- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Soluble and Solid Iron Reduction Assays with Desulfitobacterium hafniense
Published: Vol 8, Iss 17, Sep 5, 2018 DOI: 10.21769/BioProtoc.3002 Views: 6711
Reviewed by: Valentine V TrotterKarolina SubrtovaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of Transferrin- and Non-transferrin-bound Iron Uptake by Mouse Tissues
Supak Jenkitkasemwong [...] Mitchell D. Knutson
Sep 5, 2016 9145 Views

Quantification of Bacterial Polyhydroxybutyrate Content by Flow Cytometry
Antonio Lagares (Jr.) and Claudio Valverde
Dec 5, 2017 8134 Views

A SsrA/NIa-based Strategy for Post-Translational Regulation of Protein Levels in Gram-negative Bacteria
Gonzalo Durante-Rodríguez [...] Pablo I. Nikel
Jul 20, 2020 4380 Views
Abstract
There is a pressing need to develop sustainable and efficient methods to protect and stabilize iron objects. To develop a conservation-restoration method for corroded iron objects, this bio-protocol presents the steps to investigate reductive dissolution of ferric iron and biogenic production of stabilizing ferrous iron minerals in the strict anaerobe Desulfitobacterium hafniense (strains TCE1 and LBE). We investigated iron reduction using three different Fe(III) sources: Fe(III)-citrate (a soluble phase), akaganeite (solid iron phase), and corroded coupons. This protocol describes a method that combines spectrophotometric quantification of the complex Fe(II)-Ferrozine® with mineral characterization by scanning electron microscopy and Raman spectroscopy. These three methods allow assessing reductive dissolution of ferric iron and biogenic mineral production as a promising alternative for the development of an innovative sustainable method for the stabilization of corroded iron.
Keywords: Reductive dissolution of ferric ironBackground
Since the Iron Age, iron has been used to produce everyday utensils. Therefore, archaeological iron findings are an extremely important testimony of the past and should be preserved. However, due to its reactivity, iron can be easily corroded and archaeological iron objects risk to be completely damaged. When buried, iron artifacts develop a complex corrosion layer according to the environmental conditions of the burial site. After excavation, conditions change and the corrosion layer becomes unstable. To avoid complete destruction, archaeological iron objects require a rapid stabilization treatment. Currently, available stabilization treatments do not provide long-term protection and have substantial drawbacks, such as toxicity, low efficiency, and production of large amount of waste (Scott and Eggert, 2009; Rimmer et al., 2012). Consequently, it is necessary to develop new technologies to stabilize archaeological iron artifacts.
Exploiting a microbial metabolism is increasingly considered for the development of more efficient, sustainable and eco-friendly treatments in conservation-restoration (Ranalli et al., 2005; Cappitelli et al., 2006 and 2007; Jonkers, 2011; Joseph et al., 2011, 2012 and 2013; Bosch-Roig and Ranalli, 2014). Our research team is developing a treatment based on the reductive dissolution of ferric iron under anaerobic conditions (Kooli et al., 2018; Comensoli et al., 2017). The unstable corrosion products are converted into more stable biogenic minerals (i.e., magnetite and vivianite), as a byproduct of bacterial iron reduction. This conversion would stabilize the corrosion layer of the object.
In order to study the suitability of the chosen bacteria, iron reduction has to be carefully monitored. Several methods are available to quantify iron. Inductive coupled plasma mass spectrometry (ICP-MS) is useful to measure trace elements with concentrations of less than 1 ppm (Meissner et al., 2004). However, it requires expensive equipment and does not provide information on the oxidation state of iron if not combined with chromatographic separation devices such as high-performance liquid chromatography (HPLC), ion chromatography (IC), gas chromatography (GC), and capillary electrophoresis (CE) (Thomas, 2013). A spectrophotometric method to measure Fe(II) uses the metal-ligand ortho-phenanthroline (Fortune and Mellon, 1938). This compound is now considered carcinogenic (Whittaker et al., 2001). Therefore, for this protocol we selected the spectrophotometric quantification of Fe(II) with the Ferrozine® assay. This simple and reliable method requires standard lab equipment and can be used to analyze many samples. In addition, the characterization of biogenic minerals was made based on their appearance, morphology and molecular composition. For these analyses, we used scanning electron microscopy and Raman spectroscopy.
This Bio-protocol consists of three main steps (Figure 1): A. Biomass production; B. Incubation with iron sources; C. Validation of iron reduction.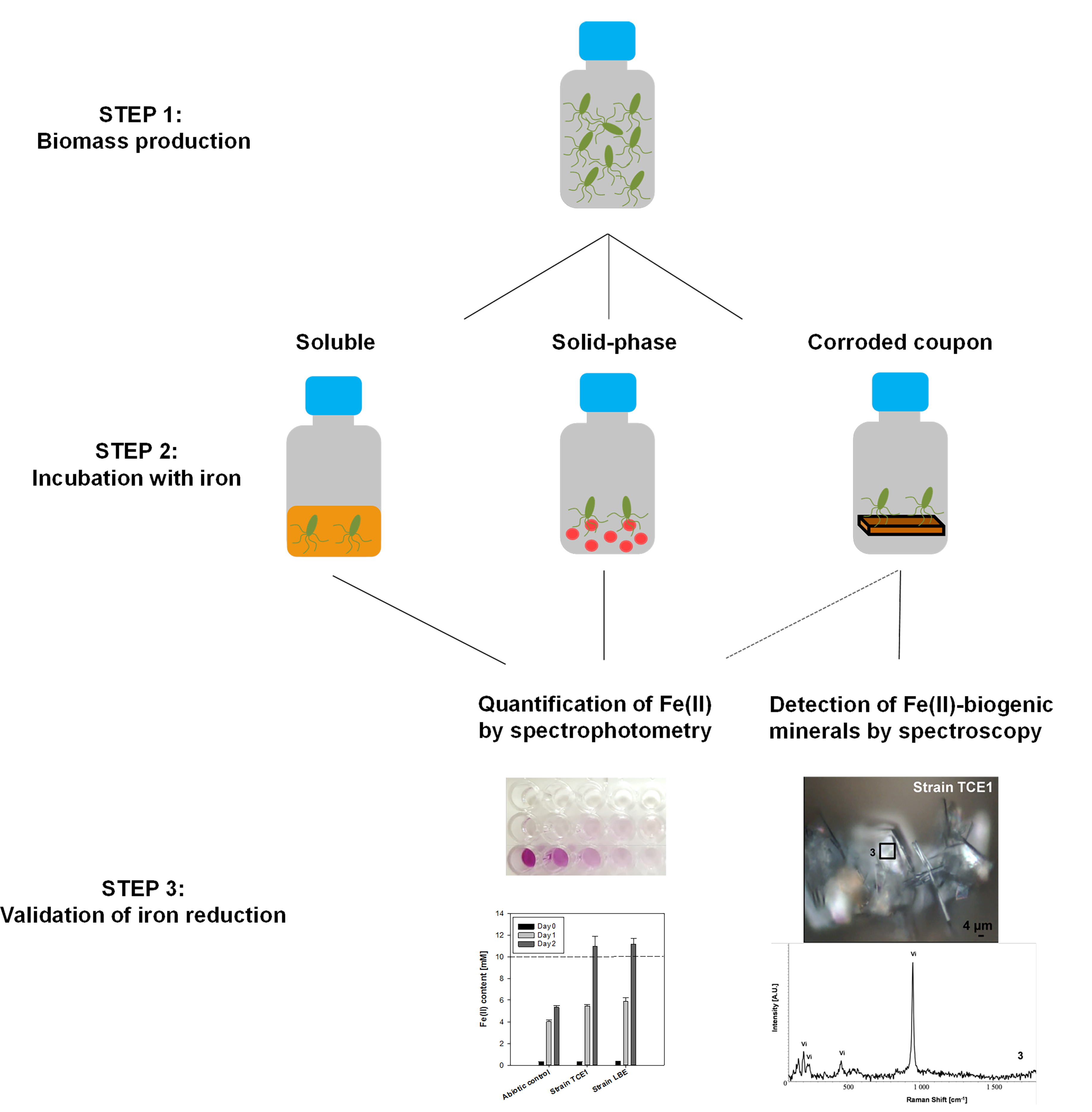
Figure 1. Graphical summary of the overall structure of this bio-protocol
Materials and Reagents
- 1.7 ml Eppendorf centrifuge tubes (Corning, Axygen®, catalog number: MCT-175-C )
- Syringes
1 ml (CODAN, catalog number: 621640 )
5 ml (CODAN, catalog number: 625607 )
20 ml (CODAN, catalog number: 627602 ) - Needle for syringes (Henke-Sass, Wolf, catalog number: 4710005016 )
- 1,000, 500, 100 and 50 ml serum bottle for anaerobic bacterial culture (DWK Life Sciences, Wheaton, catalog number: W012467A [100 ml])
- 100 ml serum bottle with large bottleneck (Merck, catalog number: STBMRFA12 )
- Rubber stoppers for serum bottles (VWR, special request)
- Metal caps for serum bottle (Thermo Fisher Scientific, catalog number: C4020-3A )
- Serum bottle seal crimper (DWK Life Sciences, Wheaton, catalog number: 224322 )
- 0.2 μm sterile filter (SARSTEDT, catalog number: 83.1826.001 )
- 96-well polypropylene microplate (SARSTEDT, catalog number: 82.1581 )
- 96-well microcentrifuge tube flipper rack with Lid (Fisher Scientific, catalog number: 11710344 )
- Desulfitobacterium hafniense strain TCE1 (Gerritse et al., 1999)
- Desulfitobacterium hafniense strain LBE (Comensoli et al., 2017)
- Ethanol (Thommen Furler, catalog number: 180-VL54K )
- Corroded iron coupons (steel coupons presenting a natural corrosion layer produced after outdoor exposure in the city of Zurich, Switzerland)
- Adhesive Carbon Tape 12 mm x 20 m (Agar Scientific, catalog number: AGG3939A )
- N2 gas cylinder (Carbagas, catalog number: I4001 )
- NH4HCO3 (Sigma-Aldrich, catalog number: A6141 )
- NaHCO3 (Sigma-Aldrich, catalog number: S5761 )
- K2HPO4•3H2O (Sigma-Aldrich, catalog number: P5504 )
- NaH2PO4•2H2O (Sigma-Aldrich, catalog number: 71505 )
- Peptone (BD, catalog number: 211677 )
- Resazurin sodium salt (Sigma-Aldrich, catalog number: R7017 )
- Cyanocobalamin (Acros Organics, catalog number: 405920010 )
- Riboflavin (Sigma-Aldrich, catalog number: R4500 )
- Thiamine-hydrochloride (AppliChem, catalog number: A0955 )
- Biotin (Thermo Fisher Scientific, Alfa Aesar, catalog number: A14207 )
- P-aminobenzoate (sodium salt) (Sigma-Aldrich, catalog number: A9878 )
- Pantothenate (sodium salt) (Sigma-Aldrich, catalog number: P3161 )
- Folic acid•2H2O (Sigma-Aldrich, catalog number: F7876 )
- Lipoic acid (Sigma-Aldrich, Fluka, catalog number: 62320 )
- Pyridoxine hydrochloride (Acros Organics, catalog number: 150770500 )
- Nicotinic acid (Sigma-Aldrich, catalog number: N4126 )
- EDTA disodium salt•2H2O (Sigma-Aldrich, catalog number: E1644 )
- FeCl2•4H2O (Sigma-Aldrich, catalog number: 44939 )
- MnCl2•4H2O (Sigma-Aldrich, catalog number: M3634 )
- CoCl2•6H2O (Sigma-Aldrich, catalog number: C8661 )
- ZnCl2 (Sigma-Aldrich, catalog number: 793523 )
- CuCl2•2H2O (Sigma-Aldrich, catalog number: C3279 )
- AlCl3 (Sigma-Aldrich, catalog number: 237051 )
- H3BO3 (Sigma-Aldrich, catalog number: B6768 )
- Na2MoO4•2H2O (Sigma-Aldrich, catalog number: 331058 )
- NiCl2•6H2O (Sigma-Aldrich, catalog number: N6136 )
- CaCl2•2H2O (Sigma-Aldrich, catalog number: 223506 )
- MgCl2•6H2O (Sigma-Aldrich, catalog number: M2393 )
- Na2S•9H2O (Sigma-Aldrich, catalog number: 208043 )
- Sodium DL-lactate 60% solution (Sigma-Aldrich, catalog number: L1375 )
- Disodium fumarate (Sigma-Aldrich, catalog number: F1506 )
- HCl 37% (S-20) (Honeywell International, catalog number: 30721-1L-GL )
- MilliQ water
- Fe(II)-ammonium sulfate (Honeywell International, Fluka, catalog number: 09720 )
- Fe(III)-citrate (Sigma-Aldrich, Fluka, catalog number: 44941-250G )
- 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES) (Sigma-Aldrich, catalog number: H3375-250G )
- NaOH (Sigma-Aldrich, catalog number: 71690 )
- Goethite: α-FeO(OH) (Sigma-Aldrich, catalog number: 71063-100G ) (alternative source of solid Fe(III)-phase to akaganeite)
- Fe2O3 (Sigma-Aldrich, catalog number: 529311-5G ) (alternative source of solid Fe(III)-phase to akaganeite)
- Growth medium for D. hafniense (see Recipes)
- N2-degassed H2O
- Sterile serum bottles
- Solution of sodium DL-lactate 40% (v/v)
- Solution of disodium fumarate 16% (v/v)
- Reducing agent solution 1 M
- Resazurin solution 0.5 g/L
- Vitamin solution 1
- Vitamin solution 2
- Vitamin solution 3
- Vitamin solution 4
- Trace elements solution
- Carbonate solution
- Solution A (basal medium)
- Solution B (vitamin solution)
- Solution C (buffering/reducing solution)
- Solution D
- Soluble Fe(III)-citrate (35 g/L) – 100 ml (see Recipes)
- HCl solutions to adjust pH
- NaOH solutions to adjust pH
- Fe(III) solution
- Solid Fe(III) suspension (see Recipes)
- Solid Fe(III) source
- Preparation of the suspension of solid Fe(III)-phase (akaganeite or goethite)
- Ferrozine® reagents (see Recipes)
- HCl solution 5 M
- Stock solution of Fe(II) 1 M for calibration curve
- Ferrozine® reagent
Equipment
- 1 L graduated flasks (SciLabware, catalog number: 1132/26 )
- Magnetic bars (Sigma-Aldrich, BRAND, catalog numbers: Z328774 , Z328812 )
- Stainless steel spatula (Sigma-Aldrich, catalog number: HS15909 )
- Balance (Mettler-Toledo International, catalog number: PG5002 )
- P20 pipetman (Gilson, catalog number: F123600 )
- P200 pipetman (Gilson, catalog number: F123601 )
- P1000 pipetman (Gilson, catalog number: F123602 )
- pH meter
- Bunsen burner (FIREBOY Plus) (Integra Biosciences, catalog number: 144000 )
- Autoclave (Fedegari Autoklav FOB5/TS) (VITARIS, catalog number: 260000-FED , serial number: NBD801AV)
- Orbital shaker (Kühner, model: SMX1200 )
- Hotplate and magnetic Stirrer (Heidolph Instruments, catalog number: MR2002 )
- Spinbar® Magnetic Stir bar (Sigma-Aldrich, SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: Z126942-1EA )
- Spectrophotometer cuvettes (Sigma-Aldrich, catalog number: C5291-100EA )
- Spectrophotometer UV-visible (GENESYSTM 10S) (Thermo Fisher Scientific, catalog number: 840-208100 )
- Microplate reader (Biochrom, Asys Hitech, catalog number: UVM 340 )
- pH meter (Benchtop Meter AE150) (Fisher Scientific, catalog number: 15524693 )
- Biosafety cabinet equipped with UV lamp at 254 nm (Azbil Telstar, catalog number: Bio II Advance )
- Chemical fume hood
- Desiccator (BRAND, catalog number: 65815 )
- Scanning electron microscope (SEM) (Philips ESEM XL30 FEG environmental scanning electron microscope equipped with an energy-dispersive X-ray analyzer (Philips)
- Raman Microscope (HORIBA, JOBIN YVON, LabRAM Aramis microscope equipped with a Nd:YAG laser of 532 nm and controlled by LabSpec NGS spectral software. HORIBA, JOBIN YVON, catalog number: LabRAM Aramis, 3 lasers and xyz stage)
- Vortex
- Vacuum pump
- Fridge
Procedure
- Biomass production
- An estimation of the volume of the medium and the number of serum bottles is needed according to the number of bacterial strains to investigate. Include always an abiotic control. To test the ability of the strains TCE1 and LBE of D. hafniense, prepare 3 different bottles of medium (abiotic control, strain TCE1 and strain LBE). For the composition, see recipe point B. Growth medium for D. hafniense.
- Prepare the inoculum using normal growth medium for D. hafniense and incubate the bacterial strains in standard conditions (30 °C under agitation at 100 rpm) for approximatively 3 days.
- Final OD600 of the inoculum should be in the range of 0.1-0.15. To verify the OD600, take a 1 ml sample from the culture using a sterile syringe and transfer it to a spectrophotometer cuvette. Measure OD600.
- Using a sterile syringe, add aseptically 40 ml of inoculum (5%) to 800 ml of growth medium. For this volume, a 1 L serum bottle is required (Figure 2).
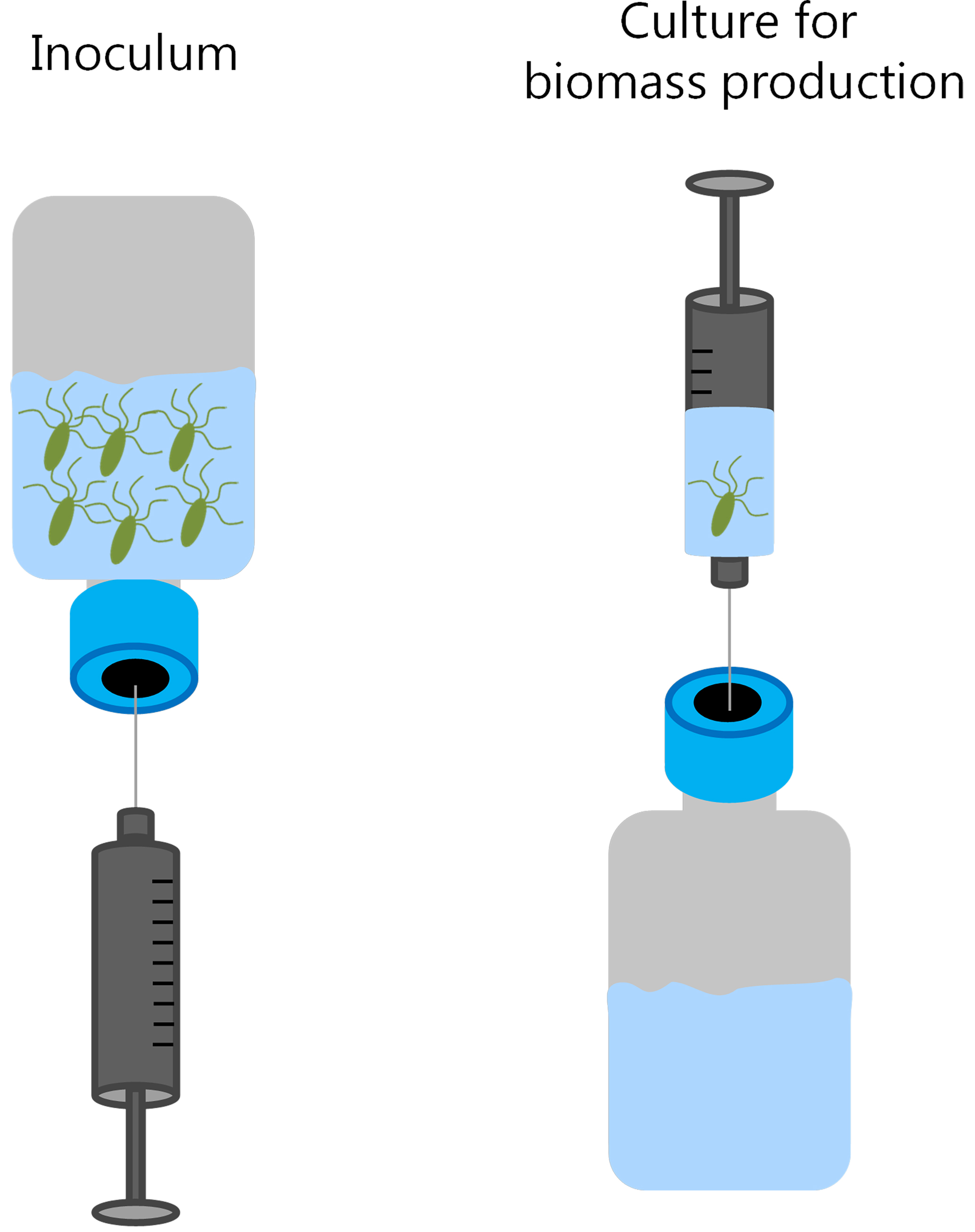
Figure 2. Scheme showing the inoculation procedure for the production of the bacterial biomass
- Incubate the cultures at 30 °C under agitation at 100 rpm until reaching an OD600 between 0.12 and 0.18 for both the strains.
- Incubation with iron sources
- Prepare the required number of empty 50 ml sterile serum bottles in N2 atmosphere (see Recipe 2). Include triplicates for each iron source, strain used, and abiotic controls. Autoclave.
- To seal standard serum bottles, insert rubber stoppers and cover them with metal caps with hollow opening. When sealed, the hollow opening allows sampling with syringes during the experiment. Finally, use the seal crimper to tie and fix the metal caps to the serum bottles.
- For the experiments with strains LBE and TCE1, prepare 18 empty 50 ml sterile serum bottles in N2 atmosphere.
- In 9 of the empty bottles add aseptically 1.5 ml of Fe(III)-citrate solution (see Recipes). In the other 9 bottles add aseptically 1.5 ml of akageneite suspension (see Recipes).
- To test bacterial reduction on corroded coupons, sterilize 9 corroded coupons by spraying a solution of ethanol 70% (v/v), followed by exposure to UV radiation (20 min each side at 254 nm) under sterile conditions. Perform the sterilization procedure inside a biosafety cabinet.
- Add the 9 coupons in serum bottles with large bottlenecks, seal the bottles, vacuum the headspace and replace the atmosphere using N2 Autoclave.
- Once all the bottles are autoclaved (Steps B1 and B6), add aseptically 20 ml of either, the sterile medium (abiotic control), or the biomass prepared in Procedure A. The procedure is done for the different iron sources (Fe(III)-citrate, akageneite, iron coupons). These proportions are calculated to obtain a culture with a starting concentration of 10 mM of soluble and solid Fe(III) phases. In the culture amended with the corroded coupons, iron concentration is unknown.
- To keep bacteria and the iron sources well mixed, incubate the serum bottles at 30 °C under agitation at 100 rpm. Incubate the bottles until a black precipitate is formed (7 days of incubation with D. hafniense). During incubation, the medium changes color from orange/green to black in the cultures amended with soluble Fe(III)-citrate and akaganeite suspension. The formation of black precipitates is an indication of iron reduction. The same phenomenon can be observed in the cultures amended with iron coupons, as the surface of the coupons and medium turn black.
- During the 7 days of incubation, collect daily 0.5 ml supernatant sample from each treatment and replicate. Make sure to take a representative sample. When the black precipitates form, insert the needle of the syringe in the rubber stopper of the serum bottle. Overturn the serum bottle. Shake gently and collect 0.5 ml of sample.
- Transfer the sample into a 1.7 ml Eppendorf tube.
- To all the tubes, add 50 μl of 5 M HCl.
- Mix by vortexing for 5 sec and incubate for 15 min at room temperature. This step will allow the dissolution of iron by the acid (HCl) and prevent the oxidation of Fe(II) ions.
- Freeze all the samples at -20 °C.
- Validation of iron reduction
- Quantification of Fe(II) by spectrophotometry
The Ferrozine® reagent will become violet upon reaction with Fe(II). Color intensity is proportional to the concentration of Fe(II). Performing a calibration curve will then allow to quantify Fe(II) content in the samples.- Switch on the microplate reader and set the wavelength at 562 nm.
- Take the standard solutions for the calibration curve from the fridge (see Recipes).
- Thaw the frozen samples (Steps B9-B12) taken from the cultures before Fe(II) quantification at room temperature.
- Mix by vortexing for 5 sec.
- Transfer 10 μl of the reaction mixtures (standard solution or samples) to a 96-well microplate.
Note: when taking the samples make sure to mix them well with a vortex before pipetting. - Add 90 μl of Ferrozine® reagent to the microplate wells.
- Do not forget to perform blank samples using the standard growth medium of D. hafniense.
- Measure absorbance at 562 nm in the following 2 min using the microplate reader. If the absorbance values are higher than the value of the more concentrated sample in the calibration curve (in our case 0.239 nm for the 1000 μM standard), prepare a dilution of the corresponding sample starting from the original sample and repeat the measure from Step C1d.
- Measure each replicate and perform 2-3 measures for each sample.
- Characterization of Fe(II)-biogenic minerals
- When the cultures become black and a precipitate is observed, remove the coupons from the culture and sterilize them by spraying a solution of ethanol 70% (v/v), followed by exposure to UV radiation (20 min on each side). With the bacterium D. hafniense, coupons can be removed from serum bottles after 7 days.
- Store the treated coupons under vacuum in a desiccator to avoid changes in the oxidation state of the biogenic minerals produced during the experiment.
- To study the morphology, the distribution and the elemental composition of the newly formed biogenic minerals, analyze coupons with SEM by simply positioning them inside the microscope chamber. To fix coupons to the sample holder use the carbon tape as illustrated in Figure 3. Observe samples in secondary electrons mode at an acceleration potential of 10-25 keV.
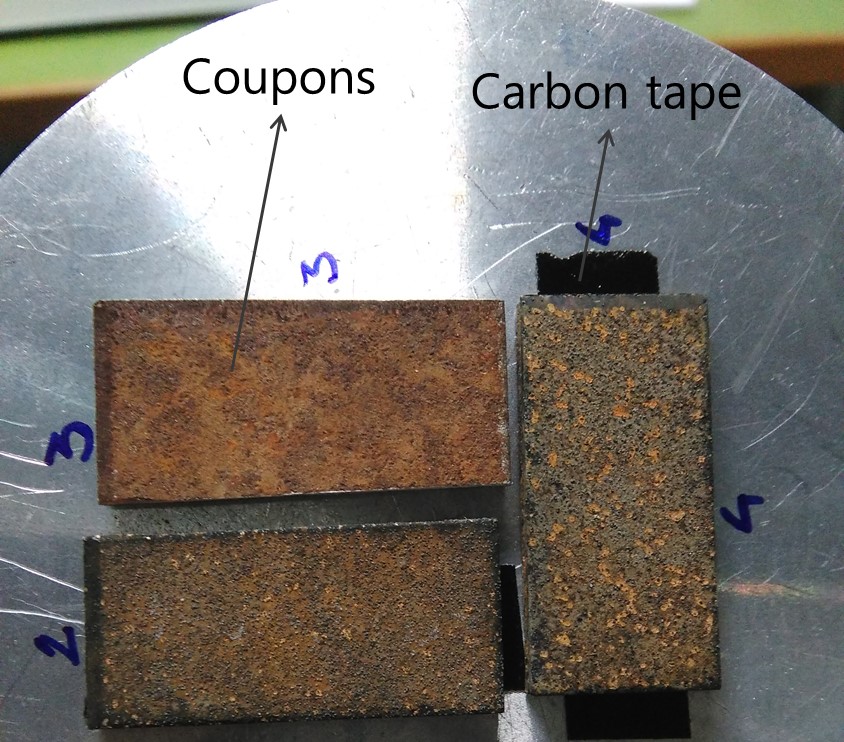
Figure 3. Sample holder with coupons and carbon tape, prepared for the SEM analysis
- To study the molecular composition of the newly formed Fe(II)-biogenic minerals, perform a Raman spectroscopy analysis directly on the surface of the coupons. To do so, simply position the coupons under the lowest objective and focus on the area to be analyzed. Change the objective and make the focus again until reaching the 400x magnification. Use the following set-up to obtain good quality spectra and to avoid burning of the surface of the sample: laser at 532 nm at power lower than 1 mW (600 g/mm), spectral interval between 100 and 1,600 cm-1 and 1,000 μm hole, 100 μm slit and 5 accumulations of 100 sec.
- Quantification of Fe(II) by spectrophotometry
Data analysis
- Calculation of Fe(II) content in mM
Convert the absorbance values in Fe(II) concentrations using the calibration curve with μM as unit, as illustrated in Figure 4. Consider only the absorbance values that are in the range of the calibration curve (in our case between 0.011 and 0.239). If the measured absorbance is lower than that, consider Fe(II) content as 0 μM. If values are higher, dilute the original sample and repeat the measurement until absorbance is within the linear range of the calibration curve.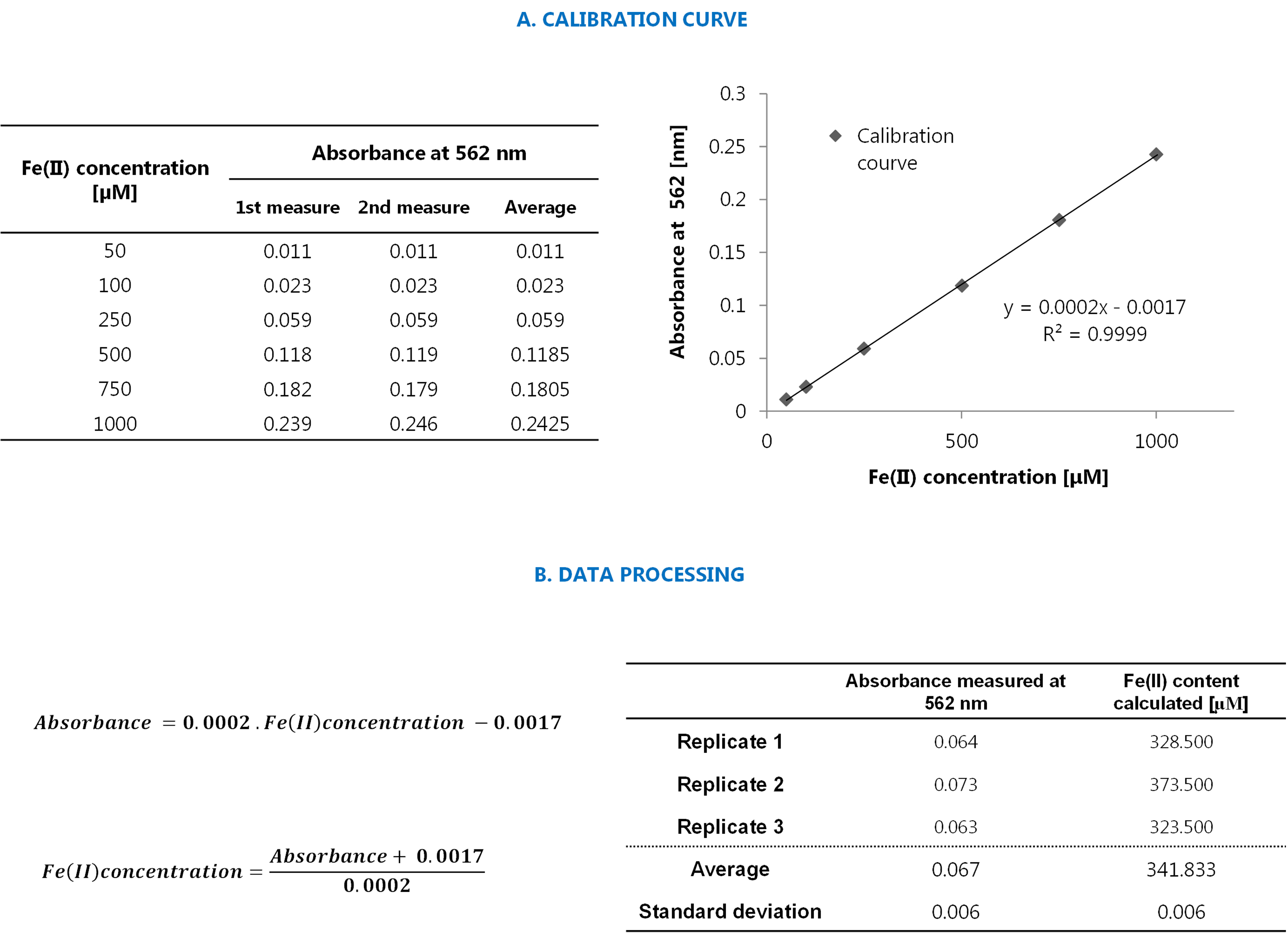
Figure 4. Procedure for the quantification of Fe(II) with the Ferrozine® reactive. A. Example of the calibration curve. On the left a table with the numeric data is presented; on the right the corresponding graph showing the equation and correlation coefficient of the calibration curve is shown. Data represent average values of duplicates. The increase in absorbance was linear between 50 and 1,000 μM and the correlation coefficients were 0.9999. B. Example of data processing. On the left, the equation extrapolated from the calibration curve is shown, and on the right, an example of data processing is presented. Data are from abiotic controls amended with Fe(II)-citrate sampled at day 0.
When all the absorbance values are converted to Fe(II) content, data can be presented in histograms as shown in Figure 5.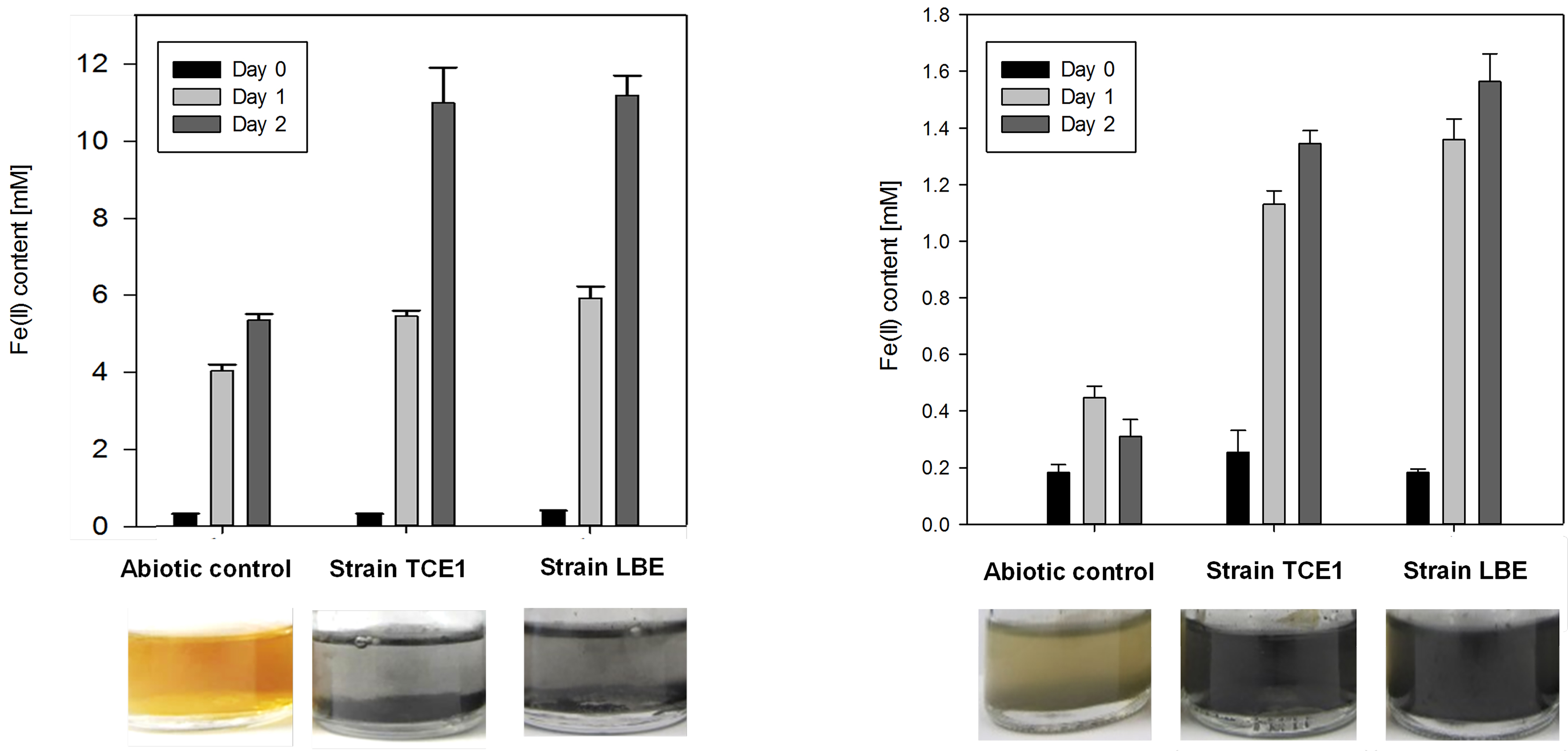
Figure 5. Quantification of Fe(II) content with the Ferrozine® assay. Graph represents Fe(II) content in the abiotic control as well as in cultures amended with soluble Fe(III)-citrate (left) and solid Fe(III) (right) as calculated from the calibration curve. - Characterization of biogenic minerals
Morphology of the biogenic crystals can be studied directly by observing the SEM micrographs. In order to identify the newly formed biogenic minerals, compare the obtained spectra (recorded on the surface of the treated coupons) with reference spectra found in the software library as well as with Raman shifts presented in literature (Frost et al., 2002; Monnier et al., 2011; Rémazeilles et al., 2013). Figure 6 shows an example of the results obtained for the corroded coupons treated with D. hafniense strains TCE1 and LBE.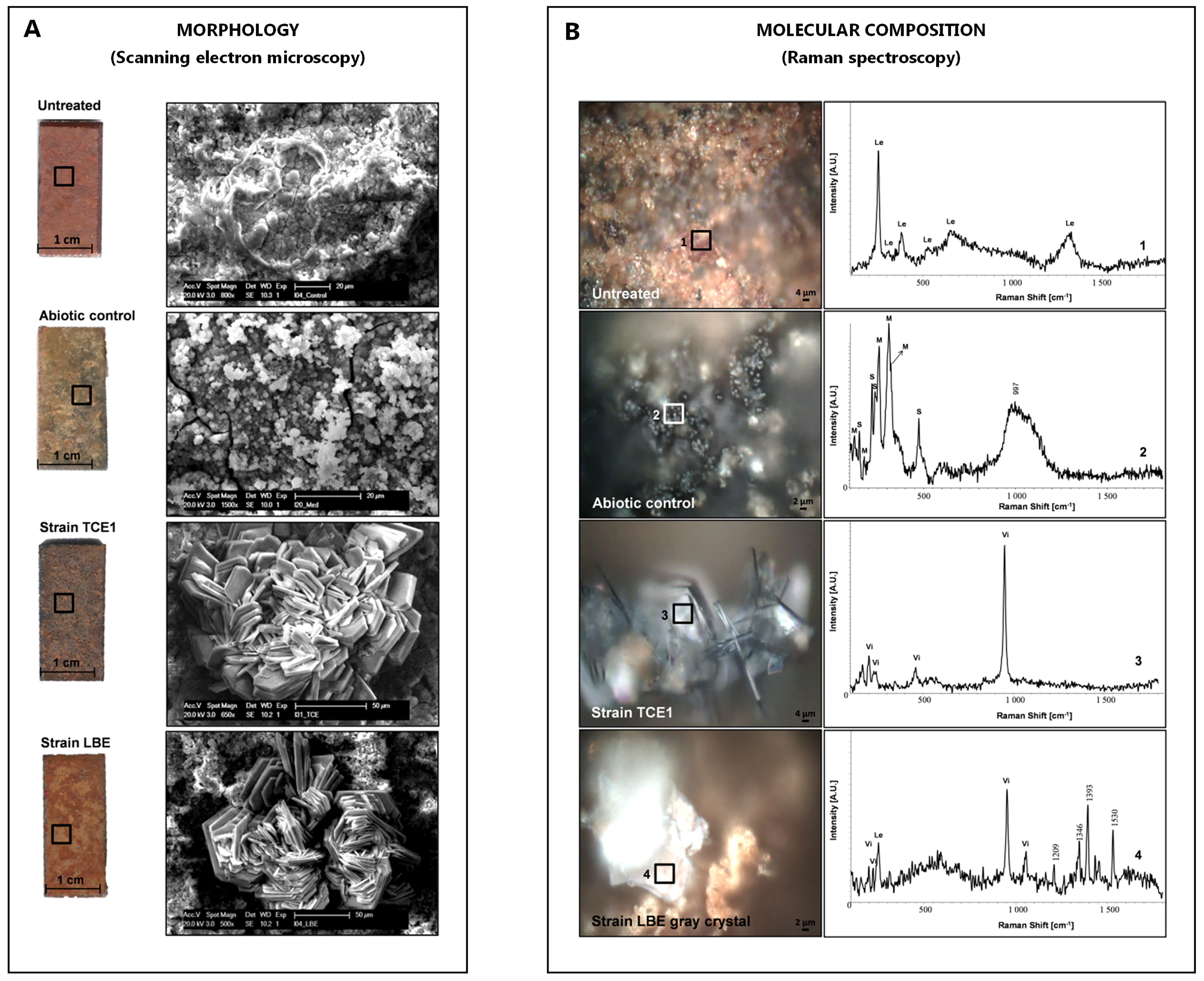
Figure 6. Iron reduction tests on corroded iron coupons treated with cultures of D. hafniense strains TCE1 and LBE. A. Morphology of the newly formed biogenic minerals. Left column: appearance of the coupons; right column: corresponding SEM images taken in the area indicated by the black square on the pictures of the coupons. B. Molecular composition of the surface of the coupons after incubation. Left column: area analyzed by Raman spectroscopy (black squares); and right column: corresponding Raman spectra. Minerals are identified as: 1: Lepidocrocite (Le), 2: Mixture of poorly crystallized mackinawite (M) and elemental sulfur (S), 3: Vivianite (Vi), and 4: Mixture of vivianite (Vi) and lepidocrocite (Le).
Notes
- Reproducibility
The collection of culture samples is a delicate step. In fact, due to the formation of iron precipitates during incubation, it is difficult to collect representative samples. Therefore, before sampling the cultures, makes sure to mix them well, otherwise the amount of iron in the sample will not be representative. - Abiotic control
Media for growing anaerobic bacteria are often complex and contain reducing agents such as Na2S. Therefore, in order to exclude abiotic reduction of Fe(III), it is essential to perform abiotic controls with all the iron sources tested. - Ferrozine® reagent
The intensity of the mixture containing the Ferrozine® reagent and the samples changes over time. Therefore, it is important to measure the absorbance precisely after 2 min of incubation, and to use the same procedure for all the samples. - Storage of treated coupons
The stability of the newly-formed biogenic minerals is unknown. So, until identification with Raman spectroscopy, store the iron coupons in a desiccator to avoid changes in the oxidation state of the biogenic minerals produced during the treatment.
Recipes
- Growth medium for D. hafniense
- N2-degassed H2O
- Boil, 500 ml of MilliQ water, with a hotplate stirrer
- Cool down under N2, distribute 80 ml to serum bottle, gas exchange for N2, autoclave (120 °C, 20 min)
- Store at room temperature up to 12 months
- Sterile serum bottles
Gas exchange for N2 and autoclave
Store at room temperature up to 12 months - Solution of sodium DL-lactate 40% (v/v)
- Dilute the 60% stock solution to 40% with MilliQ water
- Distribute 100 ml to serum bottle, gas exchange for N2, autoclave
- Store at 4 °C up to 12 months
- Solution of disodium fumarate 16% (v/v)
- Add 80 g of disodium fumarate to 500 ml of MilliQ water
- Distribute 100 ml to serum bottle, gas exchange for N2, autoclave
- Store at room temperature up to 12 months
- Reducing agent solution 1 M
- Wash crystals of Na2S•9H2O with N2-degassed H2O to remove the already oxidized part of the crystals
- Dry crystals with a tissue paper
- Weight 24.02 g of this compound (dry weight)
- Dissolve it in 100 ml of degassed-MilliQ water
- Filter sterilize into serum bottles with a 0.2 μm filter
- Gas exchange for N2
- Store at 4 °C up to 12 months
- Resazurin solution 0.5 g/L
Add 0.1 g of resazurin sodium salt to 200 ml of MilliQ water
Store at 4 °C up to 12 months - Vitamin solution 1
- Add 250 mg of cyanocobalamin to 1 L of MilliQ water
- Filter sterilize into a sterile serum bottle, gas exchange for N2
- Store at 4 °C up to 12 months
- Vitamin solution 2
- Add 50 mg of riboflavin to 1 L of MilliQ water
- Filter sterilize into a sterile serum bottle, gas exchange for N2
- Store at 4 °C up to 12 months
- Vitamin solution 3
- Add 100 mg of thiamine-hydrochloride to 1 L of MilliQ water
- Filter sterilize into a sterile serum bottle, gas exchange for N2
- Store at 4 °C up to 12 months
- Vitamins solution 4
- Add all of the components to 1 L of MilliQ water
50 mg of biotin
250 mg of p-aminobenzoate (sodium salt)
50 mg of pantothenate (sodium salt)
20 mg of folic acid•2H2O
50 mg of lipoic acid
100 mg of pyridoxine-hydrochloride
550 mg of nicotinic acid - Filter sterilize into sterile serum bottle with a 0.2 μm filter, gas exchange for N2
- Store at 4 °C up to 12 months
- Add all of the components to 1 L of MilliQ water
- Trace elements solution
- Dissolve 500 mg of EDTA in 900 ml of MilliQ water, adjust the pH to 7.0 with HCl, then add the following compounds:
2 mg of FeCl2•4H2O
100 mg of MnCl2•4H2O
190 mg of CoCl2•6H2O
70 mg of ZnCl2
2.55 mg of CuCl2•2H2O
5.52 mg of AlCl3
6 mg of H3BO3
41.4 mg of Na2MoO4•2H2O
24 mg of NiCl2•6H2O - Add MilliQ water to 1 L
- Store at 4 °C up to 12 months
- Dissolve 500 mg of EDTA in 900 ml of MilliQ water, adjust the pH to 7.0 with HCl, then add the following compounds:
- Carbonate solution
- Add 9.01 g of NH4HCO3 and 76.11 g of NaHCO3 to 1 L of MilliQ water
- Boil, cool down under N2/CO2 (4:1), distribute 49 ml to each serum bottle, gas exchange for N2/CO2 (4:1), autoclave
- Store at RT up to 12 months
- Solution A (basal medium)
- Add all of the components to 1 L of MilliQ water:
0.958 g of K2HPO4•3H2O
0.218 g of NaH2PO4•2H2O
0.1 g of Peptone
1 ml of Resazurin solution 0.5 g/L - Boil, cool down under N2/CO2 (4:1), distribute to serum bottles, gas exchange for N2/CO2 (4:1), autoclave
- Store at room temperature up to 12 months
- Add all of the components to 1 L of MilliQ water:
- Solution B (vitamin solution)
To 20 ml of anaerobic sterile MilliQ water, add aseptically the following solutions with syringes:
1 ml Trace elements solution
1 ml Vitamins solution 1
1 ml Vitamins solution 2
1 ml Vitamins solution 3
1 ml Vitamins solution 4
Store at 4 °C up to 3 months - Solution C (buffering/reducing solution)
To 49 ml of carbonate solution, add 1 ml of reducing agent solution
Store at 4 °C up to 3 months - Solution D
- Add 4.40 g of CaCl2•2H2O and 4.06 g of MgCl2•6H2O to 1 L of MilliQ water
- Distribute 200 ml in serum bottle, gas exchange for N2 and autoclave
- Store at 4 °C up to 12 months
To 45 ml of solution A, add the following components aseptically by syringe:
1.25 ml of solution B
2 ml of solution C
1.25 ml of solution D
1 ml of lactate solution
1 ml of fumarate solution
The pH should be between 7.0 and 7.6
To verify the pH, collect a 1 ml sample with a syringe and measure on a pH meter - N2-degassed H2O
- Soluble Fe(III)-citrate (35 g/L) – 100 ml
- HCl solutions to adjust pH
- Fill a part of a 100-ml graduated flask with MilliQ water
- Put 41.5 ml of HCl 37%
- Fill up to 100 ml with MilliQ water
- Perform dilutions in order to obtain a range of concentrations from 5 M (starting solution) to 0.01 M
- Store at room temperature up to 6 months
- NaOH solutions to adjust pH
- Dissolve 20 g of NaOH pellets in 100 ml of MilliQ water
- Perform dilutions in order to obtain a range of concentrations from 5 M (starting solution) to 0.01 M
- Store at room temperature up to 6 months
- Fe(III) solution
- Dissolve 3.5 g of Fe(III)-citrate in 100 ml of milliQ water. To facilitate dissolution, add a magnetic bar and mix the solution with a magnetic stirrer at 80 °C. This step can take 1-2 h. When the powder is completely dissolved no residual particle should be visible, the color of the solution becomes yellow and the pH is extremely acidic (pH 1-2)
- Adjust the pH of the solution to 7 by adding drops of NaOH. After this procedure, the solution becomes brown-orange in color
- To remove oxygen mark with an indelible pen the level of the solution in the flask, add extra MilliQ water to the solution, and let the solution boil until all the added water is evaporated (help yourself with the pen mark to detect the original volume of the solution). Then cool down the solution by flushing N2/CO2, and seal the serum bottle with rubber stoppers and metal caps using the serum bottle seal crimper. Sterilize the solution by autoclaving (120 °C, 20 min).
- Store at 4 °C up to 6 months
- HCl solutions to adjust pH
- Solid Fe(III) suspension
- Solid Fe(III) source
- In the original experiment, akaganeite (FeO0.833(OH)1.167Cl0.167) was used. Akaganeite was provided by the Swiss National Museum. This compound was synthesized following the protocols by Schwertmann and Cornell (2008). However, for this test any kind of insoluble Fe(III)-oxides or Fe(III)-oxyhydroxides can be used to prepare the suspension (i.e., Fe2O3 or α-FeO(OH), Sigma-Aldrich)
- Store at 4 °C up to 6 months
- Preparation of the suspension of solid Fe(III)-phase (akaganeite or goethite)
- For akaganeite suspension (10 g/L – 100 ml):
Add 1.0 g of akaganeite to 100 ml of MilliQ water - For goethite suspension (13 g/L – 100 ml):
Add 1.3 g of goethite to 100 ml of MilliQ water - Control the pH of the suspension and adjust it with drops of HCl or NaOH solutions to pH 7
- Repeat all the steps already described for the preparation of the soluble-Fe(III) solution in order to remove oxygen and sterilize the solution
- Store at 4 °C up to 6 months
- For akaganeite suspension (10 g/L – 100 ml):
- Solid Fe(III) source
- Ferrozine® reagents
- HCl solution 5 M
Same procedure previously described (B1. HCl solutions to adjust pH) - Stock solution of Fe(II) 1 M for calibration curve
- Clean a graduated flask of 1 L with HCl (5 M)
- Wash with MilliQ water
- Fill with a part of MilliQ water
- Add 41.55 ml of HCl 37% (S-20)
- Add 392.14 mg of Fe(II)-ammonium sulfate
- Fill up to 1 L with MilliQ water
- Dilute the stock solution to obtain the following concentrations: 50, 100, 250, 500, 750 and 1,000 μM.
- Aliquot and store at 4 °C up to 6 months (protect from light)
- Ferrozine® reagent
- Clean a graduated flask of 1 L with HCl (5 M)
- Wash with MilliQ water
- Fill with a part of MilliQ water
- Add 11.9 ml of HEPES buffer (final concentration 50 mM)
- Clean the flask wall with MilliQ water
- Add 1 g of Ferrozine®
- Fill up to 1 L with MilliQ water
- Adjust the pH with drops of NaOH solution to 7
- Store at 4 °C up to 2 months
- HCl solution 5 M
Acknowledgments
The authors are grateful to the Swiss National Science Foundation for the Ambizione grant (PZ00P2_142514, 2013-2016, Pi: Edith Joseph). The authors also want to acknowledge the research conservation laboratory of the Swiss National Museum for the help in conducting Raman investigations (Dr. Marie Woerle and Dr. Tiziana Lombardo) and providing the iron coupons used in the experiments.
This protocol is a modified version of the method described by Comensoli et al. (2017). In addition, the composition of the culture media was adapted from Gerritse et al. (1999), while the Ferrozine® assay employed to quantify Fe(II) ions in liquid solutions was adapted from Stookey (1970).
Competing interests
The authors have no conflicts of interest or competing interests to declare.
References
- Bosch-Roig, P. and Ranalli, G. (2014). The safety of biocleaning technologies for cultural heritage. Front Microbiol 5: 155.
- Cappitelli, F., Toniolo, L., Sansonetti, A., Gulotta, D., Ranalli, G., Zanardini, E. and Sorlini, C. (2007). Advantages of using microbial technology over traditional chemical technology in removal of black crusts from stone surfaces of historical monuments. Appl Environ Microbiol 73(17): 5671-5675.
- Cappitelli, F., Zanardini, E., Ranalli, G., Mello, E., Daffonchio, D. and Sorlini, C. (2006). Improved methodology for bioremoval of black crusts on historical stone artworks by use of sulfate-reducing bacteria. Appl Environ Microbiol 72(5): 3733-3737.
- Comensoli, L., Maillard, J., Albini, M., Sandoz, F., Junier, P. and Joseph, E. (2017). Use of bacteria to stabilize archaeological iron. Appl Environ Microbiol 83(9).
- Fortune, W. B. and Mellon, M. G. (1938). Determination of iron with o-phenanthroline: a spectrophotometric study. Ind Eng Chem 10(2): 60-64.
- Frost, R. L., W. Martens, P. Williams. and J. T. Kloprogge. (2002). Raman and infrared spectroscopic study of the vivianite-group phosphates vivianite, baricite and bobierrite. Mineral Mag 66(6): 1063-1073.
- Gerritse, J., Drzyzga, O., Kloetstra, G., Keijmel, M., Wiersum, L. P., Hutson, R., Collins, M. D. and Gottschal, J. C. (1999). Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl Environ Microbiol 65(12): 5212-5221.
- Jonkers, H. M. (2011). Bacteria-based self-healing concrete. Heron 56(1/2)
- Joseph, E., Cario, S., Simon, A., Worle, M., Mazzeo, R., Junier, P. and Job, D. (2011). Protection of metal artifacts with the formation of metal-oxalates complexes by Beauveria bassiana. Front Microbiol 2: 270.
- Joseph, E., Letardi, P., Comensoli, L., Simon, A., Junier P., Job, D. and Wörle, M. (2013). Assessment of a biological approach for the protection of copper alloys artefacts. In: Hyslpop, E., Gonzalez, V., Troalen, L. and Wilson, L. (Eds.). Conference Proceedings of Metal 2013, Interim Meeting of the ICOM-CC Metal WG. Historic Scotland, Edinburgh, 203-208.
- Joseph, E., Simon, A., Mazzeo. R., Job. Daniel and Wörle, M. (2012). Spectroscopic characterization of an innovative biological treatment for corroded metal artefacts. Raman Spectroscopy in Art and Archaeology 43(11): 1612-1616.
- Kooli, W. M., Comensoli, L., Maillard, J., Albini, M., Gelb, A., Junier, P. and Joseph, E. (2018). Bacterial iron reduction and biogenic mineral formation for the stabilisation of corroded iron objects. Sci Rep 8(1): 764.
- Meissner, K., T. Lippert, A. Wokaun and D. Guenther (2004). Analysis of trace metals in comparison of laser-induced breakdown spectroscopy with LA-ICP-MS. Thin Solid Films 453: 316-322.
- Monnier, J., L. Bellot-Gurlet, D. Baron, D. Neff, I. Guillot and P. Dillmann. (2011). A methodology for Raman structural quantification imaging and its application to iron indoor atmospheric corrosion products. J Raman Spectrosc 42(4): 773-781.
- Ranalli, G., Alfano, G., Belli, C., Lustrato, G., Colombini, M. P., Bonaduce, I., Zanardini, E., Abbruscato, P., Cappitelli, F. and Sorlini, C. (2005). Biotechnology applied to cultural heritage: biorestoration of frescoes using viable bacterial cells and enzymes. J Appl Microbiol 98(1): 73-83.
- Rémazeilles, C., K. Tran, E. Guilminot, E. Conforto and P. Refait. (2013). Study of Fe (II) sulphides in waterlogged archaeological wood. Stud Conserv 58(4): 297-307.
- Rimmer, M., Watkinson, D. and Wang, Q. (2012). The efficiency of chloride extraction from archaeological iron objects using deoxygenated alkaline solutions. Stud Conserv 57(1): 29-41
- Schwertmann, U. and Cornell, R. M. (2008). Iron oxides in the laboratory: preparation and characterization. John Wiley & Sons.
- Scott, D. A. and Eggert, G. (2009). Iron and steel in art: corrosion, colorants, conservation. Archetype Publications.
- Stookey, L. L. (1970). Ferrozine - a new spectrophotometric reagent for iron. Anal Chem 42(7): 779-781.
- Thomas, R. (2013). Practical guide to ICP-MS: a tutorial for beginners. CRC Press.
- Whittaker, P., Seifried, H. E., San, R. H., Clarke, J. J. and Dunkel, V. C. (2001). Genotoxicity of iron chelators in L5178Y mouse lymphoma cells. Environ Mol Mutagen 38(4): 347-356.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Comensoli, L., Maillard, J., Kooli, W. M., Junier, P. and Joseph, E. (2018). Soluble and Solid Iron Reduction Assays with Desulfitobacterium hafniense. Bio-protocol 8(17): e3002. DOI: 10.21769/BioProtoc.3002.
Category
Microbiology > Microbial metabolism > Other compound
Biochemistry > Other compound > Ion > Iron
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










