimate of xylem air content. A strong relationship exists between air flow outside the branch segment and the amount of emboli in the branch xylem (Pereira et al., 2016; Zhang et al., 2018). The vacuum method presents a simple, low cost, fast and practical method to measure plant embolism. Additionally, the pneumatic method does not require rehydrating (flushing water) through samples in which the effects of drought are being studied.
Materials and Reagents
- Pneumatic apparatus
- Adapter Luers (Cole-Parmer, catalog numbers: EW-30800-06 and EW-30800-24)
- 1 L Kitasato flask (Prolab, catalog number: PL287)
- Silicone tubing (3 mm ID and 5.2 mm OD and 4.9 mm ID and 9.7 mm OD, larger or smaller sizes depending on sample diameter, with preferences for thick walled tubes, as they seal better after clamping)
- Rigid tubing (Cole-Parmer, catalog number: EW-30600-62)
- Vacuum source, either a syringe or vacuum pump (Prolab, catalog numbers: 032357 and VAC29-110, respectively)
- Three-way stopcock (Cole-Parmer, catalog number: EW-30600-07)
- Vacuum reservoir (a container or tube with rigid walls to store vacuum; 1-10 ml volume is usually enough)
- Vacuum meter, 30 to 110 kPa recommended (Honeywell, catalog numbers: 142PC05D or 26PCCFA6D or MPX5100AP; NXP Semiconductors; Netherlands. Farnell, catalog numbers: 1386589, 731766 and 1457156, respectively)
- Sample preparation and handling
- Plastic glue
- Plastic paraffin film (Prolab, catalog number: PM996)
- Plastic clamps (Cole-Parmer, catalog number: RZ-06832-02) and/or zip ties
Equipment
- Voltage meter
- Pliers
- Sharp razors
- Voltmeter or voltage logger (1 mV precision for 142PC05D or MPX5100AP, 0.01 mV precision for 26PCCFA6D)
- Alternative: Pneumatic Shield for Arduino UNO microcontroller board (Plant Technology and Environmental Monitoring–PLANTEM), to use with 26PCCFA6D (see details in the Figure 4)
- Xylem water potential measurement
- Pressure chamber (PMS Instruments, model: PMS 1000, or other)
- Notebook and USB digital microscope (Jiusion, or other)
Note: We discourage the use of magnifying glasses because of safety issues.
Procedure
Pneumatic measurements require construction of the pneumatic apparatus and measurement of air discharge and xylem water potential. Each step is described below and in Video 1.
- Pneumatic apparatus (Figure 1)
The pneumatic apparatus consists basically of a container filled with vacuum where the branch will be connected, a meter to read vacuum pressure and a vacuum source to fill the reservoir.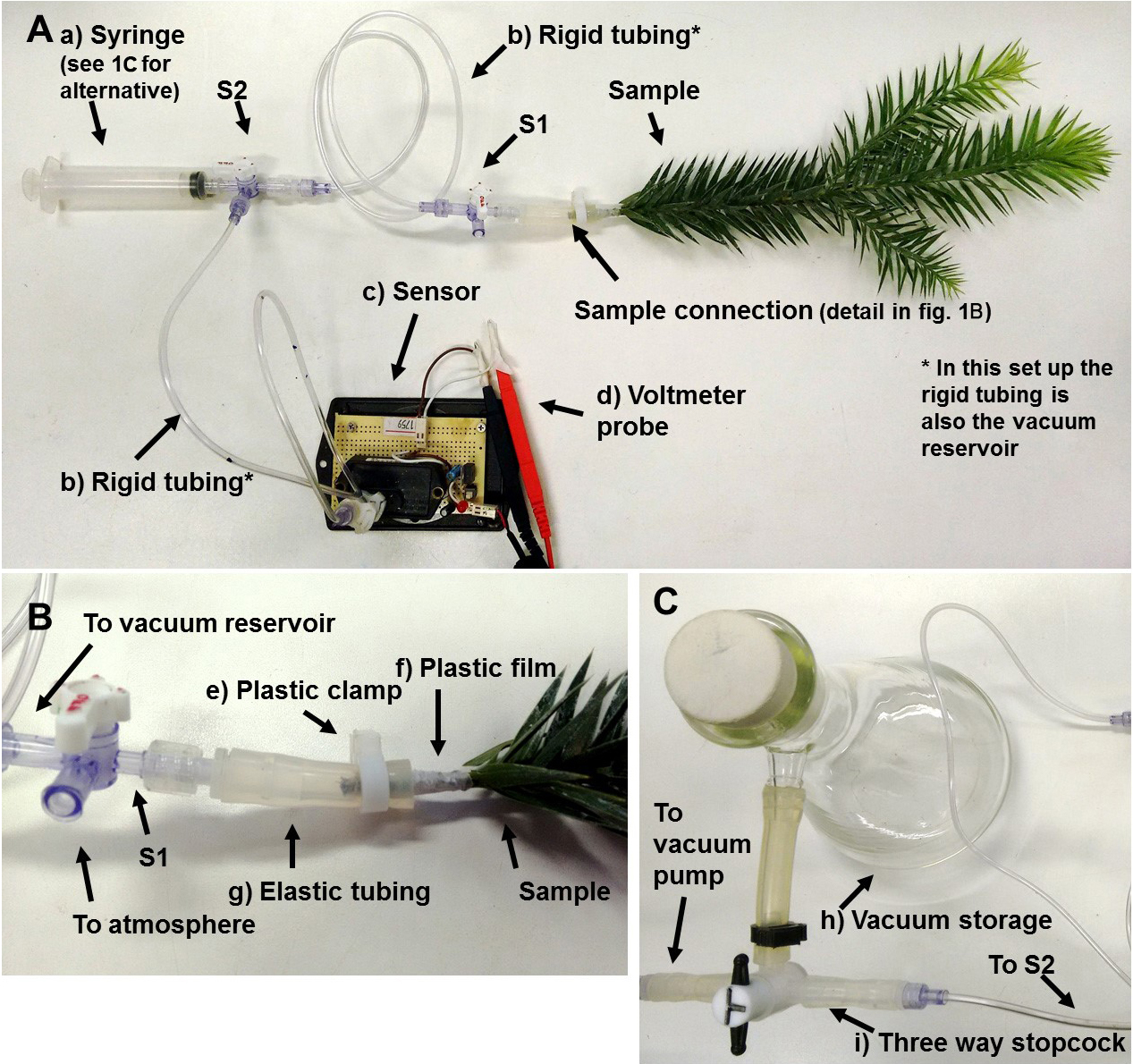
Figure 1. Pneumatic apparatus. A. Vacuum apparatus. a) common syringe used as vacuum source; b) rigid tubing also used as the vacuum reservoir; c) vacuum meter (142PC05D) coupled to a 12 V power source and a voltage regulator; d) voltmeter probe connected to a sensor output. B. Branch connection details. e) plastic clamp to tighten the elastic tubing to the sample; f) plastic film (and glue if necessary) to avoid leakage; g) elastic tubing that deforms when pressed by the plastic clamp. C. Alternative setup for the vacuum source. A vacuum pump is used to store vacuum in the vacuum storage (h; a 1 L Kitasato flask in this example). The vacuum storage is used to apply vacuum to the vacuum reservoir through a third three-way stopcock (i). S1 and S2 are the three-way stopcock 1 and 2, respectively.- Connect the vacuum reservoir to the first three-way stopcock (S1). Connect a silicone tubing (enough to fit the branch stem tight) to one exit of S1 and leave the other exit of S1 connected to the atmosphere (Figures 1A and 1B).
- Connect the vacuum reservoir to the second three-way stopcock (S2). Connect the vacuum meter to one exit of S2 and the vacuum source to the other exit of S2 (Figure 1A).
- Choose a vacuum reservoir volume (Vr; L) that ensures enough precision in the vacuum meter. Vr must be either measured or calculated from the tubing and stopcock datasheets (each rigid tubing [Cole-Parmer, USA] has 1.304 ml). A few milliliters of vacuum reservoir is usually enough (the tubing of the apparatus alone may be enough as a vacuum reservoir).
- The vacuum source can be either a syringe or a vacuum pump. Large quantities of vacuum can be stored in a container (Kitasato flasks, for example) and used to replenish the apparatus vacuum reservoir for easier use (Figure 1C).
- The vacuum meter may be read: with a voltmeter; with a voltage logger coupled to a computer; or with the Pneumatic Shield and Arduino UNO microcontroller.
- All tubing, except the one connecting the branch, must be rigid to avoid volume change under vacuum. The tube connecting the branch to S1 should be elastic so the branch can be tightly connected. We recommend using Luer lock connections for easy use and to prevent leakage.
- Air discharge measurements (Figure 2)
Air discharged from the branch (AD) is the amount of air leaving the branch to the vacuum reservoir. Air pressure changes in the vacuum reservoir are measured and converted to volume using the ideal gas law. AD increases with the amount of emboli of the branch segment being measured until the branch is fully embolized. Multiple AD points must be measured with the same sample in different xylem water potentials so the data can then be standardized and an embolism resistance curve can be estimated in the next section.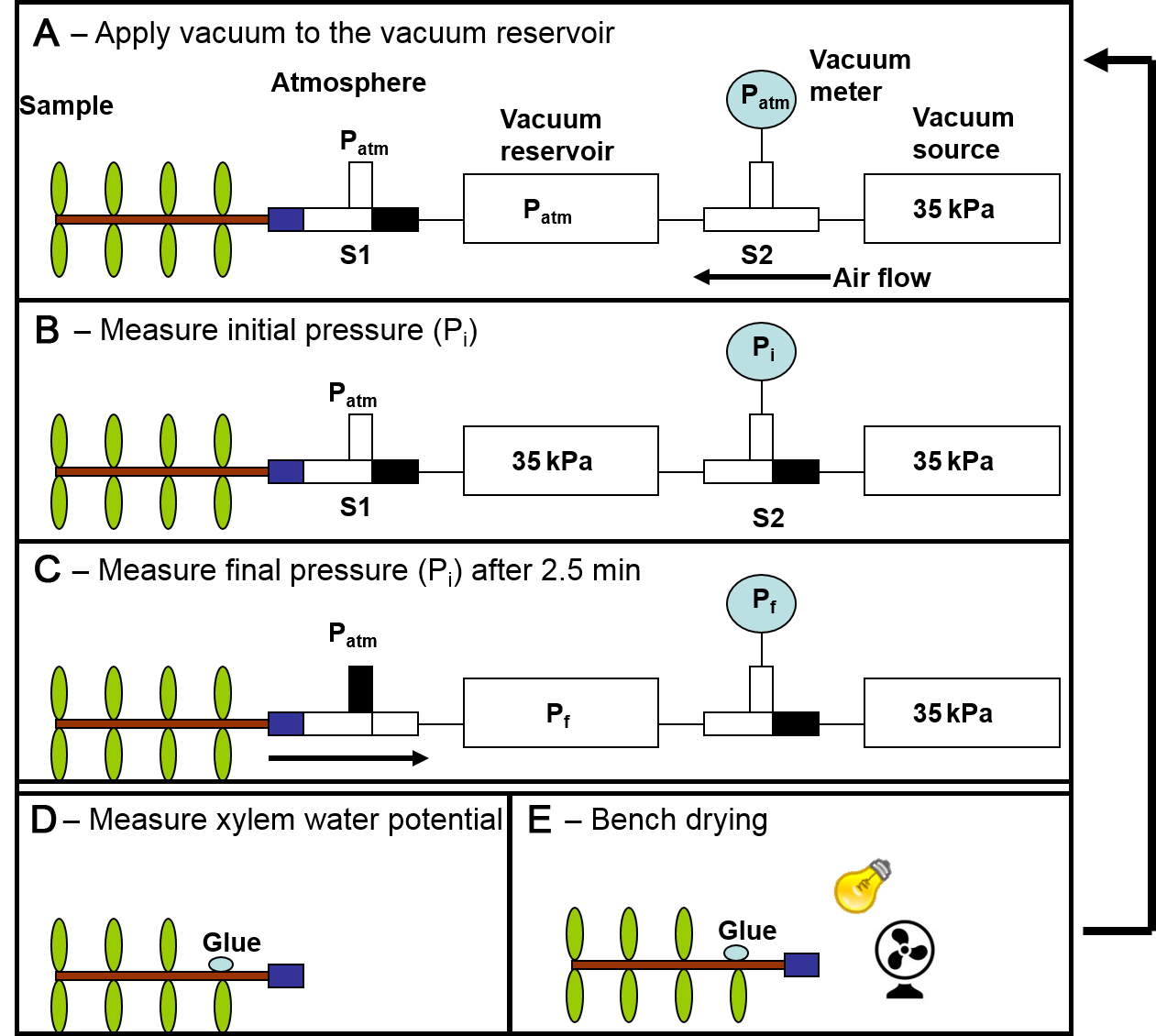
Figure 2. Air discharge curves measurement. Steps to construct and air discharge curve. 1) Fill the vacuum reservoir with air. 2) Measure initial pressure. 3) Apply pressure to sample, wait 2.5 minutes and measure final pressure. 4) Measure sample water potential. 5) Let sample to desiccate. Repeat step one after further desiccation.- Select a fully hydrated and non-embolized branch segment with some leaves.
- Cut the branch from the plant with a sharp razor.
- Connect the branch to the elastic tubing in S1 using a plastic clamp/plastic film to ensure no or insignificant leakage.
- Open the branch to the atmosphere in S1 and close the branch to the vacuum reservoir in S2 (Figure 2.1).
- Reduce the vacuum reservoir pressure to 35-40 kPa (atmospheric pressure minus 55-60 kPa) using the vacuum source and close the vacuum reservoir to the vacuum source in S2 (Figure 2.1).
- Read the initial pressure (Pi; kPa) at the vacuum reservoir (Figure 2.2).
- Close the branch segment to the atmosphere in S1 and connect it to the vacuum reservoir in S2 (Figure 2.3).
- After 2.5 min, read the final pressure at the vacuum reservoir (Pf; Figure 2.3).
- Calculate the number of moles of air Δn (mole) discharged from the branch using the ideal gas law:
(1) Δn = PiVr/RT – PfVr/RT;
where R is the gas constant (8.314 kPa L mol-1 k-1); T is apparatus/room temperature (K) during measurement, and Vr is the reservoir volume (L). - (Optional) Transform moles of air discharged to the volume of air discharged at atmospheric pressure (AD; μl) using the ideal gas law:
(2) AD = (ΔnRT/Patm) x 106
where Patm is atmospheric pressure (kPa) and 106 is to change from L to μl. - Measure the xylem water potential (Ψx) of the branch sample and seal the leaf cut with glue (Figure 2.4). See “Note 11” regarding sample handling and leaf and xylem water potential equilibrium issues.
- Leave the branch to dry in the bench (Figure 2.5).
- Bag the sample for leaf water potential to equilibrate with Ψx.
- Start again from Step B5 to obtain another data point. The last data point for a branch sample should be one where it is fully embolized as all data will be standardized by this last point.
- The branch must be connected to the atmosphere whenever a measurement is not happening to ensure the initial air pressure inside the branch is atmospheric. After an AD measure, leave the branch connected to the atmosphere for at least 2.5 min before another measure to ensure no vacuum remains inside the branch.
- The branch may shrink as it dehydrates, and the connection to S1 may get loose. Tighten the connection to ensure no leakage.
Data analysis
Data processing requires the use of pneumatic measurements to estimate the percentage loss of conductance (PLC). PLC is proportional to the percentage of maximum air discharged (PAD) of a branch sample. An example spreadsheet and an R script using base packages (R Core Team, 2011 [version 2.1.2]) for data processing and analysis are presented in the annex (“data.example.xls” and “data.analysis.r”). To obtain PAD, AD must be standardized for each branch sample (see Figures 3A and 3B).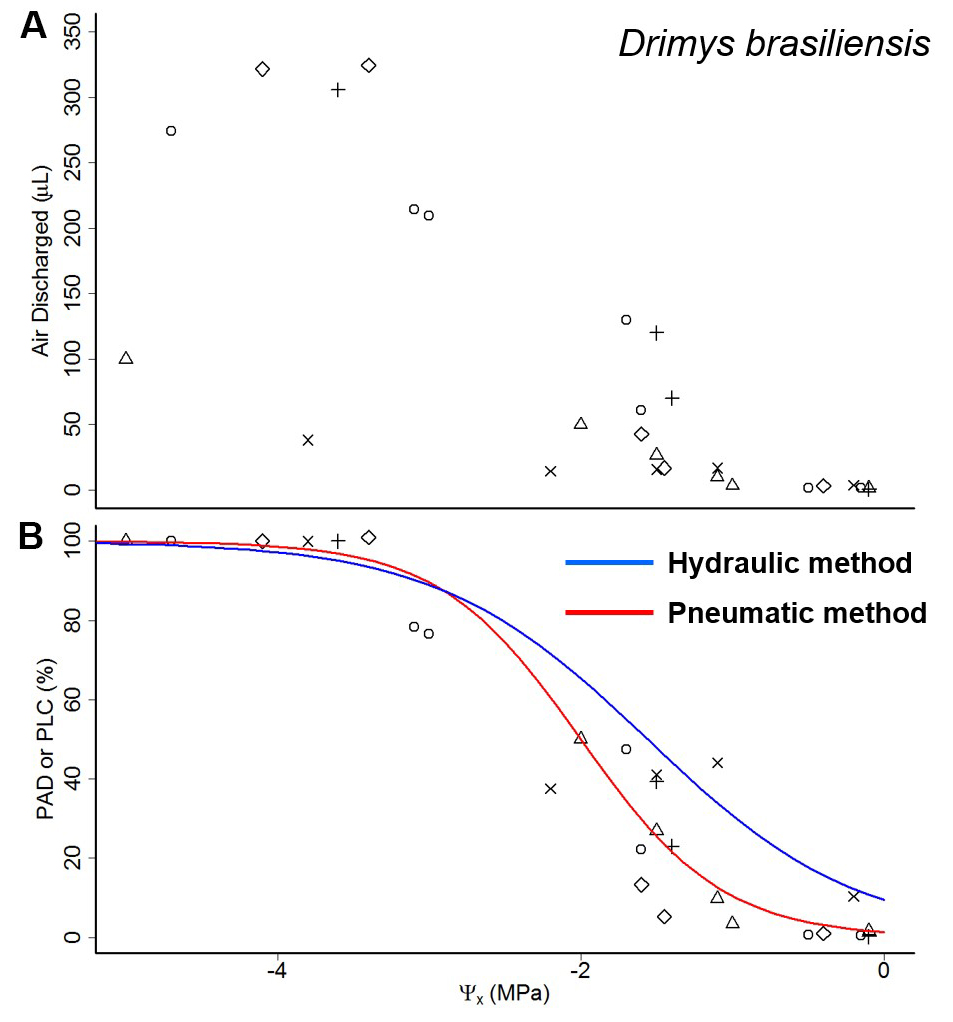
Figure 3. Air discharge and percentage air discharge curves. A. Example of air discharge measurements from branch samples in different xylem water potentials (Ψx). B. Air discharge measures from (A) were standardized by maximum air discharge to obtain percentage maximum air discharged (PAD) as a function of Ψx. Red line is the regression for the PAD data. Blue line is the percentage loss of conductivity (PLC; points not shown) curve obtained with the hydraulic method. Different symbols represent data from different samples in the pneumatic method. The branch lost its leaves at around minus 3.5-4.5 MPa.
Data.example.xlsx. Example spreadsheet for writing data and calculating AD and PAD.
Data.analysis.docx. R script with routines for processing and analyzing data collected with the pneumatic method.
- Standardize each AD measurement (ADi; where i represents each individual measurement) by the maximum AD (ADmax) of the branch segment being measured:
(3) PAD = 100 x (ADi/ADmax);
where PAD is the percentage of maximum air discharged from the sample and is related to PLC. - For some species, even when fully hydrated and with no embolism present, AD may be significant, possibly due to natural air spaces in the xylem. In this case, the initial AD (ADini) must be subtracted from each AD measurement so PAD is related to PLC:
(4) PAD = 100 x (ADi – ADini)/(ADmax – ADini) - The construction of the embolism curves follow the standard methods used to calculate PLC curves (fitting sigmoidal curves, exponential curves, etc.) but using the relationship between PAD and Ψx to estimate the PLC curves. A quality curve must present a plateau at high water potentials where PAD changes very little, a transition zone, and a second plateau where PAD stops changing (Figure 3B).
Notes
- We recommend using the Pneumatic Shield for Arduino UNO microcontroller developed by Plant Technology and Environmental Monitoring (PLANTEM, Brazil, Figure 4) as it allows for two samples to be measured at the same time and removes the need for manually taking notes on vacuum meter voltage, effectively doubling measurement velocity.
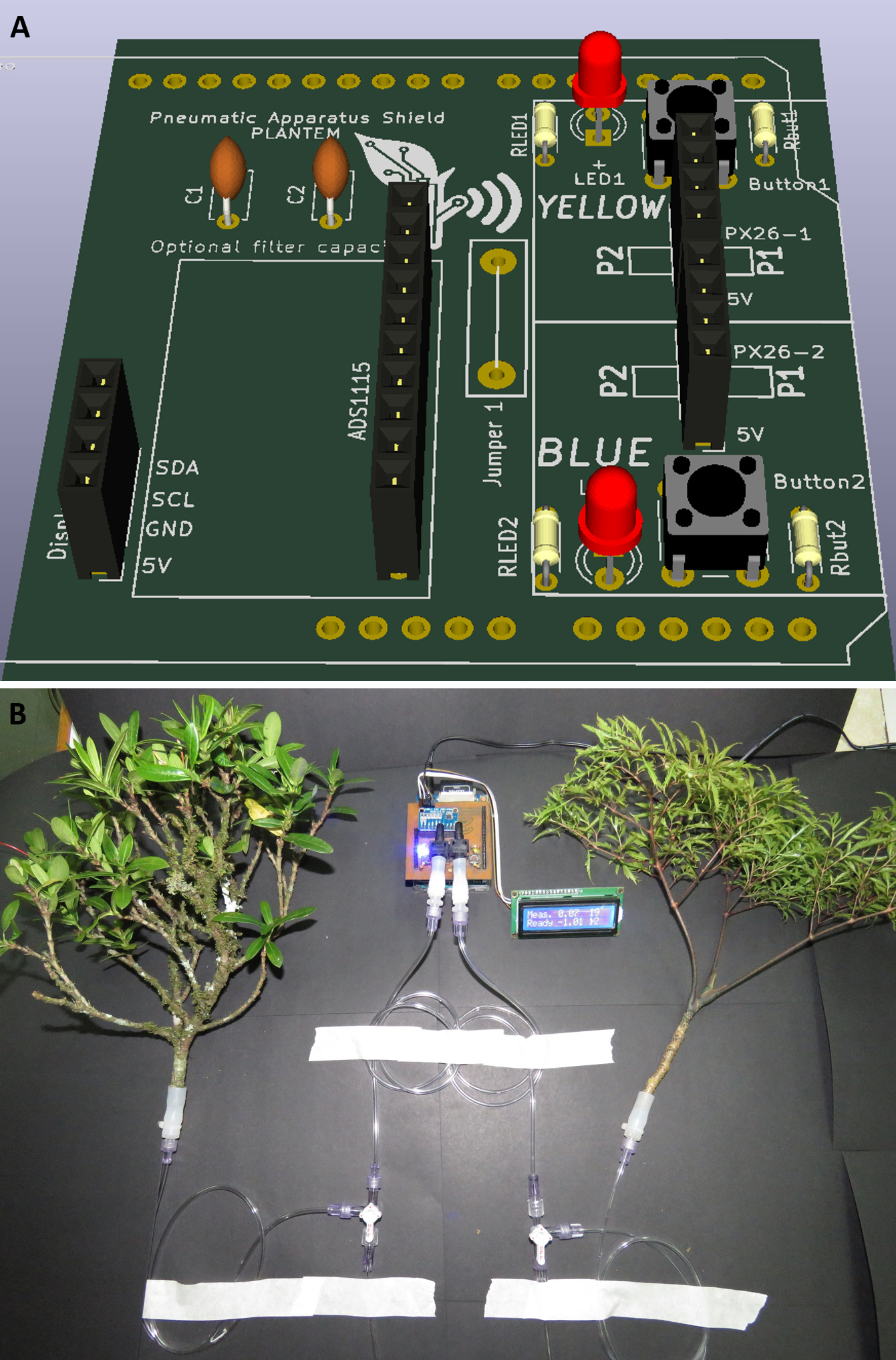
Figure 4. Pneumatic apparatus shield. A. Model of Pneumatic Shield for Arduino UNO developed by PLANTEM. B. Pneumatic Shield prototype is automatically logging two vacuum meters (26PCCFA6D). In this example, the left branch is being measured (indicated by blue LED) while the data is displayed in the LCD display and logged to an SD card. Measurement starts when a button associated with each vacuum meter is pressed, and data is logged each second. - Stopcocks also have air spaces inside them. If for one measurement you close the stopcock one direction and the other using a different direction the total amount of air may be slightly different.
- Leakage: If leakage is significant, it can be calculated and subtracted from each measurement.
- Sensor voltage supply: a simple voltage regulator can be used to supply power the vacuum meter. We recommend further controlling sensor input voltage using an LM78XX voltage regulator (usually LM7810 or 7812) as noise in input voltage will change voltmeter output. This is particularly important when using the lower output voltage 26PCCFA6D sensor. An LM78XX circuit and an assembled sensor (142PC05D) are shown in Figure 5 below:
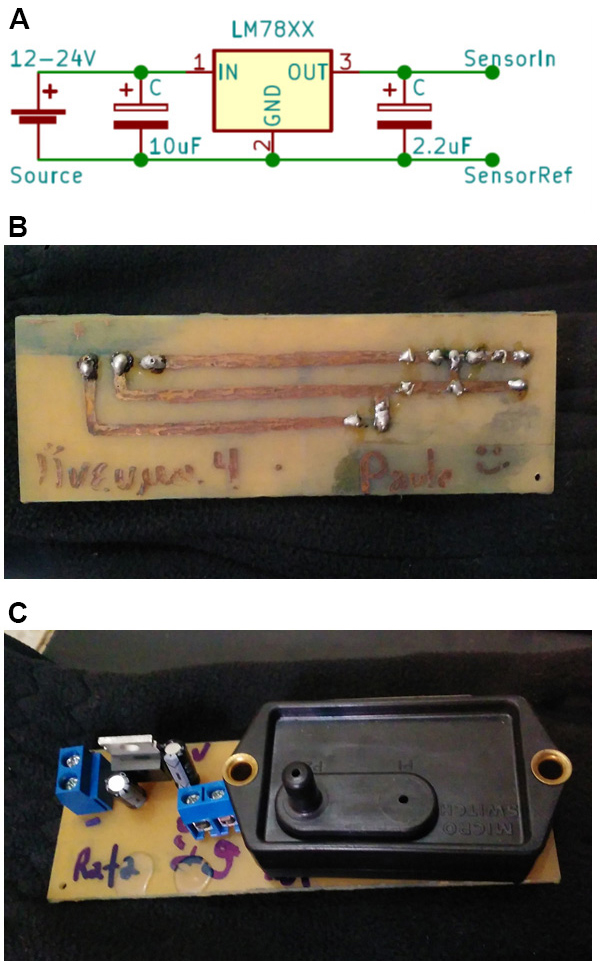
Figure 5. Pressure sensor and a simple voltage regulator. A. An LM7810 voltage regulator setup. B and C. Circuit board used in C). The circuit in B) was hand-drawn using a permanent marker on a virgin printed circuit board and corroded with ferric perchlorate. Holes were made for the components, and then they were welded on the board. 7810 is the LM7810 voltage regulator. C1 and C2 are electrolytic capacitors used to filter noise and reduce transient changes in input voltage. - The vacuum meter has very low power consumption. It can be powered from a 12 V car battery for days in the field or remote areas. In this case, a voltage regulator is fundamental.
- AD measurements can be expressed in mols of air or equivalent volume at atmospheric pressure. We recommend the expression in volume as it is in the same unit as anatomical measures and equipment descriptions.
- We use PAD instead of AD for estimating embolism because variability between samples of the same species can be substantial. If there is enough control of the samples so variability is small or the entire curve is made of one branch sample, AD can be used directly to estimate embolism.
- We use a discharge time of 2.5 min because we found it is enough to ensure precision. Shorter or longer times can be used. AD is not linear in time, so care should be taken when comparing different times with the same species/samples. If you want to measure all the volume of air inside a sample, you can use a long discharge time or extrapolate the discharge curve to infinity, find out the pressure at equilibrium and use it to calculate the total air space inside the sample.
- The branch connection (Figure 1B) can be removed from S1 without removing the sample from the elastic tubing for easier handling of multiple samples. For example, if you measure 10 samples on the same day, use 10 individual connections (Luer lock, elastic tubing, plastic clamp and sample setup) and simply connect the sample that you are going to measure each time to S1.
- Many plant species produce resins or latex in the bark/xylem. Secretion of resin/latex clogs the vessels and changes air discharge. We recommend verifying if this occurs in each species studied. If it occurs, cutting the branch with a sharp razor, waiting 1 h while the sample is bagged for the cessation of resin/latex secretion, and cutting the stem again prior to connecting to the apparatus usually solves the problem.
- To ensure xylem and leaf water potential to equilibrate, so leaf water potential can be used as an indicator of xylem water potential, before measuring leaf water potential the sample must stay the minimum amount of time possible outside of the plastic bag. The best way to do this is to measure the air discharge while the sample is bagged and afterward remove the bag to measure the water potential.
- The sample connection (Figure 1B) can hold a significant amount of air that can inflate AD measurements. We found this can be corrected by discounting the first second of measurement. Discounting the first second of measurement leads to a small underestimation of the total air inside the plant, but this has no effect on estimating PAD values, as they are relative. An alternative to measuring AD without discounting the first second is to discount the sample connection volume from the total air discharge. The total amount of discharged air (Δn), in mols, can then be calculated as:
Δn = (nr + nsc) – nf
where nr and nsc are the initial amount of air inside the reservoir and the sample connector, respectively, which sums to the total amount of air in the apparatus before measurement. nf is the final amount of air in the apparatus. As nsc is at atmospheric pressure prior to measurement, the above equation [equivalent of equation (1) in this situation] becomes:
Δn = (PiVr/RT + PatmVsc/RT) – PfVr/RT
where Vsc is the volume of the sample connection. All other procedures are equal. - Units, terms and definitions used in the protocol:
AD – air discharged from the branch segment (μl)
ADmax – maximum air discharged from the branch segment (μl). It is the AD of the sample when it is fully cavitated
ADini – air discharged when fully rehydrate and without cavitation (μl)
Pi – initial pressure of the vacuum reservoir (kPa)
Pf – final pressure of the vacuum reservoir (kPa)
Patm – atmospheric pressure (101.3 kPa at sea level approximately)
PAD – percentage of maximum air discharged from the branch
PLC – percentage loss of hydraulic conductance of the xylem
S1 – three-way stopcock connecting the branch segment to the vacuum reservoir and the atmosphere
S2 – three-way stopcock connecting the vacuum reservoir to the vacuum source and vacuum meter
R – gas constant (8.314 kPa L mol-1 k-1)
T – apparatus/room temperature (K)
Vr – vacuum reservoir volume (L)
Δn – change in the number of mols in the vacuum reservoir (ni minus nf; mol)
Ψx – xylem water potential (MPA)
Acknowledgments
This research was funded by FAPESP/Microsoft research (grant 11/52072-0) awarded to R.O. We thank the UNICAMP post-graduate programs in Ecology and Plant Biology and the Higher Education Co-ordination Agency (CAPES) scholarship award to P.R.L.B. We thank Newton International Fellowship (grant NF170370) who funded P.R.L.B. in the final writing of this manuscript and São Paulo Research Foundation for the fellowship granted to L.P. (FAPESP, Grant No. 2017/14075-3). We thank David Bartholomew for reviewing and proofreading the manuscript.
Competing interests
The authors declare they have no conflict of interest.
References
- Beikircher, B. and Mayr, S. (2016). Avoidance of harvesting and sampling artefacts in hydraulic analyses: a protocol tested on Malus domestica. Tree Physiol 36(6): 797-803.
- Brodribb, T. J. (2017). Progressing from 'functional' to mechanistic traits. New Phytologist 215: 97-112.
- Choat, B., Jansen, S., Brodribb, T. J., Cochard, H., Delzon, S., Bhaskar, R., Bucci, S. J., Feild, T. S., Gleason, S. M., Hacke, U. G., Jacobsen, A. L., Lens, F., Maherali, H., Martinez-Vilalta, J., Mayr, S., Mencuccini, M., Mitchell, P. J., Nardini, A., Pittermann, J., Pratt, R. B., Sperry, J. S., Westoby, M., Wright, I. J. and Zanne, A. E. (2012). Global convergence in the vulnerability of forests to drought. Nature 491(7426): 752-755.
- Larter, M., Pfautsch, S., Domec, J. C., Trueba, S., Nagalingum, N. and Delzon, S. (2017). Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callitris. New Phytol 215(1): 97-112.
- Melcher, P. J., Michele Holbrook, N., Burns, M. J., Zwieniecki, M. A., Cobb, A. R., Brodribb, T. J., Choat, B. and Sack, L. (2012). Measurements of stem xylem hydraulic conductivity in the laboratory and field: Measurements of stem xylem hydraulic conductivity. Methods Ecol Evol 3: 685-694.
- Pereira, L., Bittencourt, P. R., Oliveira, R. S., Junior, M. B., Barros, F. V., Ribeiro, R. V. and Mazzafera, P. (2016). Plant pneumatics: stem air flow is related to embolism - new perspectives on methods in plant hydraulics. New Phytol 211(1): 357-370.
- R Core Team (2011). R: A language and environment for statistical computing. Vienna, Austria: the R Foundation for Statistical Computing.
- Rowland, L., da Costa, A. C., Galbraith, D. R., Oliveira, R. S., Binks, O. J., Oliveira, A. A., Pullen, A. M., Doughty, C. E., Metcalfe, D. B., Vasconcelos, S. S., Ferreira, L. V., Malhi, Y., Grace, J., Mencuccini, M. and Meir, P. (2015). Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528(7580): 119-122.
- Sperry, J. S., Donnelly, J. R. and Tyree, M. T. (1988). A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ 11: 35-40.
- Sperry, J. S and Love, D. M. (2015). What plant hydraulics can tell us about responses to climate-change droughts. New Phytol 207: 14-27.
- Trifilò, P., Raimondo, F., Lo Gullo, M. A., Barbera, P. M., Salleo, S. and Nardini, A. (2014). Relax and refill: xylem rehydration prior to hydraulic measurements favours embolism repair in stems and generates artificially low PLC values. Plant Cell Environ 37(11): 2491-2499.
- Wheeler, J. K., Huggett, B. A., Tofte, A. N., Rockwell, F. E. and Holbrook, N. M. (2013). Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ 36(11): 1938-1949.
- Zhang, Y., Lamarque, L. J., Torres-Ruiz, J. M., Schuldt, B., Karimi, Z., Li, S., Qin, D. W., Bittencourt, P., Burlett, R., Cao, K. F., Delzon, S., Oliveira, R., Pereira, L. and Jansen, S. (2018). Testing the plant pneumatic method to estimate xylem embolism resistance in stems of temperate trees. Tree Physiol 38(7): 1016-1025.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bittencourt, P. R. L., Pereira, L. and Oliveira, R. S. (2018). Pneumatic Method to Measure Plant Xylem Embolism. Bio-protocol 8(20): e3059. DOI: 10.21769/BioProtoc.3059.
Category
Plant Science > Plant physiology > Abiotic stress
Plant Science > Plant physiology > Water transport
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










