- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Drosophila Endurance Training and Assessment of Its Effects on Systemic Adaptations
(*contributed equally to this work) Published: Vol 8, Iss 19, Oct 5, 2018 DOI: 10.21769/BioProtoc.3037 Views: 7688
Reviewed by: Sunanda MarellaDivya SitaramanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
![Proboscis Extension Reflex in <em>Apis mellifera</em> [Honeybee] with Only One Antenna](https://en-cdn.bio-protocol.org/imageup/arcimg/20171129091737284.jpg?t=1770531543)
Proboscis Extension Reflex in Apis mellifera [Honeybee] with Only One Antenna
Yu Guo [...] Runsheng Chen
Dec 5, 2017 7315 Views

Flight and Climbing Assay for Assessing Motor Functions in Drosophila
Steffy B Manjila and Gaiti Hasan
Mar 5, 2018 12673 Views

Testing for Assortative Mating by Diet in Drosophila melanogaster
Philip T Leftwich and Tracey Chapman
Oct 20, 2018 5667 Views
Abstract
Exercise induces beneficial systemic adaptations that reduce the incidence of age-related diseases. However, the molecular pathways that elicit these adaptations are not well understood. Understanding the molecular mechanisms that underlie the exercise response can lead to widely beneficial therapies. Large populations, relatively short lifespan, and easily modifiable genetics make Drosophila a well-suited model system for complex, longitudinal studies. We have developed an enforced climbing apparatus for Drosophila, known as the Power Tower, for the study of systemic exercise adaptations. The Power Tower takes advantage of the fly’s natural instinct for negative geotaxis, an innate behavior to run upwards after being tapped to the bottom of their vial. Flies will continuously run either to the point of exhaustion or until the machine is turned off, whichever comes first. After 3 weeks of exercise, male Drosophila adapt to training with a number of conserved, easily quantifiable physiological improvements similar to those seen in mammalian models and humans. Here, we describe a useful endurance training protocol and a suite of post-training assessments that effectively quantify training effects.
Keywords: Drosophila melanogasterBackground
Endurance exercise reduces the incidence of nearly every age-related disease (Ciolac, 2013). Endurance training has potent effects on cardiovascular function, energy metabolism, and mobility which are highly conserved from flies to humans (Piazza et al., 2009; Booth et al., 2015; Wilson et al., 2015). A better understanding of genetic mediators of exercise may lead to therapeutics that can benefit individuals that are unable to exercise due to illness or injury. Drosophila is a well-equipped model organism to help identify genetic mediators of exercise due to its short lifespan, easily modifiable genetics, and well-defined exercise response (Piazza et al., 2009).
We created a machine called the Power Tower, which utilizes Drosophila’s natural instinct for negative geotaxis to induce endurance training (Tinkerhess et al., 2012a). Wild-type male Drosophila respond to endurance training with increased endurance, speed, flight ability, resistance to cardiac stress, and increased lysosomal activity when compared to unexercised control flies (Piazza et al., 2009; Sujkowski et al., 2012; Tinkerhess et al., 2012b; Sujkowski et al., 2017). Here, we describe an updated endurance training protocol and a suite of post-training assessments that are useful for analyzing exercise adaptations. This suite of assessments has led to the identification of genes required for the exercise response in Drosophila, such as spargel (PGC-1α homolog) (Tinkerhess et al., 2012b) and to the identification of essential physiological mediators of the exercise response such as the biogenic amine octopamine (Sujkowski et al., 2017).
Our ramped endurance training protocol consists of three weeks of training, with flies being exercised daily for five consecutive days (Table 1), followed by two days of rest. Exercise adaptations can be assessed using a suite of five complementary assessments: fatigue, longitudinal climbing assessment, flight, cardiac pacing, and LysoTracker staining. The fatigue, flight, and longitudinal climbing assessments examine the flies’ endurance, flying ability, and speed, respectively. Cardiac pacing evaluates the heart’s ability to resist stress (Wessells and Bodmer, 2004). LysoTracker staining measures lysosomal activity, which increases in adipose tissue with exercise (Sujkowski et al., 2012). Together, these assessments constitute a collection of diverse responses to chronic exercise that can be used to evaluate potential genetic or pharmaceutical exercise mimetics.
Materials and Reagents
- Microscope Slides (Fisher Scientific, FisherbandTM, catalog number: 12-550-343 )
- Cover Slips (Fisher Scientific, FisherbandTM, catalog number: 12-548B )
- Aluminum Foil
- Drosophila Vials (VWR, catalog number: 89092-722 )
- Clear Polycarbonate Sheet (McMASTER-CARR, catalog number: 85585K25 )
- Plastic Funnel (Fisher Scientific, FisherbrandTM, catalog number: 10-500-3 )
- Polycarbonate Cylinder (McMASTER-CARR, catalog number: 8585K62
- Acrylic Cylinder Tube (36” length, 4” diameter) (McMASTER-CARR, catalog number: 8486K943 )
- Polystyrene Weighing Dish (Fisher Scientific, FisherbrandTM, catalog number: S67091A )
- Drosophila (Bloomington Drosophila Stock Center: https://bdsc.indiana.edu/)
- Carbon Dioxide
- Tangle Trap (BIOCONTROL NETWORK, catalog number: 268941 )
- Mineral Oil (Fisher Scientific, BioReagents, catalog number: BP26291 )
- FlyNap (Carolina Biological Supply, catalog number: 173025 )
- Signa Gel Electrode Gel (Parker Laboratories, SIGNAGEL®, catalog number: 15-60 )
- LysoTracker Green (Thermo Fisher Scientific, Molecular Probes®, catalog number: L7526 )
- Phosphate Buffered Saline (pH 7.0)
- VectaShield (Vector Laboratories, catalog number: H-1000 )
- Fly Food made by experimenter (see Recipes)
Equipment
- Power Tower
- 40” x 24” Plywood Board (Base)
- AC/DC Gearmotor (W.W. Grainger, model: 1LRA6 )
Manufacturer: Dayton, model: 1LRA6 . - Rigid Shaft Coupling (Climax Metal Products, model: ISCC-025-025-S )
- 12” x 5” x 1½” Boards x 4
- 12” x 5” x ¾” Plywood Board
- AC/DC Speed Control (Dayton, model: 4Z827 )
- 20 AMP Fuse (Cooper Bussmann, item number: 286547)
- Bent ¾” Square Tube Stock
- 14” x 11” Plywood Board x 2 (Platforms)
- Door Hinge
- Drawer Sliders x 4
- Styrofoam Pad x 2
- C-Clamp (W.W. Grainger, model: 2HUK2 )
Manufacturer: WESTWARD, model: 2HUK2 . - Nalgene Unwire Test Tube Racks x 4 (Thermo Fisher Scientific, ResmerTM, catalog number: 5970-0013PK )
- Shelf Bracket x 4
- Skateboard Wheel
- Jaece Identi-plugTM Plastic Foam Stopper (Jaece Industies, catalog number: L800-B2 )
- Incubator
- Square Rectangular Grid Screen (W.W. Grainger, model: 49N590)
Manufacturer: DIRECT METALS, model: 12002E063Y-48X96 . - 10" Bungee Cord (Home Depot Product Authority, model: 56052 )
- Bel-Art No Wire Test Tube Rack (SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: F18745-0004 )
- Traceable Nano Timer (Fisher Scientific, FisherbrandTM, catalog number: 14-649-83 )
- Ring Stand x 2 (Eisco Labs, catalog number: CH0653E1RD4 )
- Claw Clamps (Fisher Scientific, FisherbrandTM, catalog number: 05-769-7Q )
- Chain Clamps x 2 (VWR, catalog number: 21573-275 )
- Three-prong Extension Clamp (Fisher Scientific, FisherbrandTM, catalog number: 05-769-7Q )
- Isolated Square-Wave Stimulator (Phipps & Bird, catalog number: 7092-611 )
- Olympus Camera (OLYMPUS, model: SP-570 UZ )
- Dissecting Microscope (OLYMPUS, model: SZ61 )
- Fluorescent Microscope (Leica Microsystems, model: DMI6000 B )
- Hemostat Straight (Specialized Products, catalog number: 083X020 )
Software
- ImageJ (National Institutes of Health, Bethesda, Maryland, USA)
Procedure
- Power tower setup
- See Figure 1 for Power Tower assembly. For a video of the Power Tower in motion, see Video 1.
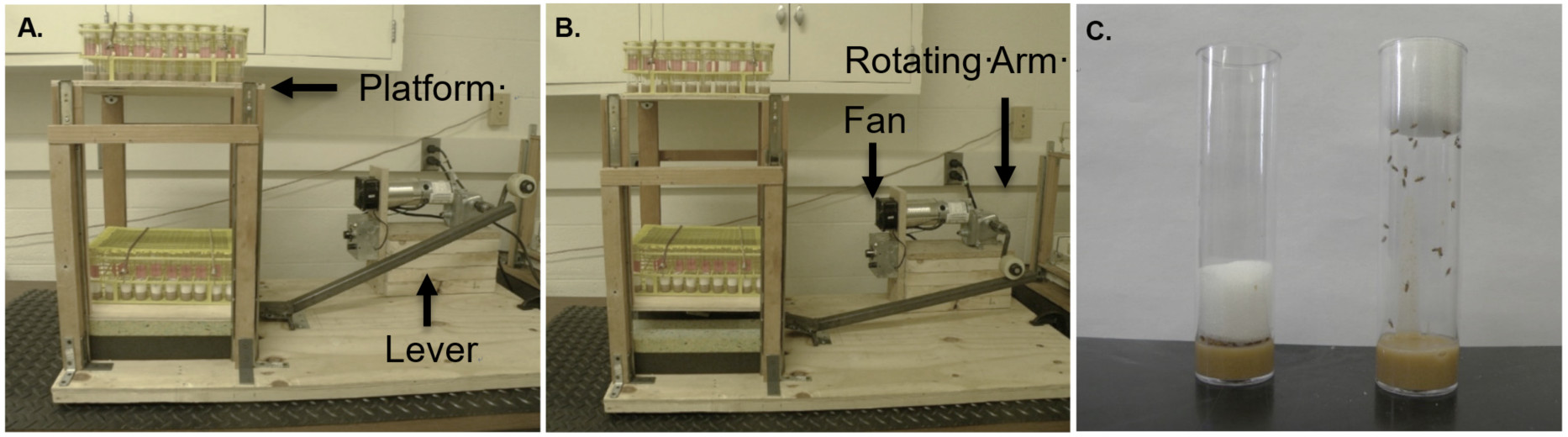
Figure 1. The Power Tower initiates negative geotaxis in Drosophila. A. The Power Tower in its resting position. The lower tier of the Power Tower contains vials with the foam stopper pushed down to prevent any running. The vials on the top tier have the foam stopper up, which allows the flies to run. B. The rotating arm presses down on the lever, which elevates the Power Tower platforms. When the rotating arm releases the lever, the platforms drop, which knocks the flies off the sides of the vials onto the food. After the platforms drop, flies begin to climb. C. Flies in the exercised group go on the machine with their foam stopper up (right), while flies that are unexercised have their foam stopper pushed down (left).Video 1. Power Tower exercise device - The Power Tower is placed on a rectangular 40”x 24” plywood board that is secured to a table with a C-clamp. A 4.5 RPM Grainger gearmotor rests on top of four 2” x 6” boards and is held in place on top of a 12” x 5” x ¾” plywood board with three bolts. The motor, fuse, and speed control are wired together in a juncture box that is secured to the side of the four boards with two screws.
- Use two 14” x 11” plywood boards to create two platforms. Place two vial racks (40 vials per rack; 160 vials/Power Tower) on each platform and secure them in place by a screw in each corner.
Note: The number of vial racks can be increased to three to accommodate more vials. We have added three vial racks to some of our Power Towers so that a total of 240 vials can be placed on them. This allows for larger experiments to be performed, since more vials can be exercised at the same time. - Attach a bent ¾ inch square tube stock to the plywood with a door hinge at the bend. A wheel is attached to the top side of the tube stock beneath the platform allowing the above platform to roll against it.
- The platforms slide up and down on drawer sliders that are attached to the plywood base with shelf brackets. Underneath of the bottom platform, a 3-inch-thick Styrofoam pad is placed on each side of the metal tube stock to cushion the landing. Styrofoam pads can be secured by two screws on each side or glued to the base to prevent them from shifting.
- Connected to the motor is a custom-made “S” shape rotating arm with another wheel attached. The rotating arm with wheel is attached to the motor with a Grainger rigid shaft coupling.
Note: Any wheel may work. We found that the size, circumference, and durability of a skateboard wheel works best rather than smaller metal wheels. - When the motor is turned on the custom-made rotating arm with wheel will push down one side of the ¾ inch square tube stock. This gradually lifts the platforms up at the other end of the tube stock. When the rotating arm releases the bent stock tube, the platforms drop to its original position (Video 1).
- The speed can be adjusted to the desired setting. Our Power Tower is set to a speed where a drop occurs approximately every 7 sec.
- A fan is used to cool the motor. To attach the fan, a 3-inch diameter hole is cut into a vertical ½ inch piece of plywood. A 3-inch fan is attached to the plywood on one side. On the other side of the 3-inch diameter hole is a metal cylinder, which should point at the motor.
Note: We use a metal cylinder to help focus the cool air from the fan directly onto the motor.
- See Figure 1 for Power Tower assembly. For a video of the Power Tower in motion, see Video 1.
- Endurance training protocol
- Anesthetize flies using CO2 and collect 1,060 males for each genotype. Female flies have a blunted exercise response (Sujkowski et al., 2017), so males are better for evaluating genetic and pharmacological modifiers. Collect into 53 vials with each vial containing 20 flies (Figure 2). Distributing flies at lower density reduces the flies’ response to the Power Tower. To ensure age matching, all flies should be collected within three days.
Note: The minimum number of flies that needs to be collected is 860 flies per genotype. However, in this case, the same flies would be used for the pre-training fatigue, post-training fatigue and the longitudinal climbing assessment (Figure 2).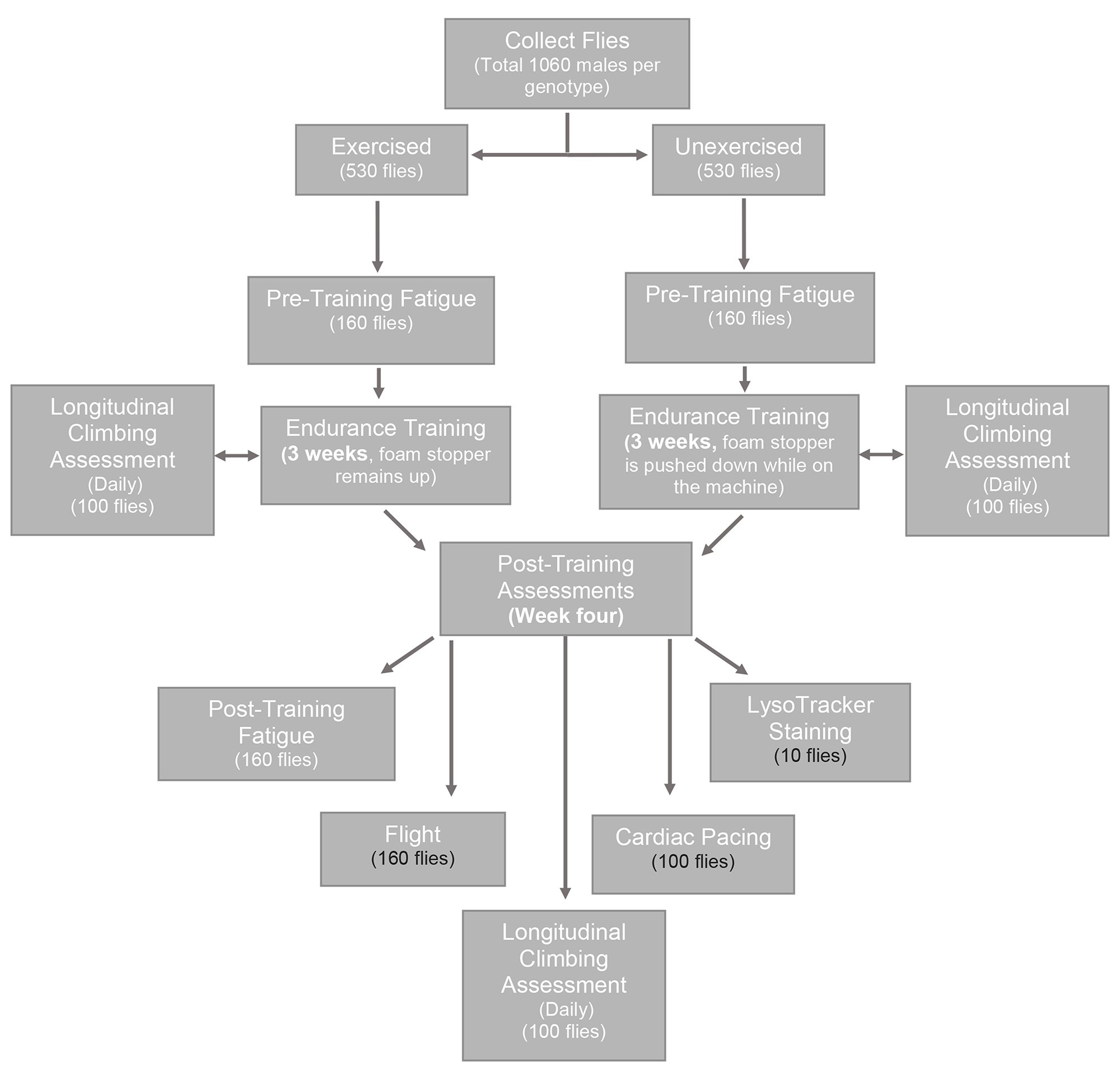
Figure 2. The work flow from collecting flies to experimental assessments. Prior to endurance training, a total of 1,060 male flies should be collected per genotype being assessed. Male flies are used because they exhibit a more robust exercise response than females. Flies should be divided into two groups, exercised and unexercised, with 530 flies in each group. After three weeks of endurance training, post-training assessments are performed during the fourth week (within one week after the last day of exercise). Lethal assessments are shown in black, then the assessment is lethal to the flies. It is possible to complete all of the experimental assessments with only 860 male flies per genotype, if flies from the longitudinal climbing assessment are also used for the fatigue assessment. - Divide each genotype into two equal groups: exercised (experimental) and unexercised (control) (Figure 2).
- Prior to exercise, flip flies into a new vial with fresh food. Flies left in used food vials tend to have a reduced response to negative geotaxis induction. The food provides a softer landing for the flies when they are knocked down by the Power Tower and reduces injury.
- Both exercised and unexercised flies are placed on the Power Tower. Unexercised flies are placed on the Power Tower to control for any non-specific machine effects. To restrict the movement of flies in the unexercised group push the foam stopper down toward the bottom of the vial leaving approximately 2-3 mm of space between the food and the foam stopper (Figure 1C). Start by continuously tapping the vial on a soft surface so flies are at the bottom of the vial. Then, push the foam stopper down towards the bottom of the vial. Pushing in the middle of the stopper with even pressure helps minimize trapped flies on the side of the vial. If flies are trapped on the side, use a tool like blunt forceps or a straight hemostat to release stuck flies. Pull the stopper towards the top of the vial until the flies become unstuck and then re-start pushing the stopper down.
- Place both the exercised and unexercised groups on the Power Tower. Our Power Tower has two tiers and there are no tier-dependent effects. Place the rectangular grid screen over the vials and secure the screen with bungee cords. Turn the Power Tower on for the required amount of time for that day (Table 1).
Note: Exercise training should be done at approximately the same time of day. We try to start our training as close to lights on as possible since this is the time flies are most active.
Table 1. Ramped Exercise Training Protocol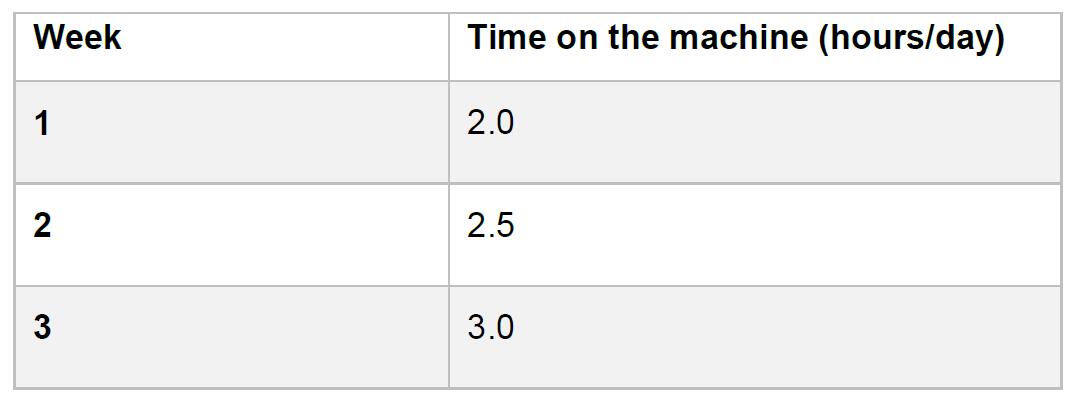
- When exercise is finished, turn the machine off and remove the flies from the Power Tower. Pull the foam stoppers to the top of vials of the unexercised group(s) by using blunt forceps or a straight hemostat and return flies to the incubator until the next training day.
- Maintain flies in a 25 °C incubator with a 12 h/12 h light/dark cycle.
- After three weeks of training perform the desired post-training assessments and complete them within one week of the last training day.
Note: Performance in post-training assessments may decrease if done more than one week after the last training day.
- Anesthetize flies using CO2 and collect 1,060 males for each genotype. Female flies have a blunted exercise response (Sujkowski et al., 2017), so males are better for evaluating genetic and pharmacological modifiers. Collect into 53 vials with each vial containing 20 flies (Figure 2). Distributing flies at lower density reduces the flies’ response to the Power Tower. To ensure age matching, all flies should be collected within three days.
- Fatigue assessment
- The fatigue assessment measures endurance and is conducted both pre- and post-exercise training (Figure 2). The post-exercise fatigue assessment is performed after the full three weeks of training.
Note: Perform the pre-fatigue at least 48 h after all flies are collected, since endurance is reduced in young flies (less than two days old). Effects from the pre-fatigue do not affect comparisons since flies from both exercised and unexercised cohorts perform the pre-fatigue assessment. Additionally, if flies are being used for other assessments, then they should rest at least 12 h before being used for other assessments to guarantee complete recovery and to ensure succeeding assessments are not impacted. - Flip eight vials of both exercised and unexercised flies from each genotype into new vials containing food.
Note: Count the number of flies in the vial prior to starting the fatigue. We start with 20 flies per vial for pre-training fatigue assessment. It is normal to experience some death during the exercise training protocol. After training, the number of flies per vial should be at least 5. If the number is lower than five, combine vials to reach an n ≥ 5. - Place those vials on the Power Tower and separate for optimal viewing. Do not push the foam stoppers down of the vials in the unexercised group. Secure the rectangular grid screen on top of the vials with bungee cords.
- Turn the Power Tower on and exercise the flies until fatigue. A vial is defined as fatigued when 80% or more of the flies have stopped climbing one centimeter or more up the vial before being knocked down again for three consecutive drops.
Note: Threshold for fatigue may be varied if experimental flies have a motor defect that diminishes their climbing ability. - Remove fatigued vials from the Power Tower while keeping the Power Tower running and record the time at which the vial was removed. Vials are treated statistically as individuals.
- Initially, monitor vials approximately once every hour. As vials appear to be nearing fatigue, they should be checked more frequently.
Note: Flies experience substantial aging during the three weeks of training, so their endurance is not higher after training than before. However, it is typically much higher than unexercised, age-matched controls. - We perform this assessment in triplicate and look for general trends between the replicates. For example, if genotype A ran significantly shorter than genotype B, we would look for this same rank order in the consecutive replicates.
Note: In our experience, the eight vials tested for this assessment accurately represents the endurance of the population.
- The fatigue assessment measures endurance and is conducted both pre- and post-exercise training (Figure 2). The post-exercise fatigue assessment is performed after the full three weeks of training.
- Flight assessment
- The flight assessment is modified from Babcock and Ganetzky (2014).
- Fasten a large cast acrylic tube to the rod of one ring stand with chain clamps and place it upright on the floor. Secure a plastic funnel to the second ring stand with a ring clamp. Affix a polycarbonate cylinder (drop tube) to the rod with a three-prong extension clamp just above the funnel and place on a countertop above the first support stand. Align the center of the drop tube with the center of the acrylic tube (Figure 3).

Figure 3. Flight assessment apparatus. Vials of flies are dropped through the cylindrical tube at the top of the apparatus. A ring stand holds this tube in place with a funnel. When the vial hits the bottom of the cylindrical tube flies will be ejected out of the funnel and into the acrylic tube that holds the polycarbonate sheet lined with Tangle-Trap. Flies will land along the tube and stick to their landing spot due to the Tangle-Trap. - Cut a polycarbonate sheet so that it is slightly longer than the height of the acrylic tube. Place the polycarbonate sheet on the countertop and coat one side with a thin layer of Tangle-Trap.
- Roll the sheet into a cylinder and insert it into the acrylic tube.
- Place a polystyrene dish filled with mineral oil on the support stand below the large cylinder, which will catch any of the flies that don’t fly to the sides.
- Tap a single vial of flies so that flies drop to the bottom of the vial. Quickly remove the foam stopper, flip the vial upside down, and drop it into the drop tube. Flies will drop into the acrylic cylinder tube and instinctively fly toward the sides where they will stick to the Tangle-Trap. Test 8 vials per cohort.
- Remove the dish of mineral oil. Photograph flies that fell through to mineral oil. Flies that fail to fly are censored to control for damage to wings from Power Tower treatment.
- Remove the polycarbonate sheet from the acrylic tube and secure it against a white background with a yard stick at the bottom.
- Photograph polycarbonate sheet with a digital camera (we use a standard Olympus digital camera). Ensure that the whole sheet is in the frame (Figure 4).
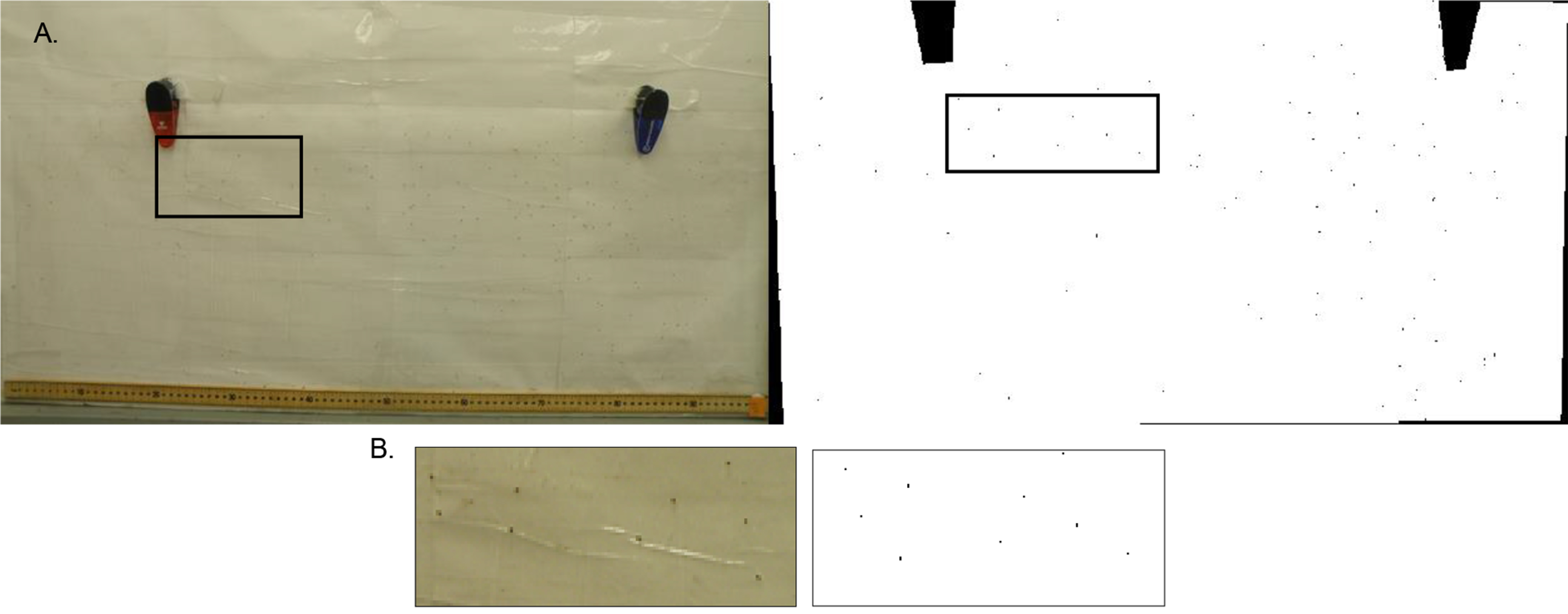
Figure 4. Example of photographs for flight assessment. A. Flight ability determines where flies land along the polycarbonate sheet. Use a digital camera to capture the landing height of the flies and use an imagining software (such as ImageJ) to adjust the contrast of the image so flies appear as black dots (right image). B. The black box on the images in (A) represent the area that is enlarged in (B). Before analysis, confirm the black dots (right) correspond with flies (left). - Remove all flies from the sheet with forceps, re-spread the Tangle-Trap so it is even, and place the sheet back into the acrylic tube.
- Repeat Steps D6-D10 for the remaining cohorts.
- Longitudinal climbing assessment
- This assessment is used to examine the longitudinal decline of climbing speed and should be done three times a week both during training and post-training (Figure 2). This method is modified from (Gargano et al., 2005).
Note: This assessment should be completed prior to the exercise training session for that day and flies should be returned to the group for training after the assessment. - Randomly select five vials from each group for this assessment.
Note: The same five vials will be used throughout the experiment in order to accurately measure the longitudinal decline of climbing speed. - Place a Bel-Art No-Wire rack against a white background or tape white printer paper around the back and sides of the rack.
- Place camera 20 cm away from the front of vial rack. Keep the distance consistent throughout the experiment.
- Flip five vials of flies from one cohort into five new empty vials.
Note: Flies climb better in fresh vials, so it is imperative to flip flies into new vials every day in order to measure climbing speed accurately. - Place the vials into the front row of the vial rack and secure them to the bottom with tape (Figure 5). Place the same vial in the identical spot of the vial rack each day the assessment is performed. This allows you to track the climbing speed of each individual vial.
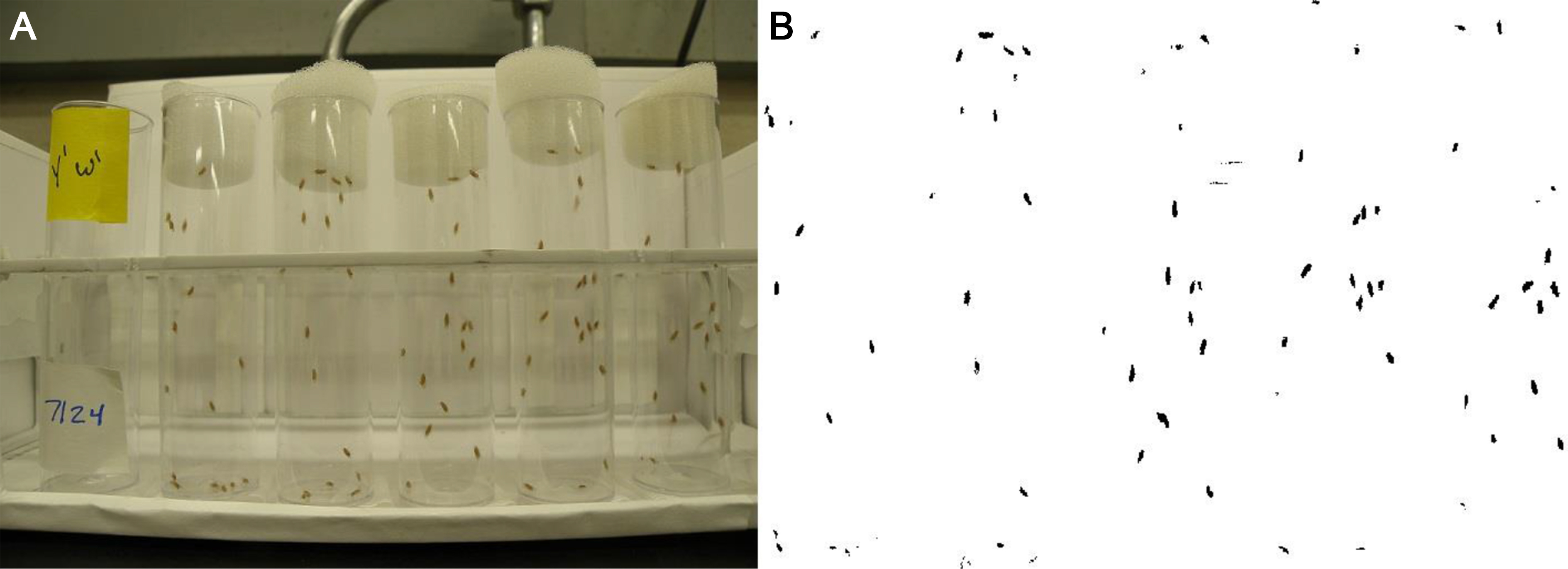
Figure 5. Longitudinal climbing assessment. A. Setup and sample photograph of the longitudinal climbing assessment. B. High contrast version of image (A) that is used for data analysis using ImageJ. - Set a timer to count down from 2 sec.
- Lift the vial rack up and hit it down onto the surface of the tabletop until all the flies drop to the bottom of the vials.
- Start the timer as soon as the flies drop to the bottom of the vials.
- Photograph immediately after the timer counts down from 2 sec.
- Repeat Steps E6-E9 three more times totaling four pictures for that group.
- Photograph 4x per cohort per day on each training day and any additional days after completion of training. The average climbing index for all five vials per picture will be calculated and used for analysis.
- This assessment is used to examine the longitudinal decline of climbing speed and should be done three times a week both during training and post-training (Figure 2). This method is modified from (Gargano et al., 2005).
- Cardiac pacing
- Cardiac pacing is an assessment for cardiac stress resistance (Wessells and Bodmer, 2004). Prepare microscope slides by covering each end with aluminum foil. Leave a small gap (approximately 2 mm) in the middle and solder electrical wires to the foil. Connect the wires to the stimulus output on an isolated square-wave stimulator and set the amplitude to 40 V and frequency to 6 Hz (Figure 6).
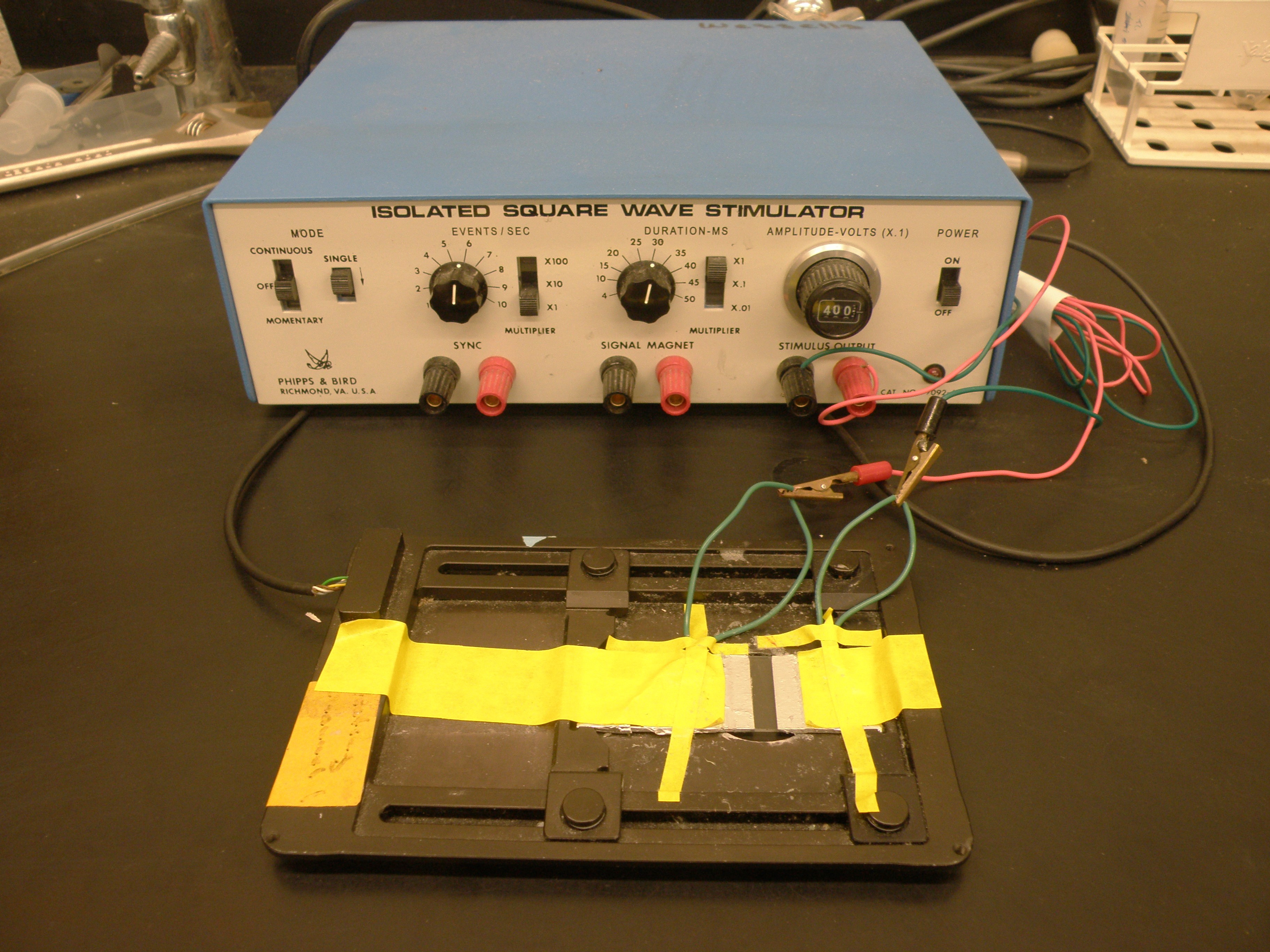
Figure 6. Cardiac pacing setup. Displayed is a temperature-controlled slide holder. The slide is held in place by tape. Electrodes are welded to the top of the slide and connected to the square wave stimulator. - Place conductive jelly along the foil in two rows and make sure the jelly does not touch (Figure 7). Replace the conductive jelly after three groups to avoid contact.
Note: After three groups, it is advised to replace the conductive jelly to avoid contact between the two rows. In warmer temperatures, the jelly is more likely to be more fluid and is more likely to touch. - Anesthetize experimental flies with FlyNap for 2 min. 100 flies per cohort will be used.
Note: Ether or carbon dioxide should not be used to anesthetize because they alter heart function. - Place flies ventral side down so their heads are in bedded in one row of jelly and their abdomens are in the other row (Figure 7).
Note: For better viewing place the flies’ ventral side up if using an inverted scope.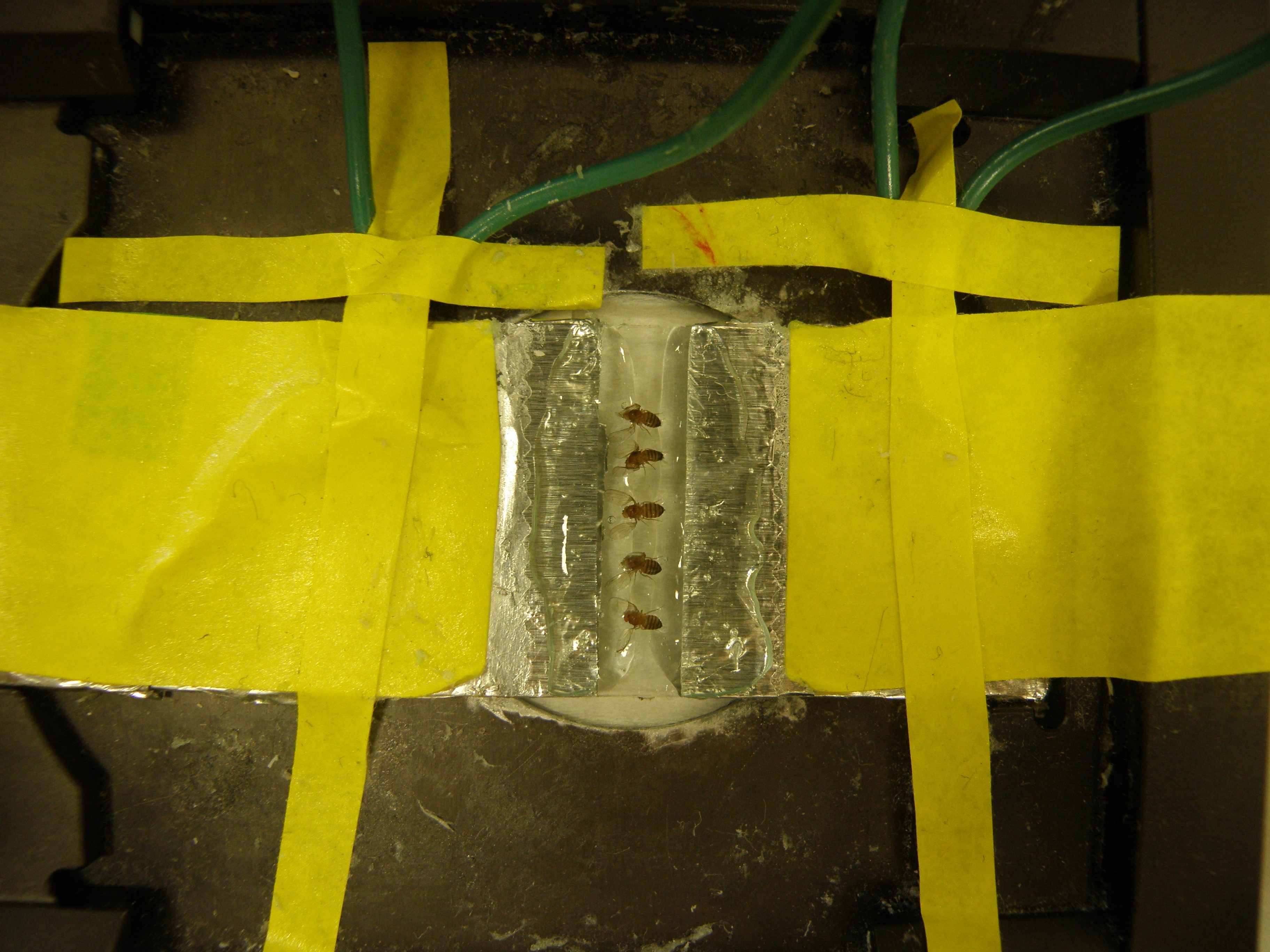
Figure 7. Flies placed in conductive jelly on cardiac pacing slide. The conductive jelly is touching the foil. The gap in the middle of the conductive jelly guarantees that the current goes through the flies, which paces the heart. - Set the stimulation to continuous and turn on the square-wave stimulator for 30 sec.
After 30 sec, turn the square-wave stimulator off and use a microscope (any microscope with 10x magnification will work) to visually score how the hearts perform. If the heart walls are not contracting (arrest) or are not moving in sync (fibrillating), the heart is scored as failing. If the heart walls are beating in synchronous, productive contractions (regardless of speed) the heart is scored as not failing. For a video of the heart beating in an intact animal, see Video 2.
Note: Young flies’ failure rate is about 25%-30% while older flies (3 weeks of age) have a failure rate around 65%-70%. Exercise is known to help preserve cardiac function. Thus, exercised wild type flies will have a lower failure rate than age-matched unexercised flies (Sujkowski et al., 2015).Video 2. Drosophila heart beating
- Cardiac pacing is an assessment for cardiac stress resistance (Wessells and Bodmer, 2004). Prepare microscope slides by covering each end with aluminum foil. Leave a small gap (approximately 2 mm) in the middle and solder electrical wires to the foil. Connect the wires to the stimulus output on an isolated square-wave stimulator and set the amplitude to 40 V and frequency to 6 Hz (Figure 6).
- LysoTracker staining
- LysoTracker staining measures lysosomal activity, which increases in adipose tissue following exercise (Sujkowski et al., 2012).
- We dissect flies as described by Vogler and Ocorr (2009), but we use PBS, pH 7.0, instead of artificial hemolymph. Briefly, dissect flies ventral side up, expose the fat bodies and remove any extraneous tissue. Work quickly, letting samples sit for no longer than 1 h at room temperature.
Note: Using PBS with a pH 7.0 is imperative for accurate staining. - Wash 1x in PBS pH 7.0.
- Dilute LysoTracker Green to 0.01 μM in PBS, apply it to the sample (dissected abdominal fat) and allow it to sit for 45 sec.
Note: LysoTracker comes in different colors and a different color of LysoTracker may be used for this experiment. If a different LysoTracker dye is used, the wavelength of light used to view the adipose tissue must match. - Wash 3x in PBS.
- Remove the heart with the adipose tissue attached and mount onto a slide using VectaShield.
Note: The adipose tissue is removed easily when pulling the heart out of the carcass. For that reason, pulling the heart out with the adipose tissue is more efficient than trying to pull out pieces of adipose tissue. It is recommended to dissect and mount at least ten heart samples with adipose tissue attached. A minimum number of 5 samples is needed for quantification. - Image slides under 500-520 nm widefield florescence with a green fluorescence protein filter at 40x (Figure 8).
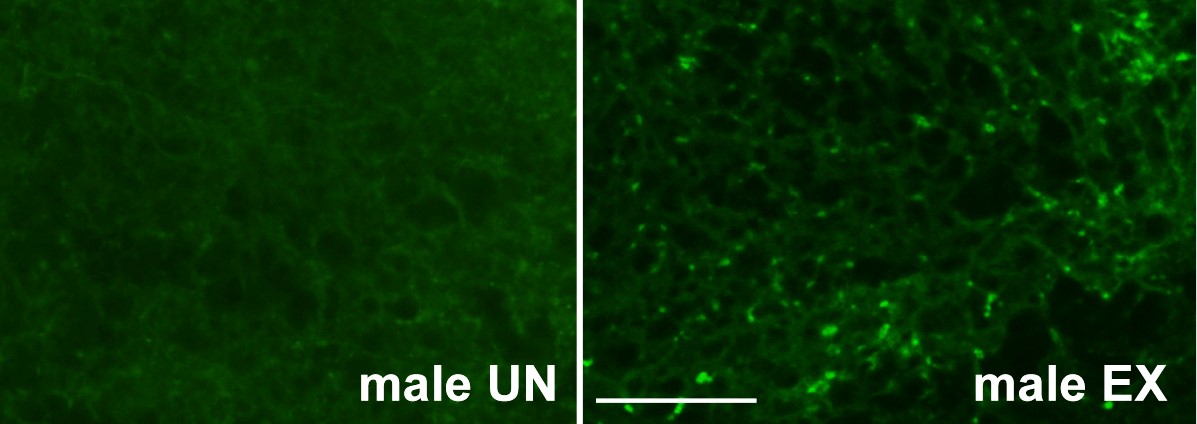
Figure 8. Image of LysoTracker staining. Displayed is a 40x image of LysoTracker staining of male unexercised and exercised wildtype flies. The bright green puncta in the male EX image indicates increased lysosomal activity. Scale bar = 50 μm.
Data analysis
To ensure repeatability and accuracy, it is recommended to repeat entire experiments along with controls in triplicate and not to compare data from various repetitions directly. Instead, look for results that are significant in multiple repetitions. This controls for any subtle environmental variations that may influence the quantitation of some assessments. While variations in the specific measured values are common, repetitions typically provide identical rank orders and statistical differences. We use ImageJ to analyze the flight assessment, climbing assessment, and LysoTracker staining. Prism is used to analyze data exported from ImageJ but any stats package can be used.
- Fatigue assessment
Each vial of 20 flies is recorded as a single datum. The time each vial reaches ‘fatigue’ is recorded in minutes and considered an event. Time-to-fatigue is plotted as a curve (sometimes called a runspan curve) and analyzed for significance by log-rank (Figure 9A). Alternatively, the mean time to fatigue can be compared between groups using either an ANOVA or a t-test. - Flight assessment
Flight is analyzed similarly to climbing using ImageJ. Photograph the unrolled sticky tube with flies still attached, being careful to keep track of which end is up. Set a scale by using the yard stick at the bottom of the picture as a reference. Adjust the contrast and brightness of the image (Figure 4), and parameters like pixel size and circularity, so only flies are detected as data points. Set the output to XY coordinates in order to determine the landing height of the flies. Export text file to a standard stats program for graphing and analysis (Figure 9B). Statistical analysis can be performed using either one-way ANOVA or t-test depending on the number of groups. - Longitudinal Climbing Assessment
High-contrast photographs are optimal for analysis. Adjust the photo’s brightness and contrast so that ImageJ will be able to clearly detect flies as dark spots on a bright background (Figure 3). Adjust parameters, like pixel size and circularity, so that ImageJ recognizes black dots as data points. Use ImageJ to calculate XY coordinates of the flies once you have identified their location on the photograph. Data can be exported as a text file to a stats program for analysis.
We find it useful to convert the height climbed into a climbing index. To generate this climbing index, we divide the vials horizontally into four equal quadrants. The quadrants are given a quartile value of one through four, with the quadrant at the top of the vial having a value of four. The number of flies in each quadrant is counted, multiplied by the quartile value, and divided by the total number of flies to generate a quadrant score. Quadrant scores are then averaged to produce the climbing index for that picture. We use a climbing index to represent the data to account for non-specific sources of variance (e.g., flies moving diagonally instead of vertically up the vial).
If the main objective is to investigate the differences in climbing speed between genotypes, then the raw data (climbing index calculate above) should be used. If investigating the decline in climbing speed, then normalized data should be used. Climbing index can be normalized to the average values from the first 2-4 days of climbing. Climbing height typically declines with age, but chronic exercise slows age-related declines in climbing speed (Piazza et al., 2009). Normalized or raw climbing indexes can be plotted as data points in a line graph. Use a two-way ANOVA with a Bonferroni post-hoc correction to determine significance (Figure 9C). - Cardiac pacing
The percent of flies that failed following pacing is reported as percent failure rate. Significance is determined by a Chi-Square test for binary variables, where ‘1’ is considered a failure event and ‘0’ is considered a pass (Figure 9D). - LysoTracker staining
Crop the image to the desired area of analysis and use scale bar to set scale appropriately. Using a threshold function, use ImageJ to calculate the density of puncta in the selected area. Adjust the image’s brightness and contrast so that green puncta will be able to be detected as data points. Adjust parameters like pixel size and circularity so that only puncta are analyzed as data points in the output file. Ensure the output file reliably reflects appearance of lysosomes in photograph. Use one-way ANOVA or a Student’s t-test to determine significance between groups (Figure 9E).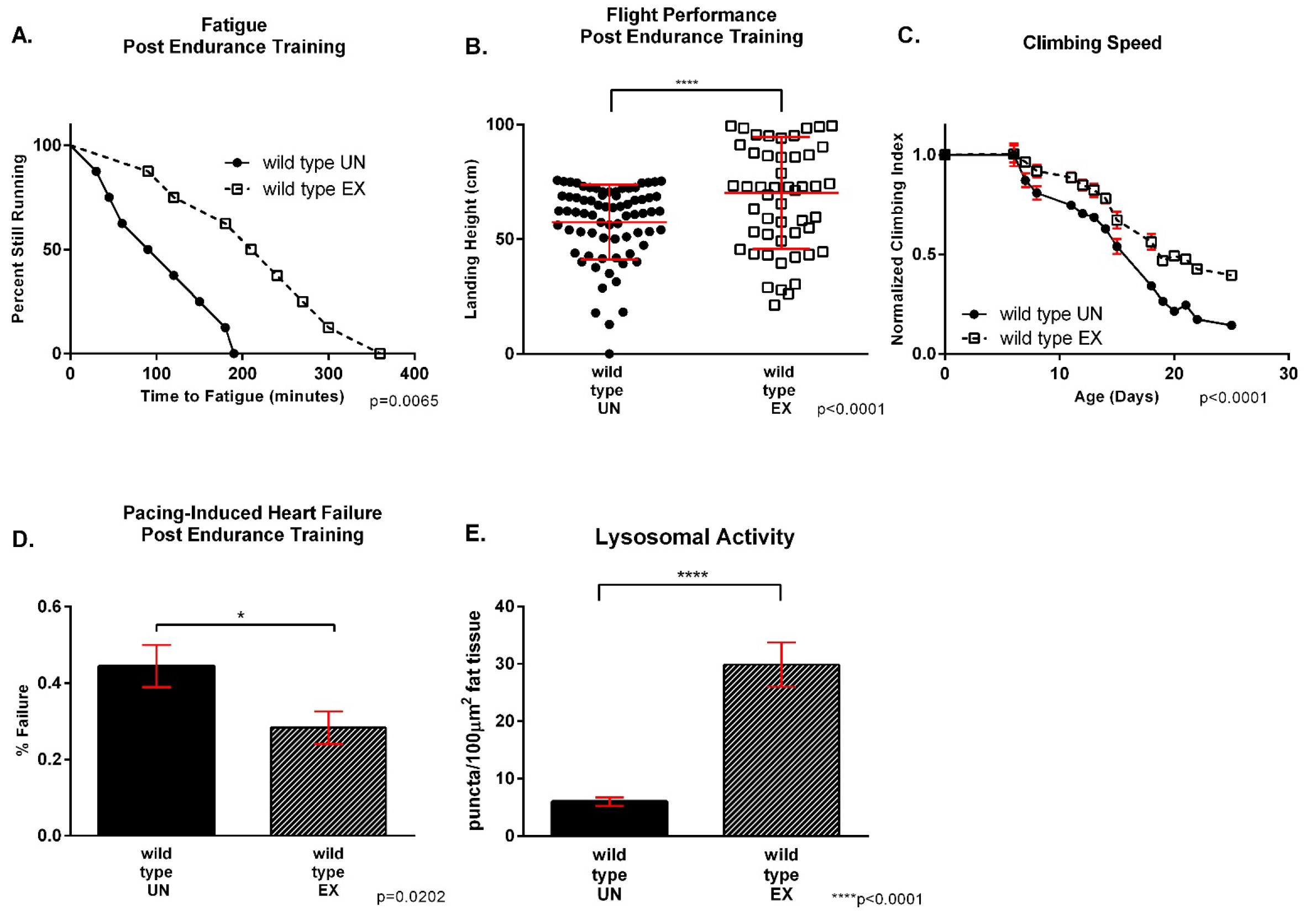
Figure 9. Example of wild type data post endurance training. A-B. Endurance trained flies run significantly longer (P = 0.0065, n = 160) and have better flight ability (P = 0.0006, n = 160) than age-matched untrained flies. C. Trained flies have increased climbing speed (P < 0.0001, n = 100). D. Trained wild type flies have increased resistance to cardiac stress (P = 0.02, n = 100). E. There is an increased number of puncta in fat tissue after LysoTracker in exercised wild type flies (P < 0.0001).
Notes
Endurance Training Response: Modifications to the ramped exercise protocol may be made, however the degree of training response may vary due to any modification. Diet, temperature, bacterial infection, genetic intervention and time on the machine are all factors that influence adaptations to exercise and may impact the training response. Additionally, various genetic backgrounds respond to endurance training to varying degrees. To get the best training response, we recommend that flies are healthy, clear of any bacterial infections, and to abort the experiment if bacteria or fungus becomes visible in vials during the training period.
Recipes
- Fly Food
10% yeast
10% sucrose
2% agar
0.1% propionic acid
0.1% methyl 4-hydroxybenzoate
Note: Variation to diet may alter exercise response.
Acknowledgments
We would like to acknowledge Tinkerhess et al. (2012a), Babcock and Ganetzky (2014), Gargano et al. (2005), and Wessells and Bodmer (2004) whose work was modified to produce the endurance protocol, flight assessment, longitudinal climbing assessment, and cardiac pacing, respectively. This work was supported by the NIH/NIA (R21 AG055712‐01 to R. W.).
Competing interests
We have no conflicts of interest to declare.
References
- Babcock, D. T. and Ganetzky, B. (2014). An improved method for accurate and rapid measurement of flight performance in Drosophila. J Vis Exp(84): e51223.
- Booth, F. W., Ruegsegger, G. N., Toedebusch, R. G. and Yan, Z. (2015). Endurance exercise and the regulation of skeletal muscle metabolism. Prog Mol Biol Transl Sci 135: 129-151.
- Ciolac, E. G. (2013). Exercise training as a preventive tool for age-related disorders: a brief review. Clinics (Sao Paulo) 68(5): 710-717.
- Gargano, J. W., Martin, I., Bhandari, P. and Grotewiel, M. S. (2005). Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 40(5): 386-395.
- Piazza, N., Gosangi, B., Devilla, S., Arking, R. and Wessells, R. (2009). Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One 4(6): e5886.
- Sujkowski, A., Bazzell, B., Carpenter, K., Arking, R. and Wessells, R. J. (2015). Endurance exercise and selective breeding for longevity extend Drosophila healthspan by overlapping mechanisms. Aging (Albany NY) 7(8): 535-552.
- Sujkowski, A., Ramesh, D., Brockmann, A. and Wessells, R. (2017). Octopamine drives endurance exercise adaptations in Drosophila. Cell Rep 21(7): 1809-1823.
- Sujkowski, A., Saunders, S., Tinkerhess, M., Piazza, N., Jennens, J., Healy, L., Zheng, L. and Wessells, R. (2012). dFatp regulates nutrient distribution and long-term physiology in Drosophila. Aging Cell 11(6): 921-932.
- Tinkerhess, M. J., Ginzberg, S., Piazza, N. and Wessells, R. J. (2012a). Endurance training protocol and longitudinal performance assays for Drosophila melanogaster. J Vis Exp(61): 3786.
- Tinkerhess, M. J., Healy, L., Morgan, M., Sujkowski, A., Matthys, E., Zheng, L. and Wessells, R. J. (2012b). The Drosophila PGC-1α homolog spargel modulates the physiological effects of endurance exercise. PLoS One 7(2): e31633.
- Vogler, G. and Ocorr, K. (2009). Visualizing the beating heart in Drosophila. J Vis Exp (31): 1425.
- Wessells, R. J. and Bodmer, R. (2004). Screening assays for heart function mutants in Drosophila. Biotechniques 37(1): 58-60, 62, 64 passim.
- Wilson, M. G., Ellison, G. M. and Cable, N. T. (2015). Basic science behind the cardiovascular benefits of exercise. Heart 101(10): 758-765.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Damschroder, D., Cobb, T., Sujkowski, A. and Wessells, R. (2018). Drosophila Endurance Training and Assessment of Its Effects on Systemic Adaptations. Bio-protocol 8(19): e3037. DOI: 10.21769/BioProtoc.3037.
Category
Neuroscience > Behavioral neuroscience > Animal model > Other
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









