- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
GC-MS-Based Analysis of Methanol: Chloroform-extracted Fatty Acids from Plant Tissues
Published: Vol 8, Iss 18, Sep 20, 2018 DOI: 10.21769/BioProtoc.3014 Views: 15437
Reviewed by: Scott A M McAdamBedabrata SahaAli Parsaeimehr

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Sorghum bicolor Extracellular Vesicle Isolation, Labeling, and Correlative Light and Electron Microscopy
Deji Adekanye [...] Jeffrey L. Caplan
Oct 5, 2024 2107 Views

A New Approach to Detect and Semi-quantify All Molecular Species and Classes of Anionic Phospholipids Simultaneously in Plant Samples
Manon Genva [...] Laetitia Fouillen
Apr 20, 2025 1718 Views

PhosphoLIMBO: An Easy and Efficient Protocol to Separate and Analyze Phospholipids by HPTLC From Plant Material
Louise Fougère [...] Yohann Boutté
Sep 5, 2025 1334 Views
Abstract
Fatty acids (FAs) are carboxylic acids with long aliphatic chains that may be straight, branched and saturated or unsaturated. Most of the naturally occurring plant FAs contains an even number of carbon (C4-C24). FAs are used in food and pharmacological industries due to their nutritional importance. In addition, FAs are considered as a promising alternative for the production of biodiesel from terrestrial plant biomass. To establish commercial applications, more reliable analytical methods are needed for the identification, quantification, and composition determination of FAs. Here, we describe a relatively rapid and sensitive method for the extraction, identification, and quantification of FAs from a small quantity of plant tissue. The method includes steps of lipid extraction, conversion of lipid to fatty acid methyl esters (FAMEs) by transmethylation, identification and quantification of FAMEs using gas chromatography-mass spectrometry (GC-MS). In this protocol, an internal standard is added prior to GC-MS analysis. The amount of each FA is calculated from its peak area relative to the peak area of the internal standard.
Keywords: BiodieselBackground
Synthesis of fatty acids is important for the storage of metabolic energy. The increasing population and energy cost have emphasized the need to produce sustainable renewable fuels. The source of second-generation biofuels is non-food oilseed crops or lignocellulosic biomass mainly comprising of wastes of crop plants like perennial grasses including switchgrass, husks, straw and forest residue (Hadar, 2013). In this context, plants can serve as an excellent system to study fatty acid for nutraceutical and biodiesel aspects. Further, in biodiesel production, clean burn properties of the fuel are influenced by structural features of FAs including chain length and the degree of unsaturation (Knothe, 2005). Lignocellulosic biomass is a greener alternative to produce these products directly from cost-effective resources. FA profiling using GC-MS permits the normalization, annotation and quantification of a relatively wide variety of fatty acids in a single plant extract. The efficiency of lipid extraction depends on the polarity of the solvent. Polar lipids (such as glycolipids or phospholipids) are more soluble in polar solvents (such as alcohols) and non-polar lipids (such as triacylglycerols) are more soluble in non-polar solvents (such as chloroform). Thus, the total lipid extraction depends on the nature of the organic solvent. Bligh and Dyer (1959) established a method for total lipid extraction using the mixture of chloroform and methanol as a solvent. The total lipids were converted to fatty acid methyl ester by transmethylation (Carreau and Dubacq, 1978). In one study, it was observed that the solvent system of chloroform/methanol is very effective for lipid extraction (Sheng et al., 2011). Moreover, lipid recovery in terms of total lipid content, lipid class and FA composition of different microalgal extracts are also affected by different types of pretreatment and cell disruption techniques and solvents (Ryckebosch et al., 2012). The chloroform/methanol mixture was also found to be useful for the extraction of lipids from microalgae (Ryckebosch et al., 2012). Similarly, there are several other reports suggest that chloroform/methanol/phosphate buffer-based solvent system has a higher efficiency for the extraction of lipids from different microalgae and plants (Kumari et al., 2013; Mishra et al., 2015; Pandey et al., 2015; Patel et al., 2016). Chloroform/methanol mixture exhibits strong dissolving power for the entire range of polarity found in lipids. This mixture is also able to break up membranes and denature the proteins (Schreiner, 2006). The buffer helps to overcome the ionic adsorption effects of salt that may hinder lipid extraction in plant tissue. In most of the studies, methanolic NaOH and methanolic HCl were found to be appropriate derivatizing agents for the profiling of FAs in plants.
Based on this information, here we provide a detailed protocol for extraction of lipids, identification, and quantification of fatty acid methyl esters. We also provide a detailed formula to estimate the total saturated fatty acids (SFA), unsaturated fatty acids (MUFAs and PUFAs), unsaturation index (Poerschmann et al., 2004), degree of unsaturation (Ramos et al., 2009), atherogenic and thrombogenic indices (Simat et al., 2015). Analyses of FAs are done by GC-MS. This protocol provides a relatively rapid and reproducible method. Moreover, this method can be used to profile fatty acids from different types of plant tissues. The produced information can be useful in several contexts of nutraceutical, pharmacological and industrial purposes.
Materials and Reagents
- 5 ml, 2 ml and 1 ml glass pipettes (Borosil)
- Filter paper 40 (GE Healthcare, Whatman, catalog number: 1440-110 )
- 50 ml graduated centrifuge tube, PP (Tarsons, catalog number: 546041 )
- 2 ml GC vials and caps (Agilent Technologies, catalog number: 5190-2240 ) with 250 μl glass inserts (Agilent Technologies, catalog number: 5181-1270 )
- Pyrex culture tube, screw cap with PTFE liner (Corning, catalog number: 9826-13 )
- Plant tissue (Leaf, Stem, Root, and Fruit)
- Liquid nitrogen
- Nitrogen gas (> 99% Purity)
- HPLC-grade methanol (Merck, catalog number: 1060020500 )
- HPLC-grade chloroform (Merck, catalog number: 1024470500 )
- HPLC-grade water (Avantor Performance Materials, J.T. Baker, catalog number: 1.00577.2500 )
- HPLC-grade n-Hexane (Sigma-Aldrich, catalog number: 34859 )
- Potassium phosphate monobasic, ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: P0662 )
- Potassium phosphate dibasic, ACS reagent, ≥ 98% (Sigma-Aldrich, catalog number: P3786 )
- Sodium hydroxide (Sigma-Aldrich, catalog number: S8045 )
- F.A.M.E. Mix, C4-C24 (Sigma-Aldrich, catalog number: 18919-1AMP )
- Nonadecanoic acid (Sigma-Aldrich, catalog number: N5252 )
- Solvent extraction solution A (see Recipe 1)
- Solvent extraction solution B (see Recipe 2)
- Methanolic NaOH (see Recipe 3)
- Methanolic HCl (see Recipe 4)
- Internal standard (see Recipe 5)
Equipment
- 50 ml Flask (Borosil)
- Funnel (Borosil)
- Table-top centrifuge (Sigma Laborzentrifugen, model: 3-30KS )
- Sample concentrator (Hangzhou Allsheng Instruments, catalog number: MD200-2 )
- VacSeal liquid nitrogen dewar (Jencons-PLS, catalog number: 238-112 )
- Table-top spinix-vortex (Tarsons, catalog number: 3002 )
- Rotospin-Rotary mixer (Tarsons, catalog number: 3092 )
- Shaking water bath (JULABO, catalog number: SW23 )
- -20 °C New Brunswick premium freezers (Eppendorf)
- -80 °C New Brunswick premium freezers (Eppendorf)
- GC-MS (Shimadzu, model: GCMS-QP2010 ) coupled with mass spectrometer and equipped with an auto-sampler (AOC-5000) and flame ionization detection (FID)
- Balance (Sartorius, model: BSA224S-CW )
- The RTx-5MS capillary column (60 meters, 0.25 mm ID, and 0.5 μm df) (Rastek, catalog number: 13455 )
Software
- GC-MS solution Version 2.70, Post-run analysis
- Excel software (Microsoft office 2010)
Procedure
- Extraction of lipid from plant tissue (Figure 1)
- Collect the plant tissue (Leaf/Stem/Root/Fruit) and quickly freeze the sample in liquid N2. To perform absolute quantification of fatty acids, determine the exact fresh weight (FW) of plant material quickly and accurately before freezing the sample. Store the sample at -80 °C in foil until further use.
Critical Step: Sufficient plant material is necessary for the yield of satisfactory GC-MS signals. Usually, 100.00 to 500.00 mg tissue is enough for the analysis of fatty acid content. In this protocol, ~200 mg tissue is collected as one sample.
Pause Point: Frozen samples can be stored at -80 °C. - Homogenize ~200.00 mg of plant tissue in liquid N2 using mortar pestle and transfer the powder to a 50 ml graduated centrifuge tube.
- Add 5 ml of pre-cooled (4 °C) solvent extraction solution A (see Recipe 1) and spinix-vortex vigorously for 3 min.
- Centrifuge the sample for 10 min at 10,000 x g at 4 °C and collect the supernatant in a 50 ml flask A.
- Add 2.5 ml of pre-cooled solvent extraction solution B (see Recipe 2) to the pellet and vortex vigorously.
- Centrifuge sample for 10 min at 10,000 x g at 4 °C and transfer the supernatant in 50 ml flask A (Repeat the Steps A5-A6).
- Filter the pooled supernatant by gravity filtration method through Whatman filter paper 40 using a funnel and wash by mixing with an equal volume of potassium phosphate buffer (50 mM; pH-7.5).
- Centrifuge the mixture for 10 min at 10,000 x g at 4 °C and collect the lower organic phase into a 50 ml graduated centrifuge tube. Collect the lower phase with a glass pipette.
- Dry total lipids under N2 using a sample concentrator and determine total lipid contents gravimetrically.
Pause Point: Total dried lipid can be stored at -20 °C prior to further analysis.

Figure 1. Workflow of extraction of total lipid from plant tissue
- Collect the plant tissue (Leaf/Stem/Root/Fruit) and quickly freeze the sample in liquid N2. To perform absolute quantification of fatty acids, determine the exact fresh weight (FW) of plant material quickly and accurately before freezing the sample. Store the sample at -80 °C in foil until further use.
- Conversion of lipid to fatty acid methyl esters (FAMEs) by transmethylation (Figure 2)
- Dissolve ~1-10 mg of total lipid in 1 ml of methanolic NaOH (see Recipe 3) and transfer in Pyrex culture tube.
- Heat for 15 min at 55 °C in a water bath.
- Add 2 ml of methanolic HCl (see Recipe 4) and vortex for 20 sec.
- Again heat for 15 min at 55 °C in the water bath.
- Add 10 μl of 1 mg ml-1 nonadecanoic acid (C19:0) into the lipid samples as an internal standard.
- FAMEs extract through Rotospin-Rotary mixer (Tarson) by adding 1 ml of water (Milli Q) and subsequently add 2 ml of 100 % hexane.
- Transfer upper phase (~2 ml) into a fresh Pyrex culture tube.
- Extract FAMEs two more times each with 2 ml of 100% hexane and pool in the same Pyrex culture tube (Step B7).
- Dry the pooled FAMEs under N2 and finally dissolved in 100 μl of 100% hexane.
- Transfer the FAMEs thus eluted into glass inserts, to 2 ml clear GC vials.
Pause Point: Samples can be stored in capped vials at -20 °C for a month.
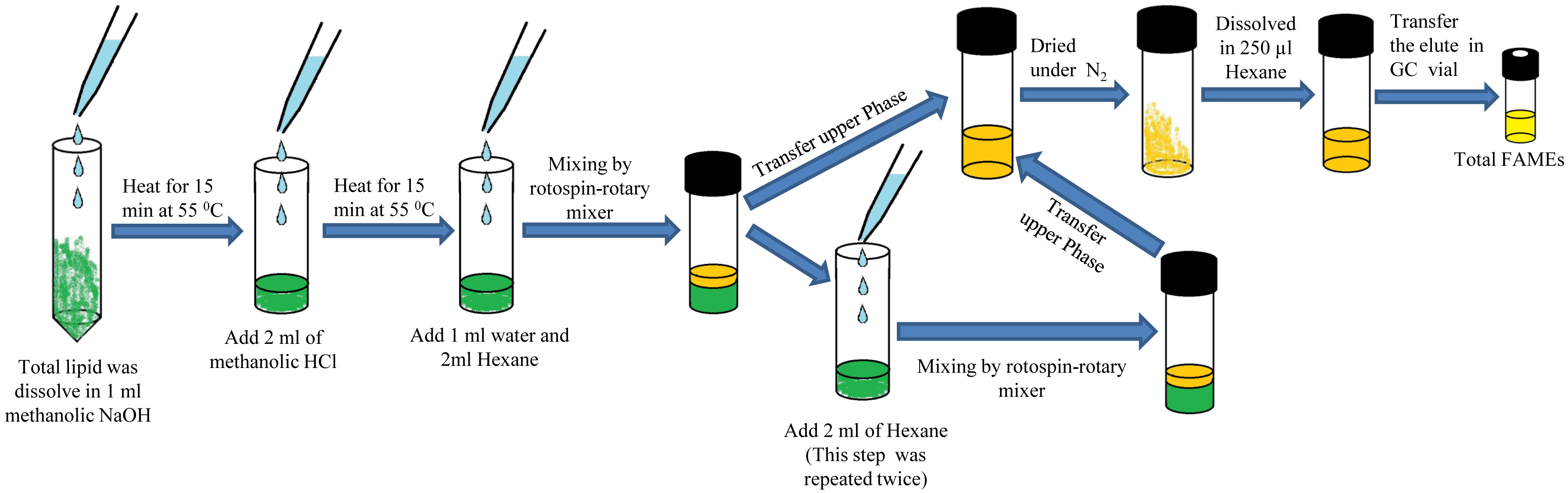
Figure 2. Workflow of conversion of lipid to fatty acid methyl esters (FAMEs) by transmethylation
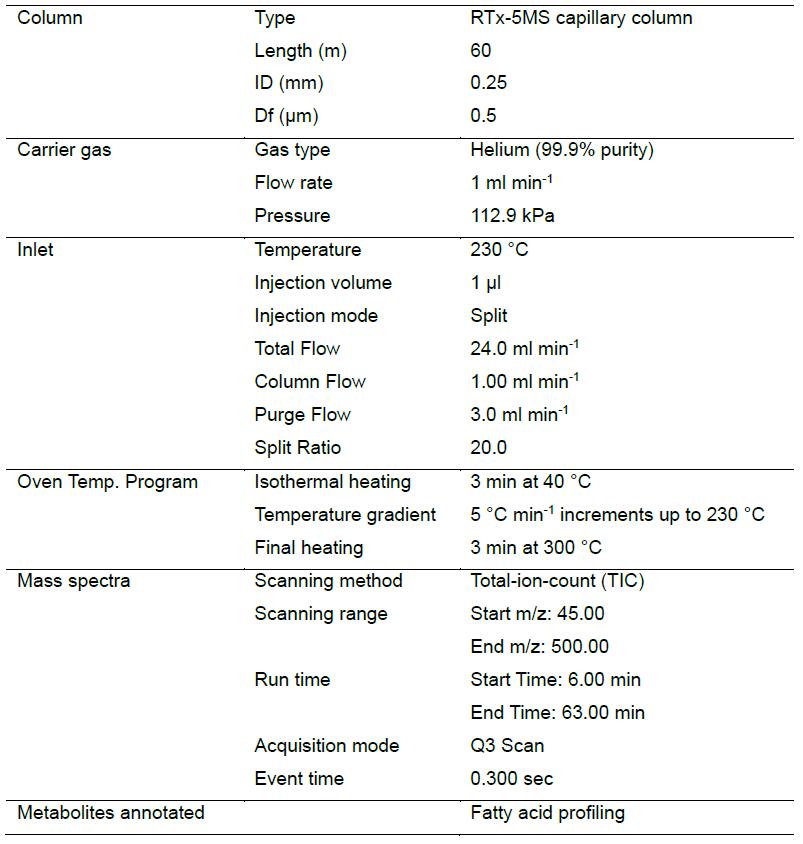
- GC-MS analysis (Figure 3)
- Carry out GC-MS analysis of FAMEs samples by gas chromatography coupled with a mass spectrometer (GC-MS QP-2010, Shimadzu, Japan) equipped with an auto-sampler (AOC-5000) using flame ionization detection (FID) and RTx-5MS capillary column (Table 1).
- Use RTx-5MS capillary column (60 meters, 0.25 mm ID, and 0.5 μm df) from Rastek, USA with 1 ml min-1 flow rate of helium (99.9 % purity) as a carrier gas and a pre-column pressure of 112.9 kPa. Set the initial column temperature regime to 40 °C for 3 min, followed by a 5 °C min-1 increment up to 230 °C followed by 40 min at 230 °C. Following this, set the injection volume, time and temperature of 1 μl, 67 min and 230 °C respectively. Operate the mass spectrometer in electron compact mode with electron energy of 70 eV and keep the temperature of the ion sources and quadrupole at 200 °C.

Figure 3. Workflow of GC-MS running and Post-run data analysisTable 1. GC-MS operating conditions for determination of fatty acid compositions
Data analysis
- Perform Peak finding, peak integration and retention time correction with the post-run analysis (GC-MS QP-2010) Shimadzu, Japan.
- Compare the corresponding FAMEs and retention time indices with standards (FAME Mix C4-C24, Supelco, USA and 7-hexadecenoic acid methyl ester, Cayman Chemicals, USA) by GC-MS post-run analysis.
- Next, normalize the integrated relative peaks against the FAME mix standard signal.
- Fatty acids were quantified by area normalization of the relative peak area of each fatty acid using Microsoft Office Excel 2010. Figure 4 shows representative differential peak areas of total ion chromatograms of standard (FAME mix) and plant leaf.
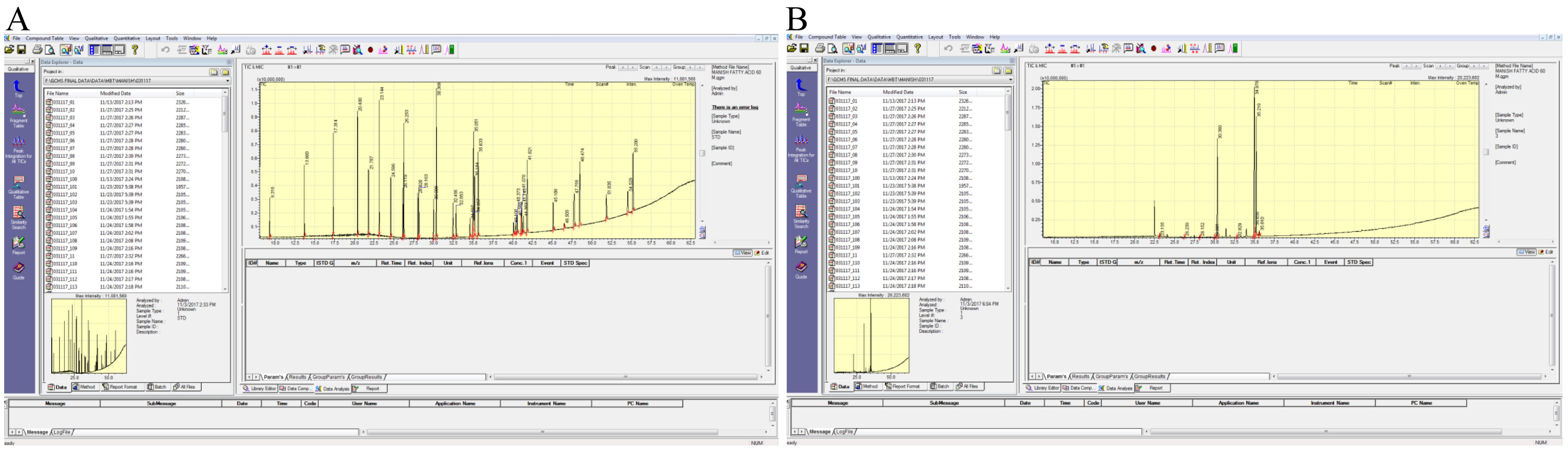
Figure 4. Representative total-ion-count (TIC) chromatograms. A. Standard; B. Leaf. - Total saturated fatty acids (SFA) and unsaturated fatty acids (MUFA and PUFA) contents are estimated by summation of percent quantity of corresponding fatty acids (Patel et al., 2016). Unsaturation index (Poerschmann et al., 2004), degree of unsaturation (Ramos et al., 2009), atherogenic and thrombogenic indices (Simat et al., 2015) were calculated using the following equations:
Monounsaturated fatty acids (MUFAs) = (Summestion of single dobule bond in long aliphatic chains)
Polyunsaturated fatty acids (PUFAs) = (Summestion of ≥ two dobule bond in long aliphatic chains)
Degree of unsaturation (DU) = (MUFA w%) + 2 × (PUFA w%)
Unsaturation index (UI) = Ʃ(FA w% × number of double bonds)
Atherogenic index (AI) = (C12:0 + 4 × C14:0 + C16:0)/(Ʃn3PUFA + Ʃn6PUFA + ƩMUFA)
Thrombogenic index (TI) = (C14:0 + C16:0 + C18:0)/((0.5 × MUFA) + (0.5 × n6PUFA) + (3 × n3PUFA) + (n3/n6))
Recipes
- Solvent extraction solution A
Chloroform:methanol, 1:2 (v/v) - Solvent extraction solution B
Chloroform:methanol, 1:1 (v/v) - Methanolic NaOH
1% NaOH in MeOH
The solution can be stored at 4 °C up to one month - Methanolic HCl
5% Conc. HCl in MeOH (Conc. HCl-36%; Molarity: 11.65)
The solution can be stored at 4 °C up to one month - Internal standard
1 mg ml-1 nonadecanoic acid (C19:0)
Acknowledgments
MKP acknowledges the National Post-doctoral Fellowship (PDF/2016/001971) from SERB-Department of Science & Technology (DST), Govt. of India for supporting this work. Core grant from National Institute of Plant Genome Research (NIPGR) is also acknowledged. SD acknowledges junior research fellowship from Department of Biotechnology (DBT), Govt. of India. We acknowledge DeLCON for research literature made available to us. This protocol has been derived from Bligh and Dyer (1959) and Carreau and Dubacq (1978) with minor modifications made by Mishra et al. (2015), Pandey et al. (2015) and Patel et al. (2016) for reproducible results.
Competing interests
The authors declare no conflicts of interest or competing interests.
References
- Bligh, E. G. and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8): 911-917.
- Carreau, J. P. and Dubacq, J. P. (1978). Adaptation of a macro-scale method to the micro-scale for fatty acid methyl transesterification of biological lipid extracts. J Chromatogr A 151(3): 384-390.
- Hadar, Y. (2013). Sources for lignocellulosic raw materials for the production of ethanol. Springer Berlin Heidelberg.
- Knothe, G. (2005). Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86(10): 1059-1070.
- Kumari, P., Bijo, A. J., Mantri, V. A., Reddy, C. R. and Jha, B. (2013). Fatty acid profiling of tropical marine macroalgae: an analysis from chemotaxonomic and nutritional perspectives. Phytochem 86: 44-56.
- Mishra, A., Patel, M. K. and Jha, B. (2015). Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J Funct Foods 13: 21-31.
- Pandey, S., Patel, M. K., Mishra, A. and Jha, B. (2015). Physio-biochemical composition and untargeted metabolomics of cumin (Cuminum cyminum L.) make it promising functional food and help in mitigating salinity stress. PLoS One 10(12): e0144469.
- Patel, M. K., Mishra, A. and Jha, B. (2016). Non-targeted metabolite profiling and scavenging activity unveil the nutraceutical potential of psyllium (Plantago ovata Forsk). Front Plant Sci 7: 431.
- Poerschmann, J., Spijkerman, E. and Langer, U. (2004). Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb Ecol 48(1): 78-89.
- Ramos, M. J., Fernandez, C. M., Casas, A., Rodriguez, L. and Perez, A. (2009). Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100(1): 261-268.
- Ryckebosch, E., Muylaert, K. and Foubert, I. (2012). Optimization of an analytical procedure for extraction of lipids from microalgae. J Am Oil Chem Soc 89(2): 189-198.
- Schreiner, M. (2006). Optimization of solvent extraction and direct transmethylation methods for the analysis of egg yolk lipids. Int J Food Prop 9(3): 573-581.
- Sheng, J., Vannela, R. and Rittmann, B. E. (2011). Evaluation of methods to extract and quantify lipids from Synechocystis PCC 6803. Bioresour Technol 102(2): 1697-1703.
- Simat, V., Bogdanović, T., Poljak, V. and Petričević, S. (2015). Changes in fatty acid composition, atherogenic and thrombogenic health lipid indices and lipid stability of bogue (Boops boops Linnaeus, 1758) during storage on ice: Effect of fish farming activities. J Food Compos Anal 40: 120-125.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Patel, M. K., Das, S. and Thakur, J. K. (2018). GC-MS-Based Analysis of Methanol: Chloroform-extracted Fatty Acids from Plant Tissues. Bio-protocol 8(18): e3014. DOI: 10.21769/BioProtoc.3014.
Category
Plant Science > Plant biochemistry > Lipid
Biochemistry > Lipid > Lipid measurement
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










