- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sendai Virus Propagation Using Chicken Eggs
Published: Vol 8, Iss 18, Sep 20, 2018 DOI: 10.21769/BioProtoc.3009 Views: 7193
Reviewed by: Longping Victor TseMasfique MehediGeorge William Carnell

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Herpes Simplex Virus Type 1 Propagation, Titration and Single-step Growth Curves
Linda Grosche [...] Beate Sodeik
Dec 5, 2019 12356 Views

Production, quantification, and infection of Amazonian Phlebovirus (Bunyaviridae)
Carolina Torturella Rath [...] Ulisses Gazos Lopes
Jul 5, 2021 4049 Views

Generation, Propagation, and Titering of Dicistrovirus From an Infectious Clone
Junzhou Shen [...] Eric Jan
Feb 20, 2025 2187 Views
Abstract
Sendai virus is a member of the family Paramyxoviridae, and an enveloped virus with a negative-stranded RNA genome. Sendai virus is not pathogenic to humans, but for mice and can cause pneumonia in mice. Easy and efficient techniques for propagating Sendai virus are required for studying virus replication, virus-induced innate- and adaptive-immunity, Sendai-virus-based virotherapy and IgA nephropathy. Here, we describe a protocol for Sendai virus propagation using chicken eggs. This traditional protocol enables us to generate a large amount of virus enough for animal experiments as well as cell culture experiments in a relatively inexpensive way.
Keywords: Sendai virusBackground
Sendai virus (SeV) is a mouse parainfluenza virus type 1 that was discovered in Sendai, Japan, in the 1950s (Ishida and Homma, 1978). The virus was once named Hemagglutinating Virus of Japan (HVJ) by the Japanese Society for Virology, but was later termed ‘newborn virus pneumonitis (type Sendai)’ (Kuroya and Ishida, 1953). The name SeV is currently most popular, and now understood to be a pathogen of mice, not humans (Karron RA, 2007). Fukumi et al. first described SeV infections of mice in 1954 (Fukumi et al., 1954). This infection can be subclinical, but SeV is also known as one of the leading causes of pneumonia in certain mouse strains (Fukumi et al., 1954; Parker et al., 1978). SeV is an excellent tool to study the following in the various fields: the pathomechanism of a murine model of IgA nephropathy (Yamashita et al., 2007; Chintalacharuvu et al., 2008), a stimulator of RIG-I/MDA5 in innate immunity (Fensterl et al., 2008; Chattopadhyay et al., 2010, 2011 and 2013; Yamashita et al., 2012a, 2012b and 2013), oncolytic SeV-based virotherapy (Saga and Kaneda, 2015), a respiratory infection (Hermesh et al., 2010 and 2012), and a vector for AIDS vaccine (Ishii and Matano, 2015). SeV is uniquely sensitive to interferon-associated responses, and grows to high titers in both chicken eggs and in FDA-approved mammalian cell lines, an advantage for vaccine production. This protocol provides a method for SeV propagation using chicken eggs. This method can be applied for viral propagation for other viruses such as influenza virus.
Materials and Reagents
- 3 ml disposable transfer pipette (Bioland Scientific, catalog number: TPP02-11 )
- Centrifuge bottles, 250 ml (Fisher Scientific, catalog number: 05-564-1)
Manufacturer: Thermo Fisher Scientific, catalog number: 3141-0250 . - Bucket with ice
- 25 G 5/8 needle (Fisher Scientific, catalog number: 14-826AA)
Manufacturer: BD, catalog number: 305122 . - 1 ml syringes (BD, catalog number: 309659 )
- 18 G needle (Fisher Scientific, catalog number: 14-826-5G)
Manufacturer: BD, catalog number: 305195 . - 50 ml syringe (Fisher Scientific, catalog number: 14-955-461 )
- Pencil
- Push pin (Staples, catalog number: 480117 )
- Face mask (Cellucap Manufacturing, catalog number: 1826EL )
- Iodine Wipes (Dynarex, catalog number: B003U463PY )
- Egg holder/case (provided with embryonated eggs, or you can obtain regular ones from grocery store when you purchase regular/unembryonated eggs)
- Chicken embryonated eggs (48 eggs, 9-10 days old) (Charles River, catalog number: 10100332 )
- Duco® Cement Multi-Purpose Household Glue (ITW Consumer, Duco Cement, catalog number: 62435 )
- Sendai virus stock (ATCC, catalog number: VR-105 or catalog number: VR-907 )
- PBS with Ca2+ and Mg2+ (Thermo Fisher Scientific, catalog number: 14040133 )
- 70% ethanol
- Bleach (Essendant, catalog number: CLO30966CT )
- 0.5 M EDTA (Thermo Fisher Scientific, catalog number: R1021 )
Equipment
- Pipette (200 μl and 1,000 μl; any products are fine)
- Biosafety cabinet (any types are fine if it is BSL2 level)
- 1 L Graduated cylinder (Thermo Fisher Scientific, catalog number: 3664-1000 )
- Egg Incubator (up to 41 eggs; Farm Innovators, Digital Circulated Air Incubator with Auto Egg Turner, model: 4250 )
- Refrigerator/4 °C cold room
- High-speed Centrifuge with fixed-angle rotor of 250 ml bottles (Beckman Coulter, model: Avanti® J-E )
- Sonic water bath (Skymen Cleaning Equipment, model: JP-008 )
- Flashlight (Defiant, catalog number: HD15FL04-3 or Caliburn Lighting, catalog number: PISTOL-TRIPOD-RCH )
- Scissors (World Precision Instruments, catalog number: 14192-G )
- Forceps (World Precision Instruments, catalog number: 501974 )
Procedure
- Egg candling on Day 10
Eliminate infertile and fertile dead eggs by egg candling, mark the position of embryo, and air cell with a pencil (Figure 1). You need a dark room and concentrated light source. The best evidence of a live embryo is well-developed blood vessels.
Note: The capacity of the egg incubator is 41 eggs. If you have the egg incubator with larger capacity (e.g., 160 eggs), you can order 180 eggs, and handle 160 eggs.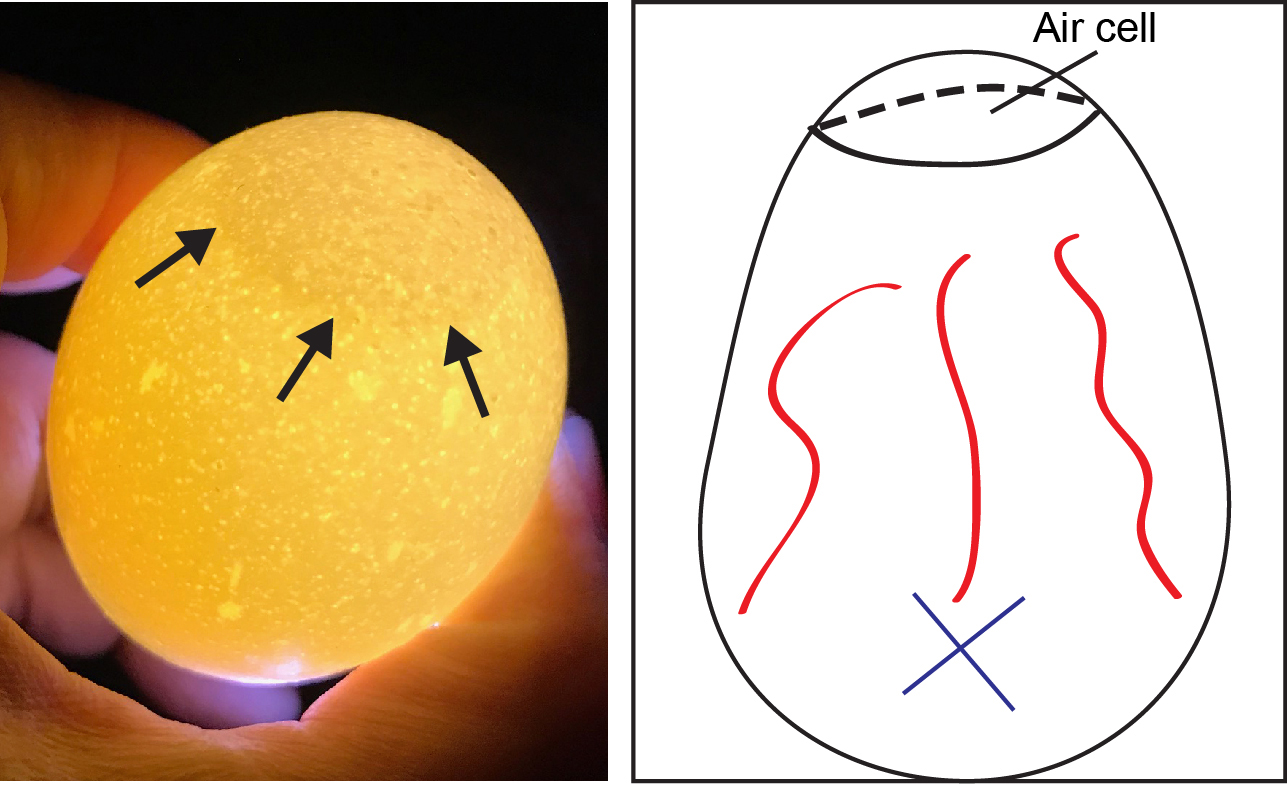
Figure 1. Chicken egg with air cell. Air cell is visualized by candling (left). Draw a line along the edge of air cell (arrows in left panel) and mark X (right) in the position of the embryo with a pencil under a strong light in dark room.
Note: This is an example of regular/unembryonated egg. You will see an embryo as a slightly dark spot and red vessels (right) by candling in the embryonated egg on Day 10. - Allantoic inoculation on Day 10
In a biosafety cabinet, dilute sterile Sendai virus stock to a concentration of 1 x 106 pfu/ml in sterile PBS with Ca2+ and Mg2+. The inoculum is 0.2 ml, containing 2 x 105 pfu. Keep tubes of virus on ice.- Wipe top (air cell side) of egg with 70% ethanol.
- Wipe top of egg with iodine and let dry.
- Puncture hole with alcohol soaked push pin at the location opposite to the position of the embryo and 10 mm below the air cell edge (Figure 2).
Note: Blood vessels usually do not reach to this area (10 mm below the air cell edge). Allantoic fluid does not leak from the punctured hole.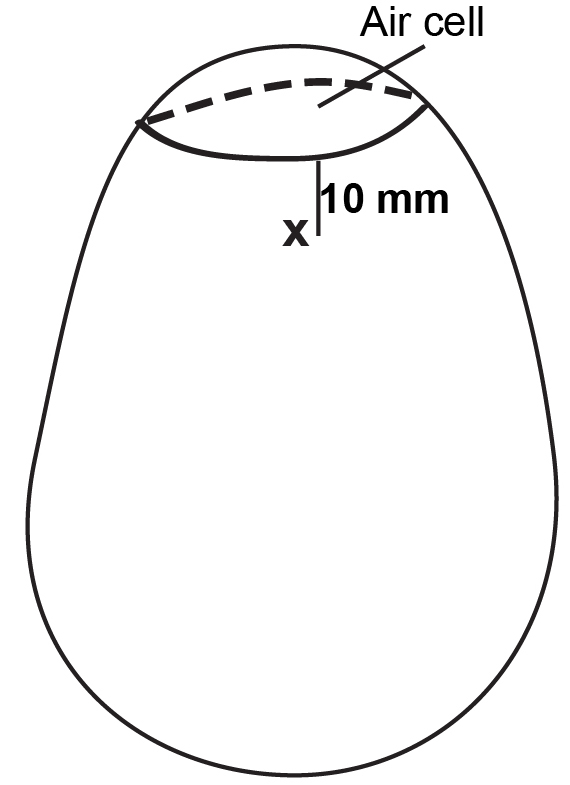
Figure 2. Chicken egg with a puncture. Make a hole with a push pin at the location 10 mm below the air cell edge and opposite to embryo. - Insert 25 G 5/8 needle to the hilt, deliver 0.2 ml inoculum. Do not inject any air.
- Seal hole with Duco cement, let dry.
- Return eggs to the incubator, turn on the turner.
Note: The egg incubator contains the function of turning eggs every four hours to cause the yolk to be repositioned away from the shell, making it safe for developing embryos.
- Egg candling on Day 12
Candle the eggs at the end of the day, mark and discard the dead ones. Place all live ones in a refrigerator/cold room. Do not stack them because they need to be cooled quickly for proper contraction of the blood vessels. Keep the eggs in the cold overnight, and process the next day. - Harvest of allantoic fluid on Day 13
From this step, all procedures need to be performed in a biosafety cabinet. Wearing a face mask is recommended because the virus can cause mild respiratory symptom in humans. Cut off shell that covers the air cell using scissors and forceps. Break the shell membrane, avoid rupturing yolk or blood vessels with an 18 G needle on a 50 ml syringe, and aspirate allantoic fluid (yellowish tinge) (Figure 3). From one large egg 10-15 ml usually can be collected. Watch for yolk (very yellow) and egg white (albumin: clear and very viscous) (Figure 4). The yolk and the egg white with viral inoculation do not show any difference from the ones of regular/unembryonated eggs from grocery store in appearance and smell. If an egg looks nasty (i.e., egg white looks very whitish or yellowish, and/or are stinky), this is a phenomenon of bacterial growth in the egg and the egg should be thrown away after immediate bleaching (disinfecting). Place the collected allantoic fluid in centrifuge tubes (250 ml) on ice immediately.
Note: On Day 13, yolk is not easily visible. Blood vessels do not reach to the air cell, and will be shrunk after cooling the eggs in the cold room; Red blood cells and shell will spin out with clarification, and not be a problem. However, membrane pieces will co-purify with the virus. Therefore, avoid to remove membrane pieces.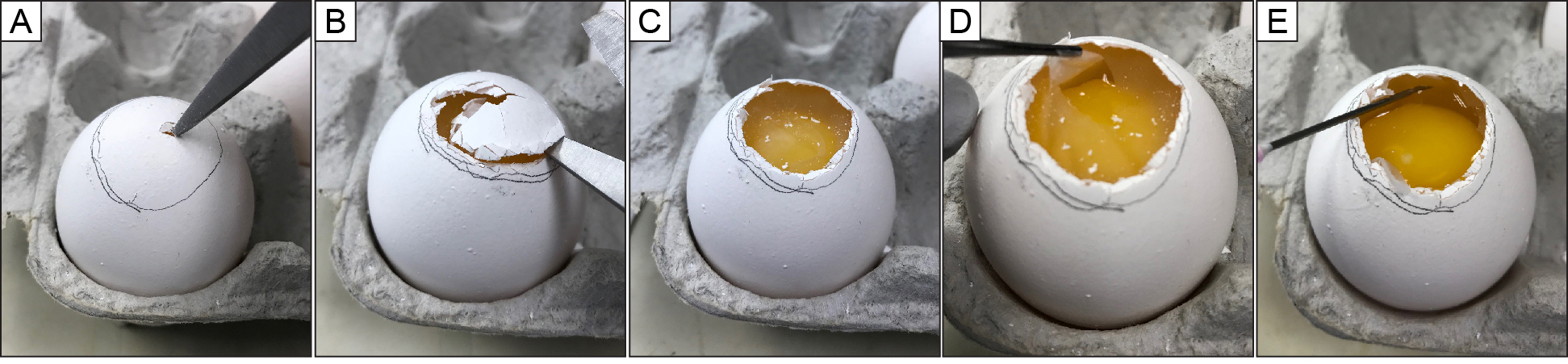
Figure 3. How to open up an egg. A. Making a hole on the shell over air cell. B. Cutting shell by scissors along but slightly inside of the line of air cell. C. Exposed air cell and intact shell membrane. D. Peeling and removal of shell membrane with forceps completely; E. Collecting allantoic fluid between shell membrane and an embryo (yolk in this picture) with an 18 G needle and 50 ml syringe.
Note: This is an example of unembryonated egg. You will see an embryo instead of yellow yolk in the actual viral propagation.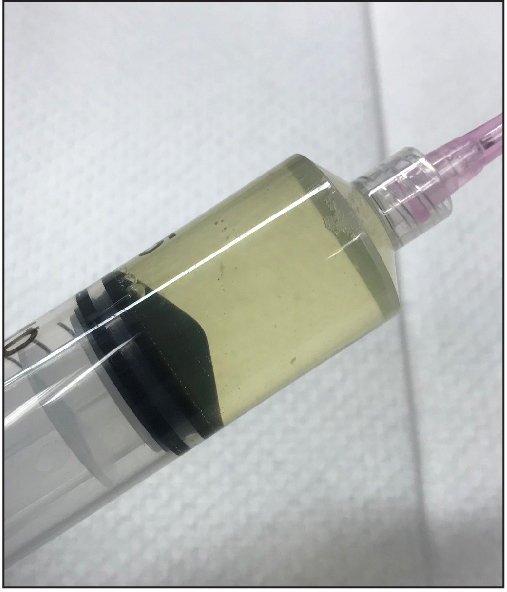
Figure 4. Collected allantoic fluid. Allantoic fluid is transparent or slightly cloudy with yellowish tinge. - Concentration of SeV by centrifugation
- Collect allantoic fluid into 250 ml centrifuge bottles (max volume 225 ml) on ice. Balance bottles.
- Spin the rotor at 2,600 x g for 25 min to remove the shell. Do not use brake.
- Decant fluid into fresh centrifuge bottles that contain 2 ml of 0.5 M EDTA. This will make the pellet easier to be re-suspended later.
- Spin the rotor at 27,000 x g for 90 min to obtain the 1st pellet. Decant fluid into a graduated cylinder for the volume measurement, and discard the fluid after immediate bleaching.
- Re-suspend the pellet in cold PBS (with Ca2+ and Mg2+) with 1 mM EDTA using a 2-3 ml disposable transfer pipette. Condense multiple bottles into one bottle. Extensive rinsing will be required to re-suspend the first pellet. Bring the volume up to about 200 ml with cold PBS (with Ca2+ and Mg2+) with 1 mM EDTA.
Note: The purpose of Steps E5 and E6 is to combine multiple pellets to be one single pellet. If you do not have many bottles, you do not need these steps. - Spin the rotor at 27,000 x g for 90 min to obtain the second pellet. Decant the fluid and discard after immediate bleaching.
- Re-suspend the pellet in about 2 ml of cold PBS (with Ca2+ and Mg2+) with 1 mM EDTA. Use another 8-10 ml to rinse the remainder, and pool into a tube.
- Sonicate (40 kHz frequency and 35 W ultrasonic power) final suspension for 2 min at room temperature in a sonic water bath to break apart viral chunk.
- Aliquot and freeze them at -80 °C. Make and freeze also two small aliquots for testing viral titer.
Notes
This protocol needs an appropriate facility (Biosafety level 2) and an approved Institutional Biosafety Committee (IBC) protocol.
Acknowledgments
Dr. Yamashita was supported by American Heart Association Scientist Development Grant (17SDG33660947) and University of California Los Angeles (UCLA) Clinical and Translational Science Institute (CTSI) Grant (UL1TR001881), and is supported by UCLA CTSI KL2 grant (KL2TR001882) and Cedars-Sinai CTSI Clinical Scholars Grant. We would like to thank Dr. Steven Emancipator for the mentoring in the early stage of Dr. Yamashita's career development and Dr. John Nedrud for previous collaboration. The protocol was adapted from our previous work (Yamashita et al., 2007; Chintalacharuvu et al., 2008).
Competing interests
Authors declare no conflicts of interest or competing interests.
References
- Chattopadhyay, S., Fensterl, V., Zhang, Y., Veleeparambil, M., Yamashita, M. and Sen, G. C. (2013). Role of interferon regulatory factor 3-mediated apoptosis in the establishment and maintenance of persistent infection by Sendai virus. J Virol 87(1): 16-24.
- Chattopadhyay, S., Marques, J. T., Yamashita, M., Peters, K. L., Smith, K., Desai, A., Williams, B. R. and Sen, G. C. (2010). Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J 29(10): 1762-1773.
- Chattopadhyay, S., Yamashita, M., Zhang, Y. and Sen, G. C. (2011). The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J Virol 85(8): 3708-3716.
- Chintalacharuvu, S. R., Yamashita, M., Bagheri, N., Blanchard, T. G., Nedrud, J. G., Lamm, M. E., Tomino, Y. and Emancipator, S. N. (2008). T cell cytokine polarity as a determinant of immunoglobulin A (IgA) glycosylation and the severity of experimental IgA nephropathy. Clin Exp Immunol 153(3): 456-462.
- Fensterl, V., White, C. L., Yamashita, M. and Sen, G. C. (2008). Novel characteristics of the function and induction of murine p56 family proteins. J Virol 82(22): 11045-11053.
- Fukumi, H., Nishikawa, F. and Kitayama, T. (1954). A pneumotropic virus from mice causing hemagglutination. Jpn J Med Sci Biol 7(4): 345-363.
- Hermesh, T., Moltedo, B., Moran, T. M. and Lopez, C. B. (2010). Antiviral instruction of bone marrow leukocytes during respiratory viral infections. Cell Host Microbe 7(5): 343-353.
- Hermesh, T., Moran, T. M., Jain, D. and Lopez, C. B. (2012). Granulocyte colony-stimulating factor protects mice during respiratory virus infections. PLoS One 7(5): e37334.
- Ishida, N. and Homma, M. (1978). Sendai virus. Adv Virus Res 23: 349-383.
- Ishii, H. and Matano, T. (2015). Development of an AIDS vaccine using Sendai virus vectors. Vaccine 33(45): 6061-6065.
- Karron RA, C. P. (2007). Parainfluenza Viruses (5th Edition). Philadelphia, PA: Lippincott Williams and Wilkins.
- Kuroya, M. and Ishida, N. (1953). Newborn virus pneumonitis (type Sendai). II. The isolation of a new virus possessing hemagglutinin activity. Yokohama Med Bull 4(4): 217-233.
- Parker, J. C., Whiteman, M. D. and Richter, C. B. (1978). Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect Immun 19(1): 123-130.
- Saga, K. and Kaneda, Y. (2015). Oncolytic Sendai virus-based virotherapy for cancer: recent advances. Oncolytic Virother 4: 141-147.
- Yamashita, M., Chattopadhyay, S., Fensterl, V., Saikia, P., Wetzel, J. L. and Sen, G. C. (2012a). Epidermal growth factor receptor is essential for Toll-like receptor 3 signaling. Sci Signal 5(233): ra50.
- Yamashita, M., Chattopadhyay, S., Fensterl, V., Zhang, Y. and Sen, G. C. (2012b). A TRIF-independent branch of TLR3 signaling. J Immunol 188(6): 2825-2833.
- Yamashita, M., Chintalacharuvu, S. R., Kobayashi, N., Nedrud, J. G., Lamm, M. E., Tomino, Y. and Emancipator, S. N. (2007). Analysis of innate immune responses in a model of IgA nephropathy induced by Sendai virus. Contrib Nephrol 157: 159-163.
- Yamashita, M., Millward, C. A., Inoshita, H., Saikia, P., Chattopadhyay, S., Sen, G. C. and Emancipator, S. N. (2013). Antiviral innate immunity disturbs podocyte cell function. J Innate Immun 5(3): 231-241.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tatsumoto, N., Arditi, M. and Yamashita, M. (2018). Sendai Virus Propagation Using Chicken Eggs. Bio-protocol 8(18): e3009. DOI: 10.21769/BioProtoc.3009.
Category
Microbiology > Microbial cell biology > Virus propagation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









