- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Human Endothelial Cell Spheroid-based Sprouting Angiogenesis Assay in Collagen
Published: Vol 8, Iss 17, Sep 5, 2018 DOI: 10.21769/BioProtoc.2995 Views: 21456
Reviewed by: Vivien Jane Coulson-ThomasThomas KorffSudan Puri

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analysis of Mouse Brain Sections by Live-cell Time-lapse Confocal Microscopy
Tao Yang [...] Bing Ye
Apr 5, 2023 1939 Views

An Improved Protocol for the Matrigel Duplex Assay: A Method to Measure Retinal Angiogenesis
Kathleen C. Brown [...] Piyali Dasgupta
Dec 5, 2023 1908 Views

Utilizing EdU to Track Leukocyte Recruitment to the Brain
Zoie K. Lipfert [...] David P. Sullivan
Dec 5, 2025 1560 Views
Abstract
Angiogenesis, the formation of new blood vessels from pre-existing ones plays an important role during organ development, regeneration and tumor progression. The spheroid-based sprouting assay is a well-established and robust method to study the influence of genetic alterations or pharmacological compounds on capillary-like tube formation of primary cultured endothelial cells. A major advantage of this assay is the possibility to study angiogenesis in a 3D environment. Endothelial cells are cultured as hanging drops to form spheroids. Those spheroids are embedded into a collagen matrix and tube formation is analyzed 24 h later. By analyzing sprout number and sprout length the effects of genetic manipulation or drug treatment on angiogenesis can be investigated.
Keywords: Endothelial cellsBackground
Blood vessels supply organs with oxygen and nutrients. In cases the local demands are not met anymore, cells secrete vascular endothelial growth factor (VEGF) to induce the formation of new blood vessels. The new vessel sprout is composed of a leading tip cell which is trailed by stalk cells (Potente and Makinen, 2017). Angiogenesis occurs under physiological conditions (e.g., growth of muscle and adipose tissue) as well as pathological conditions (e.g., wound healing, macular degeneration, and tumor growth). As such, there is a great need to decipher the basic mechanisms coordinating angiogenesis and to test compounds that interfere with pathological angiogenesis.
The spheroid-based sprouting assay, which was developed by Dr. Thomas Korff and Dr. Hellmut Augustin in the late 90s (Korff and Augustin, 1999), enables researchers to investigate the effects of drugs or genetic manipulations on sprouting angiogenesis in a fast and robust manner (Heiss et al., 2015). One great advantage of the spheroid-based sprouting assay is the analysis of sprout formation in a 3D environment. This promotes cell-cell signaling between endothelial cells. Upon stimulation with VEGF endothelial cells degrade the surrounding matrix and invade it. This mimics the situation in vivo. Thereby, this assay better reflects in vivo angiogenesis than other well known in vitro angiogenesis assays such as 2D tube formation on Matrigel (Nowak-Sliwinska et al., 2018).
Sprout number and length are read-outs for the angiogenic potential. In addition, this assay can be used to analyze competition for the tip cell position. Therefore, genetically modified endothelial cells, which are labeled with different fluorophores, are mixed before spheroid formation (Figure 1). Thereby, it can be analyzed, which genetic manipulation leads to a preference for the tip or the stalk position (Tetzlaff et al., 2018). With live-cell imaging it is possible to investigate the migration of endothelial cells and the dynamic competition of endothelial cells for the tip position.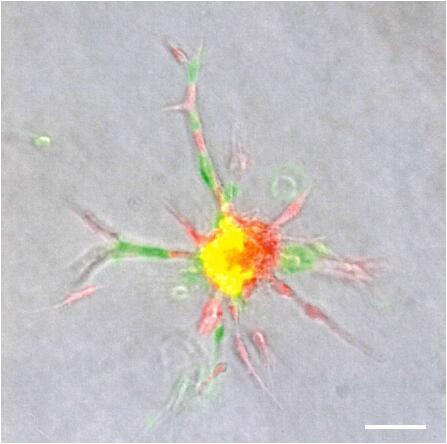
Figure 1. Endothelial cells expressing either mCherry or GFP mixed before spheroid formation. Spheroid was embedded in a collagen matrix and sprouting was analyzed 24 h later by using a fluorescence microscope. Scale bar = 100 µm.
Materials and Reagents
- Pipette tips (10 µl, 200 µl, 1,000 µl) (Starlab, catalog numbers: S1111-3700 , S1111-0706 , S1111-6701 )
- Reservoir (Thermo Fisher Scientific, catalog number: 95128093 )
- Serological pipettes (5 ml, 10 ml, 25 ml) (Corning, catalog numbers: 4487 , 4488 , 4489 )
- 10 cm square Petri dish (Greiner Bio One International, catalog number: 688102 )
- 6-well plate (Corning, catalog number: 3516 )
- 24-well plate for suspension culture (Greiner Bio One International, catalog number: 662102 )
- 15 ml conical tube (Corning, catalog number: 352096 )
- 50 ml conical tube (Corning, catalog number: 352070 )
- Human umbilical vein endothelial cells: freshly isolated from three different donors (also commercially available)
- Rat tails
- Acetic acid (Carl Roth, catalog number: 3738.2 )
- Endopan 3 Basal Medium (Pan-Biotech, catalog number: P04-0010B )
- Endopan 3 Medium plus Supplements (Pan-Biotech, catalog number: P04-0010K )
- Ethanol (VWR, catalog number: 20821.330 )
- FBS (Merck, Biochrom, catalog number: S 0615 )
- FGF2 (R&D Systems, catalog number: 234-FSE-025 )
- 10x Medium 199 (Sigma-Aldrich, catalog number: M0650 )
- Methyl cellulose, 4,000 centipoises (Sigma-Aldrich, catalog number: M0512 )
- Sodium hydroxide (Sigma-Aldrich, catalog number: 30620 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: 158127 )
- PBS (Thermo Scientific Fisher, catalog number: 14190169 )
- 0.05% trypsin-EDTA (Thermo Scientific Fisher, catalog number: 25300054 )
- VEGF-A165 (R&D Systems, catalog number: 293-VE-010 )
- Methocel stock solution (see Recipes)
- Collagen stock solution (see Recipes)
Equipment
- 500 ml bottle (Fisher Scientific, catalog number: FB800500 )
- Magnetic stirrer (Heidolph Instruments, catalog number: 505-20000-00 )
- 12-channel pipette (Volume 10-100 μl) (Eppendorf, catalog number: 3125000044 )
- Hemacytometer; Neubauer counting chamber (BRAND, catalog number: 717805 )
- Pipette-aid (BRAND, catalog number: 26304 )
- Autoclave (VWR, Tuttnauer, catalog number: 481-0585 )
- Balance (KERN & SOHN, catalog number: PBJ 4200-2M )
- Centrifuge with swinging-bucket rotor and adaptors for 15 ml and 50 ml conical tubes (Eppendorf, model: 5810 , catalog number: 5810000320)
- Humidified cell culture incubator set to 37 °C and 5% CO2 (Thermo Fisher Scientific, HeracellTM 150CU , catalog number: 50116047)
- Light microscope (Leica Microsystems, model: Leica DM IRB )
- Safety cabinet (Thermo Fisher Scientific, model: Safe 2020 , catalog number: 51026638)
- Water bath (GFL, catalog number: 1012 )
Software
- FIJI (Curtis Rueden, University of Wisconsin-Madison, Laboratory for Optical and Computational Instrumentation)
Procedure
- Preparation of hanging drops
Culture human umbilical vein endothelial cells (HUVEC) in Endopan 3 medium containing supplements and FBS until cells are confluent. This protocol is adjusted to running the experiment under three conditions: basal, VEGF stimulation, FGF2 stimulation.- Wash HUVEC twice with PBS.
- Detach cells from cell culture plate using trypsin-EDTA.
- Stop reaction using PBS containing 10% FBS, spin down cells at 200 x g for 5 min, discard supernatant and re-suspend cells in cell culture medium.
- Count cells using a hemacytometer.
Note: Twenty thousand cells are needed per condition. Due to loss of cells during the whole procedure, calculate to ensure you have an extra 20,000 cells. In total, 80,000 cells are needed for the preparation of the hanging drops. - Transfer 80,000 cells to a fresh 15 ml conical tube. Add cell culture medium (Endopan 3 medium plus supplements and FBS) to a total volume of 4 ml.
- Add 1 ml of methocel stock solution, which can improve spheroid formation.
Note: Some researchers do not use methocel at this step, however in our hands, this improves spheroid formation, which was also reported elsewhere (Leung et al., 2015). - Mix solution carefully and transfer it to a sterile multichannel pipette reservoir.
- Using a 12-channel pipette, pipet 25 μl drops of the solution onto a 10 cm square Petri dish. Take care to avoid air bubbles (Figure 2A) (Video 1). Video 1. Preparation of hanging drops. Endothelial cell-methocel-solution is pipetted on a square Petri dish for hanging drop formation.
- To form spheroids, incubate drops upside-down in a humidified cell culture incubator set at 37 °C and 5% CO2 for 24 h (Figure 2B).
Note: Hereon prepare the methocel solution containing 20% FBS which is needed on the next day. Mix carefully the methocel stock solution with 20% FBS. Avoid air bubbles. In case air bubbles are formed, check whether they disperse on the next day. If not, use another batch.
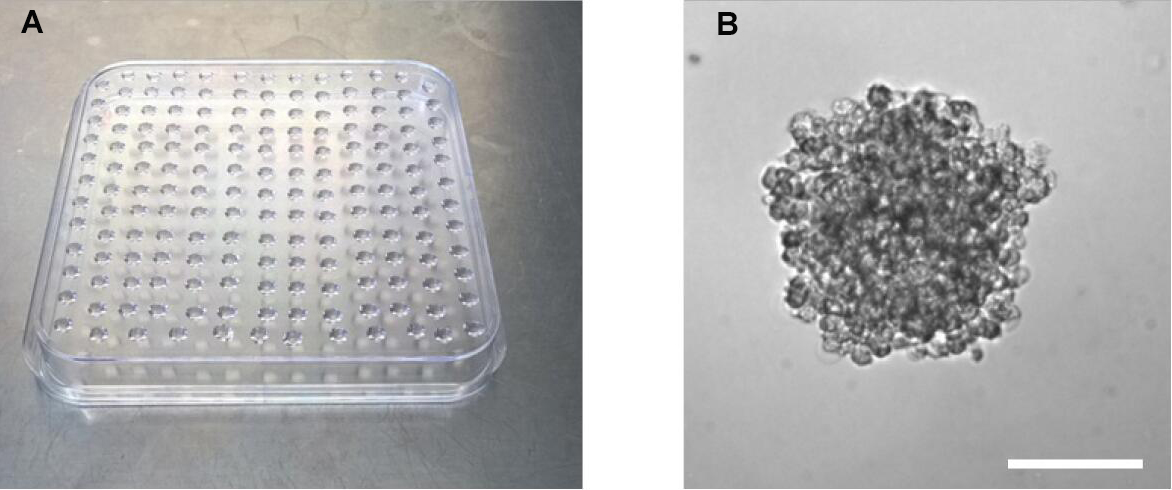
Figure 2. Preparation of hanging drops for spheroid formation. A. Petri dish with hanging drops. B. Representative image of a spheroid in a hanging drop 24 h after upside-down incubation. Scale bar = 100 µm.
- Embedding of spheroids
Note: Before continuing with the protocol, check whether spheroids formed overnight. In case multiple small cell clumps have been formed, discard them.- Place collagen stock solution, 10x Medium 199, 0.2 N NaOH and methocel containing 20% FBS on ice.
- Using a 10 ml serological pipette gently wash off the hanging drops (containing the spheroids) with 10 ml PBS by pipetting up and down. Transfer solution into a 15 ml conical tube (Video 2). Video 2. Transfer of spheroids. Hanging drops containing spheroids are washed off and solution is transferred to a 15 ml conical tube.
- Spin down spheroids at 200 x g for 5 min. Then aspirate supernatant and add 2 ml of methocel containing 20% FBS (Video 3). Video 3. Mixing of spheroids and collagen. Spheroids are suspended in methocel containing 20% FBS and mixed with collagen. Solution is added to wells of a 24-well plate.
- Prepare collagen medium: Mix 4 ml collagen stock solution with 0.5 ml 10x Medium 199 on ice. Adjust pH value by adding dropwise sterile ice-cold 0.2 N NaOH. Subsequently invert the tube carefully to ensure thorough mixing. Add NaOH until the pH indicator of the collagen medium changes the color from yellow to orange (approximately 250-500 µl).
Important: Prepare the collagen medium on ice to prevent collagen polymerization. - Add 2 ml of the collagen from Step B5 to the spheroids which were re-suspended in methocel containing 20% FBS.
Note: Mix the solution carefully. Avoid air bubbles. - Add 1 ml of the spheroid-collagen solution per well of a 24-well plate.
- Incubate the plate in a humidified cell culture incubator set at 37 °C and 5% CO2 for 30 min to allow polymerization of the collagen matrix (Figure 3).

Figure 3. Collagen matrices in which spheroids are embedded were added to wells of a 24-well suspension culture plate - Stimulate spheroids with 100 µl of basal medium, 25 ng/ml VEGF-A (in basal medium) and 25 ng/ml FGF2 (in basal medium) by adding it dropwise on the collagen matrix.
Note: The volume of the added medium can be changed if necessary. - Incubate collagen matrix for 24 h in a humidified cell culture incubator.
- Stop the sprouting assay by adding 1 ml of 10% paraformaldehyde and store plates at 4 °C (for up to 4 weeks).
Data analysis
- To analyze the pro- or anti-angiogenic effect of a gene mutation or a chemical compound, the number of sprouts and the length of the sprouts have to be determined.
- Aspirate the paraformaldehyde from the collagen matrix.
- Place the 24-well plate under an inverted microscope.
- Acquire images of 10 randomly selected spheroids.
- Count the number of sprouts per spheroid and measure the sprout length of all sprouts per spheroid using the imaging software FIJI (Figure 4).
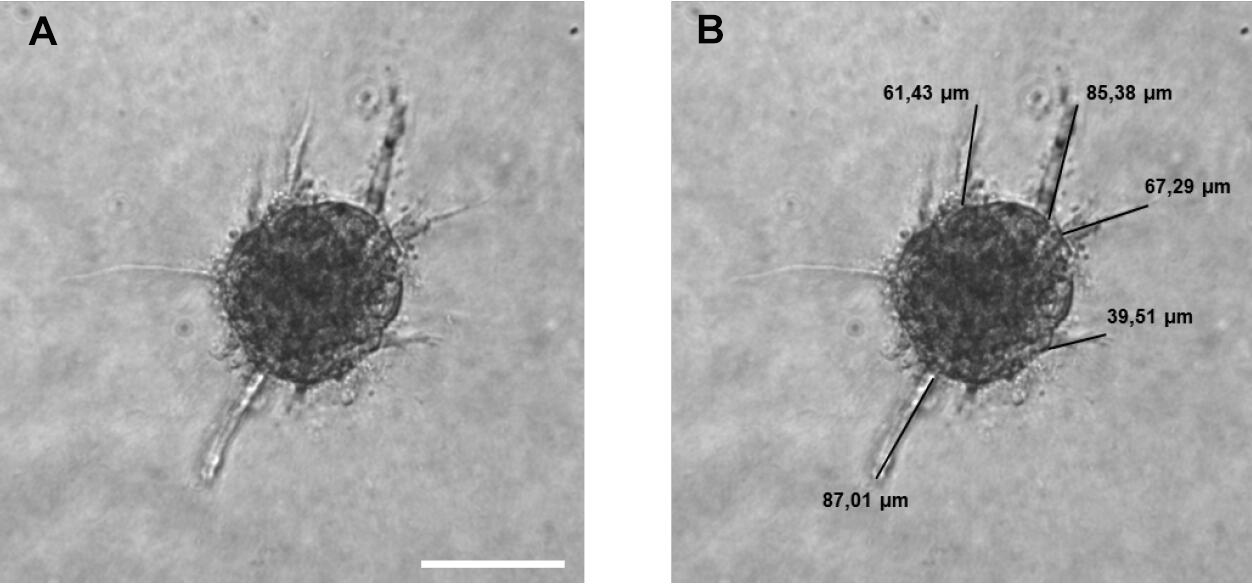
Figure 4. Sprouting analysis of a HUVEC spheroid. A. Image of a HUVEC spheroid embedded in a collagen matrix after 24 h of sprouting. B. Image analysis: Measuring the sprout length of all sprouts of the spheroid by using FIJI software. A line was manually drawn, and the length of the line was determined. Scale bar = 100 µm.
- The following parameters are used as readout for the angiogenic activity:
- Number of sprouts per spheroid.
- Average sprout length of the sprouts per spheroid.
- Cumulative sprout length of all sprouts per spheroid.
- The parameters of ten technical replicates are averaged and at least 3 biological replicates should be analyzed. Spheroids stimulated with VEGF-A or FGF2 should show a clearly increased sprouting ability compared to basal spheroids (Figure 5).
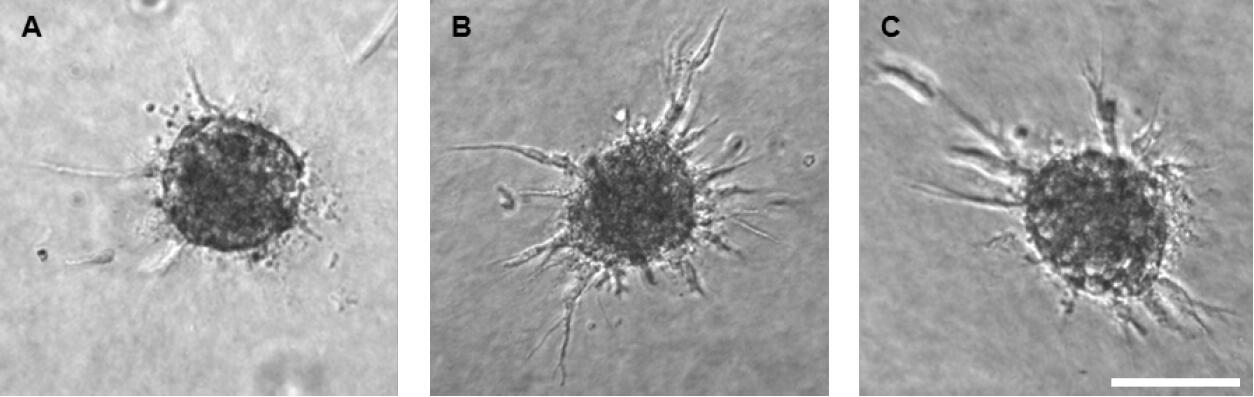
Figure 5. HUVEC spheroid embedded in a collagen matrix after 24 h of sprouting. The assay was performed under basal conditions (A), with 25 ng/ml VEGF-A stimulation (B) and with 25 ng/ml FGF2 stimulation (C). Scale bar = 100 µm.
Notes
- Methocel stock solution: Proper centrifugation of methyl cellulose solution is important to remove debris. Otherwise, cells can stick to the plate resulting in multiple small spheroids instead of a single one. Stock solution can be stored at 4 °C for up to 6 months.
- Collagen stock solution: Prepare the collagen stock solution four weeks in advance, since freshly isolated collagen can cause higher inter-experimental variation. Stock solution can be stored at 4 °C for at least 6 months.
- Embedding of spheroids: After neutralization, collagen medium should be clear.
Recipes
- Methocel stock solution
- Weigh 6 g of methyl cellulose, transfer it to a 500 ml bottle and add a clean magnetic stirrer. Autoclave it at 121 °C for 20 min
- Heat up 250 ml Endopan 3 basal medium to 60 °C and add it to the autoclaved methyl cellulose
- Stir the solution for 20 min at room temperature
- Add 250 ml Endopan 3 basal medium (room temperature) and stir the solution overnight at 4 °C
- Aliquot the stock solution into 50 ml conical tubes
- Centrifuge methocel solution for 2 h at room temperature and 5,000 x g
- Use supernatant of the methocel solution for spheroid culture
- Collagen stock solution
Note: A detailed video protocol has been published by (Bruneau et al., 2010).- Place two rat tails in 500 ml of 70% ethanol and incubate for 20 min at room temperature
- Cut the skin by using a scalpel and peel off the skin from the tail root to the tail tip
- Wash tails in 70% ethanol
- Break every second vertebral and isolate the tendons
Be careful: Do not isolate the attached connective tissue. - Collect tendons and incubate them in 500 ml of 70% ethanol for 20 min
- Dry sterilized tendons in a safety cabinet for 30-60 min
- Transfer tendons into 250 ml of 0.1 % sterile acetic acid (v/v in H2O) and incubate them for 48 h at 4 °C
- Aliquot the collagen solution and centrifuge the aliquots at 4 °C and 17,000 x g for 90 min
- Collect the clear supernatant, aliquot it into 50 ml conical tubes and store it at 4 °C
- For equilibration, add 0.5 ml of ten-fold Medium 199 to 4 ml of collagen solution, mix and incubate it for at least 15 min on ice. In case the mixed solution solidifies, dilute the collagen stock solution with 0.1% sterile acetic acid until the Medium 199-collagen-solution stays liquid.
Acknowledgments
The assay was originally developed by Dr. T. Korff and Dr. H.G. Augustin (Korff and Augustin, 1999; Pfisterer and Korff, 2016).
This work was supported by the Deutsche Forschungsgemeinschaft (SFB-TR23, project A7) and the Helmholtz society to A.F.
Competing interests
The authors declare that they have no conflict of interest.
References
- Bruneau, A., Champagne, N., Cousineau-Pelletier, P., Parent, G. and Langelier, E. (2010). Preparation of rat tail tendons for biomechanical and mechanobiological studies. J Vis Exp(41).
- Heiss, M., Hellstrom, M., Kalen, M., May, T., Weber, H., Hecker, M., Augustin, H.G. and Korff, T. (2015). Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J 29(7): 3076-3084.
- Korff, T. and Augustin, H. G. (1999). Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci 112 (Pt 19): 3249-3258.
- Leung, B. M., Lesher-Perez, S. C., Matsuoka, T., Moraes, C. and Takayama, S. (2015). Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci 3(2): 336-344.
- Nowak-Sliwinska, P., Alitalo, K., Allen, E., Anisimov, A., Aplin, A.C., Auerbach, R., Augustin, H.G., Bates, D.O., van Beijnum, J. R., Bender, R. H. F., Bergers, G., Bikfalvi, A., Bischoff, J., Böck, B. C. and Brooks, P. C. (2018). Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 21: 425-532.
- Pfisterer, L. and Korff, T. (2016). Spheroid-Based in vitro angiogenesis model. Methods Mol Biol 1430: 167-177.
- Potente, M. and Makinen, T. (2017). Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 18(8): 477-494.
- Tetzlaff, F., Adam, M. G., Feldner, A., Moll, I., Menuchin, A., Rodriguez-Vita, J., Sprinzak, D. and Fischer, A. (2018). MPDZ promotes DLL4-induced Notch signaling during angiogenesis. Elife 7: e32860.
Article Information
Copyright
Tetzlaff and Fischer. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tetzlaff, F. and Fischer, A. (2018). Human Endothelial Cell Spheroid-based Sprouting Angiogenesis Assay in Collagen. Bio-protocol 8(17): e2995. DOI: 10.21769/BioProtoc.2995.
- Tetzlaff, F., Adam, M. G., Feldner, A., Moll, I., Menuchin, A., Rodriguez-Vita, J., Sprinzak, D. and Fischer, A. (2018). MPDZ promotes DLL4-induced Notch signaling during angiogenesis. Elife 7: e32860.
Category
Developmental Biology > Cell growth and fate > Angiogenesis
Cancer Biology > Angiogenesis > Cell biology assays > Cell migration
Cell Biology > Cell movement > Cell migration
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











