- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pentylenetetrazole (PTZ)-induced Convulsion Assay to Determine GABAergic Defects in Caenorhabditis elegans
Published: Vol 8, Iss 17, Sep 5, 2018 DOI: 10.21769/BioProtoc.2989 Views: 6152
Reviewed by: Alessandro DidonnaYusuke TominaDURAI SELLEGOUNDERAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Behavioral Assay to Examine the Effects of Kavalactones on Caenorhabditis elegans Neuromuscular Excitability
Bwarenaba B. Kautu [...] M. Shawn Mengarelli
Sep 20, 2018 5230 Views

Simultaneous Monitoring Cytoplasmic Calcium Ion and Cell Surface Phosphatidylserine in the Necrotic Touch Neurons of Caenorhabditis elegans
Yoshitaka Furuta and Zheng Zhou
Oct 20, 2021 2623 Views

Aerotaxis Assay in Caenorhabditis elegans to Study Behavioral Plasticity
Qiaochu Li [...] Karl Emanuel Busch
Aug 20, 2022 2251 Views
Abstract
Pentylenetetrazole (PTZ) is a GABAA receptor antagonist and is used to monitor presynaptic defects in the release of the inhibitory neurotransmitter GABA. PTZ is a competitive inhibitor of GABA, and prevents binding of GABA on the GABAA receptors present on the surface of muscle. In the absence of GABA binding, the excitatory to inhibitory signal ratio increases resulting in a convulsive phenotype. This assay provides a fast and reliable method to detect presynaptic defects in GABAergic synaptic transmission. The assay is based on correlating the extent of convulsions with the degree of presynaptic GABA release defects.
Keywords: PTZBackground
Synapses are the asymmetric intercellular junctions that mediate synaptic transmission. They constitute the fundamental units of brain circuitry that enable execution of complex behaviors. The arrival of an action potential causes calcium influx via voltage-gated calcium channels resulting in depolarization of the plasma membrane. Upon this pre-synaptic depolarization, synaptic vesicles fuse with the plasma membrane releasing their contents into the synaptic cleft via a highly controlled process (Sudhof, 1995; Sudhof, 2004).
Locomotion is a prominent behavioral output in Caenorhabditis elegans. Neural circuits that generate coordinated dorso-ventral sinusoidal bends allow for normal locomotion in C. elegans. Locomotory behavior is orchestrated at multiple levels and involves the integration of diverse sensory cues by multiple sensory neurons that are processed by the interneurons and ultimately direct changes at the neuromuscular junctions (NMJ) (de Bono and Maricq, 2005; Bargmann, 2012). The C. elegans NMJ has three main components; the excitatory cholinergic motor neurons, the inhibitory GABAergic motor neurons and the postsynaptic muscle cells (White et al., 1976; White et al., 1986). Cholinergic signaling mediates muscle contraction while GABAergic signaling fine-tunes C. elegans locomotion by exerting a regulatory control on excitatory signaling. Any defect in GABAergic signaling results in an increased excitatory signal at the NMJ resulting in more muscle contraction.
GABA released from the presynaptic GABAergic motor neuron functions via GABAA receptors, which encode GABA-gated chloride channels (Schofield et al., 1987). GABAA receptors contain a neurosteroid or PTZ-binding site, right next to the subunit containing the GABA-binding site. In the event of reduced presynaptic GABA release and in the presence of PTZ, PTZ binds the neurosteroid-binding site hampering the binding of GABA at the GABA- binding site. A reduction in GABA binding causes a decrease in the inhibitory signaling, resulting in a convulsive phenotype called ‘head bobs’ or anterior convulsions (Williams et al., 2004; Locke et al., 2008) and depicted in Figure 1. The convulsive phenotype occurs because of an altered excitatory to inhibitory input ratio to the body-wall muscles. The PTZ assay is often used as a secondary assay in conjunction with the Aldicarb assay (Oh and Kim, 2017), to investigate the role of genes involved in GABA synaptic transmission. The results from this assay can be further supported by additional experimentation including electrophysiological recordings.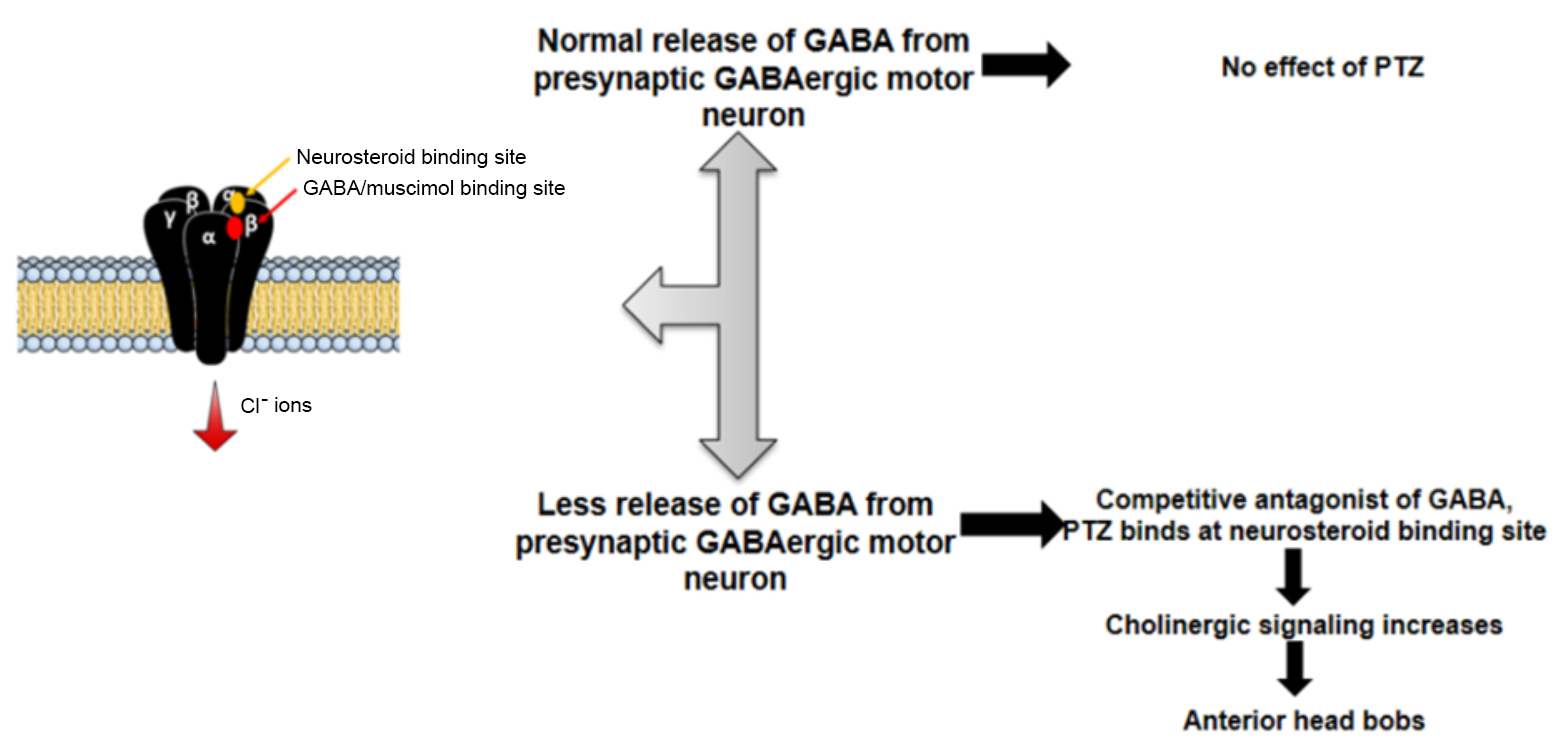
Figure 1. Mechanism of PTZ-induced anterior head bobs. PTZ is a competitive antagonist of GABA. If the presynaptic GABA release is lower than that seen in WT animals, PTZ binds at the neurosteroid binding site, thus hampering the binding of GABA at GABA-binding site. In the absence of GABA signaling, excitatory cholinergic signaling increases resulting in anterior convulsions called ‘head-bobs’. Hence, mutants showing anterior convulsions on PTZ plates suggest defective presynaptic GABA release.
Materials and Reagents
- 35 mm and 60 mm Petri dishes (35 mm: Tarsons, catalog number: 460035 ; 60 mm: Tarsons, catalog number: 460061 )
- Spreader (Tarsons, catalog number: 920081 )
- 99.99% platinum wire (Sigma-Aldrich, catalog number: 267201 )
- C. elegans: N2 (Wild type worms)(University of Minnesota, Caenorhabditis Genetic Center) and casy-1(tm718) (National Bio resource Project, Japan)
- Escherichia coli OP50 (University of Minnesota, Caenorhabditis Genetic Center)
- Pentylenetetrazole (PTZ) (Sigma-Aldrich, catalog number: P6500 )
- Ethanol (Ethyl alcohol Absolute, ACS-ISO grade) (Merck, catalog number: 100983 )
- Cholesterol (SRL Sisco Research Laboratories, catalog number: 54181 )
- Calcium chloride dihydrate (CaCl2•2H2O) (Sigma-Aldrich, catalog number: C3306 )
- Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: M7506 )
- Potassium phosphate, monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5379 )
- Potassium phosphate, dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P8281 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Bacto-agar (HiMedia Laboratories, catalog number: GRM026 )
- Bacto-peptone (SRL Sisco Research Laboratories, BactoTM, catalog number: 95292 )
- 100 mg/ml PTZ stock solution (see Recipes)
- 5 mg/ml cholesterol (see Recipes)
- 1 M CaCl2 stock solution (see Recipes)
- 1 M MgSO4 stock solution (see Recipes)
- 1 M KPO4, pH 6.0 stock solution (see Recipes)
- Nematode growth medium (NGM) agar plates (see Recipes)
Equipment
- Pipettes (Eppendorf, model: Research® plus, catalog number: 2231000224 )
- 2 L glass conical flask (DWK Life Sciences, DURAN, catalog number: 2121763 )
- Autoclave (Equitron-7431 SLEFA)
- Microscope (ZEISS, model: Stemi 2000 C )
Software
- GraphPad Prism v6 (GraphPad Software)
Procedure
- For routine growth and maintenance of C. elegans, see Wormbook chapter Maintenance of C. elegans (Stiernagle, 2006). Maintain C. elegans strains to be used in the assay in plates without contamination.
- Prepare fresh 35 mm Nematode Growth Medium (NGM) plates (see Recipes) containing 3 ml media, at least one day before the assay and store at 4 °C until use.
- Seed regular 60 mm NGM agar plates for routine maintenance with 15 μl E. coli OP50 under sterile conditions in a bacterial hood two days before the assay. Allow the plates to dry at room temperature.
- Place about 20-30 L4 stage wild-type (WT), N2, and mutant strains of interest using a pick made of platinum wire (described in Stiernagle, 2006) onto the 60 mm NGM plates seeded with E. coli OP50 and allow the worms to grow at 20 °C for about 20 h.
- On the day of assay, take out 35 mm NGM plates from 4 °C and allow them to attain room temperature. Pipette 300 μl of 100 mg/ml PTZ stock solution at the center of the plate and spread it uniformly over the entire NGM surface to obtain a final concentration of 10 mg/ml. Allow the plates to dry for 35 min with lids closed. Keep the same number of 35 mm NGM plates without PTZ to be used as control in the experiment. Spread 300 μl of Ethanol on control plates to avoid behavioral changes due to exposure of ethanol. Once the plates are dried, put a 10 μl drop of concentrated E. coli OP50 (overnight culture concentrated by centrifugation) at the center of the plate and let it dry for at least 2 h at room temperature. The plates are now ready for the assay.
- Transfer 10-15 worms of each genotype from Step 4 onto the center of the plate on food on both the control and PTZ-containing plates. Leave the plates undisturbed for 30 min at room temperature (~23 °C).
- Observe the C. elegans after the 30 min interval. Count the total number of animals on the plate and the number of C. elegans showing anterior convulsions or ‘head bobs’ (Figure 2A). Wild type C. elegans shows no response to PTZ exposure (S1 movie in Thapliyal et al., 2018). While mutant defective in GABAergic synaptic transmission show high degree of convulsions (S2 movie in Thapliyal et al., 2018). One can also observe the C. elegans after a 60 min interval to take the second reading (optional). These recordings are also done at room temperature (~23 °C).
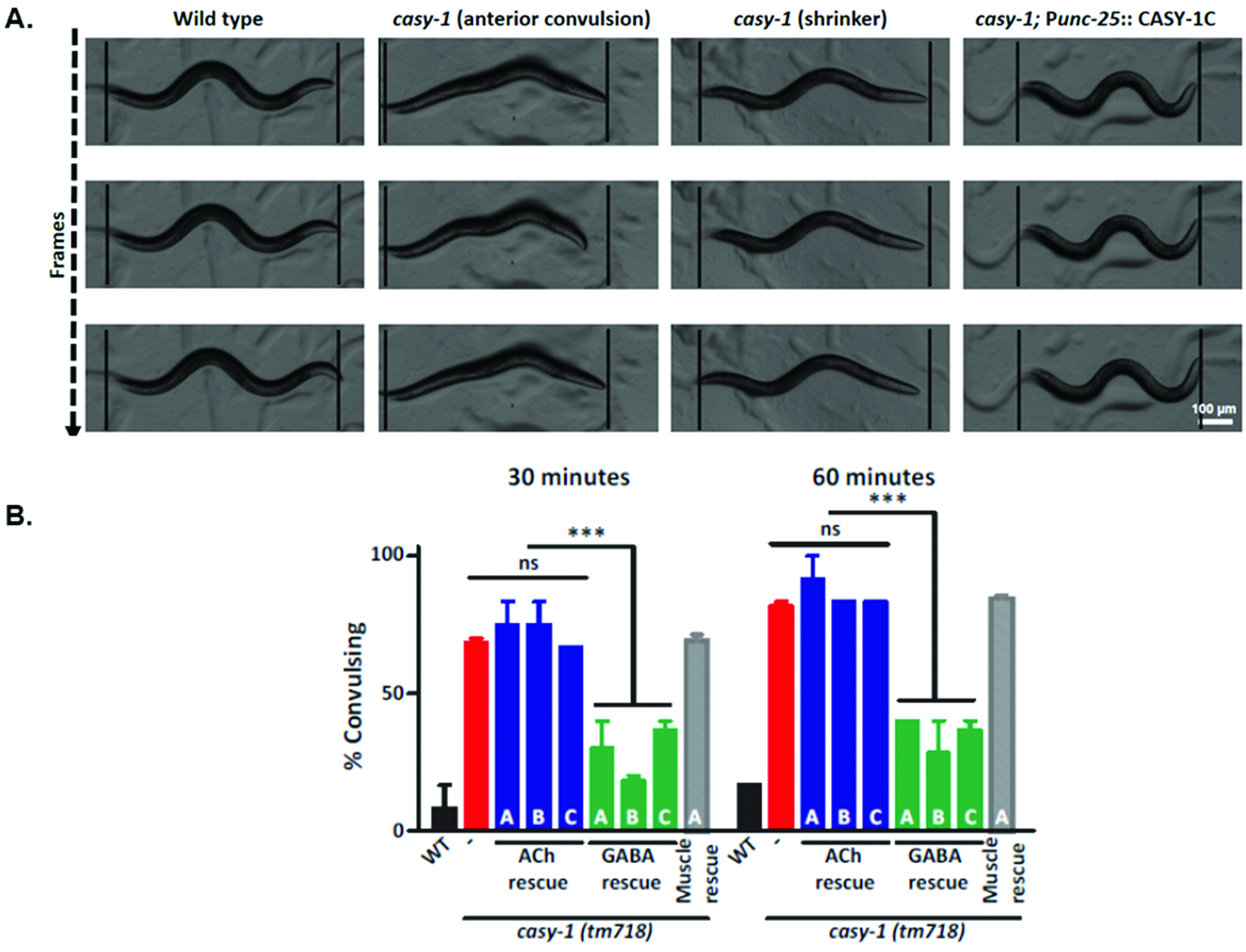
Figure 2. casy-1 mutants show increased convulsions when compared with WT C. elegans. The casy-1 mutants show higher sensitivity to the GABA receptor antagonist PTZ when compared to WT animals. A. Representative still frame images demonstrating casy-1 mutant C. elegans with anterior convulsions (head bobs) and tail shrinker phenotype. The still frame images are representative frames from movies (7 frames/sec). B. The graph shows the fraction of animals showing anterior ‘head bobs’ after 30 min and 60 min exposure to 10 mg/ml PTZ. The sensitivity to PTZ could be fully rescued by expressing casy-1 isoforms in GABAergic neurons but not in cholinergic neurons or muscle. Assays were done (~15 C. elegans/assay) at least thrice. Values that differ significantly from WT animals are indicated (*** indicates P < 0.0001 using two-way ANOVA). Data are represented as mean ± S.E.M. Part of Figure 2A and Figure 2B have been previously published in (Thapliyal et al., 2018). - Here in the present example, casy-1 represents a cell adhesion molecule that has been reported to regulate vesicle trafficking in GABAergic motor neurons (Thapliyal et al., 2018). GABA release from the presynaptic terminals is reduced in casy-1 mutants which can be easily interpreted from high degree of convulsions in the mutant upon PTZ exposure.
Data analysis
- Calculate the percentage of C. elegans showing convulsions on each plate at the 30 and 60 min time point using the formula given below:
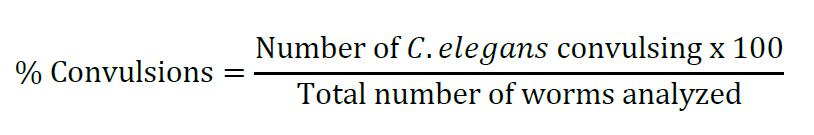
- Software: GraphPad Prism v6.
- Use Grouped graph with 3 replicate values to plot the results. At least 10-15 worms of each genotype are used per assay. Repeat the assay two more times on different days (total 3 trials) and report the summary of the statistics as shown in Figure 2B.
- Statistical analysis: A two way-ANOVA is used to determine statistical P-values.
Notes
- As a standard scientific procedure to avoid biases, the experimenter should be blinded to the genotypes of the test C. elegans. Ideally, a colleague from the lab can number the plates to prevent the experimenter from identifying the genotypes. For mutants with visual phenotypic defects, an additional mutant line with a similar phenotypic defect and unrelated to the content of the study must be added.
- The sensitivity of the C. elegans to PTZ could change depending upon the variability in experimental conditions (room temperature, humidity etc.) or differences in the PTZ stock obtained from the company. However, the overall readout of the experiment usually remains the same.
- In our hands, more variability in convulsions was seen in C. elegans containing extrachromosomal arrays (rescue lines). This could be due to differences in array lines and should not be concluded as a generalized phenomenon.
Recipes
- 100 mg/ml PTZ Stock Solution
Dissolve 100 mg PTZ in 1 ml volume of absolute ethanol
This solution is prepared fresh before each experiment - 5 mg/ml cholesterol
Add 500 mg of cholesterol in 100 ml of 95% ethanol and mix by rotating on a rotator at room temperature. It takes a few hours to dissolve
Store at 4 °C - 1 M CaCl2 stock solution
Dissolve 14.7 g of CaCl2•2H2O in 100 ml ddH2O and autoclave for 15 min at 121 °C
Store at 4 °C - 1 M MgSO4 stock solution
Dissolve 12.04 g of MgSO4 in 100 ml ddH2O and autoclave for 15 min at 121 °C
Store at 4 °C - 1 M KPO4, pH 6.0 stock solution
- Mix 108.3 g KH2PO4 and 35.6 g K2HPO4 in 500 ml ddH2O
- Adjust the pH to 6.0 by adding NaOH, finally make up the volume to 1 L
- Aliquot the solution and autoclave for 15 min at 121 °C
- Store at 4 °C
- Nematode growth medium (NGM) agar plate
- Add 3 g NaCl, 16 g Bacto-agar, and 2.5 g Bacto-peptone in 975 ml of ddH2O in a 2 L flask
- Autoclave for 50 min at 121 °C
- Let the NGM agar cool to 55-60 °C. Add 1 ml of 5 mg/ml cholesterol, 1 ml of 1 M CaCl2, 1 ml of 1 M MgSO4, and 25 ml of 1 M KPO4, pH 6.0
Acknowledgments
This protocol has been adapted from Thapliyal et al. (2018). ST thanks the Council of Scientific and Industrial Research (CSIR) for a graduate fellowship. KB is an Intermediate Fellow of the DBT-Wellcome Trust India Alliance and thanks the Alliance for funding support (grant number IA/12/500516).
Competing interests
Authors declare no conflict of interest or competing interest.
References
- Bargmann, C. I. (2012). Beyond the connectome: how neuromodulators shape neural circuits. Bioessays 34(6): 458-465.
- de Bono, M. and Maricq, A. V. (2005). Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 28: 451-501.
- Locke, C., Berry, K., Kautu, B., Lee, K., Caldwell, K. and Caldwell, G. (2008). Paradigms for pharmacological characterization of C. elegans synaptic transmission mutants. J Vis Exp (18): 837.
- Oh, K. H. and Kim, H. (2017). Aldicarb-induced paralysis assay to determine defects in synaptic transmission in Caenorhabditis elegans. Bio-protocol 7(14): e2400.
- Schofield, P. R., Darlison, M. G., Fujita, N., Burt, D. R., Stephenson, F. A., Rodriguez, H., Rhee, L. M., Ramachandran, J., Reale, V., Glencorse, T. A. and et al. (1987). Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature 328(6127): 221-227.
- Stiernagle, T. (2006). Maintenance of C. elegans. WormBook: 1-11.
- Sudhof, T. C. (1995). The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature 375(6533): 645-653.
- Sudhof, T. C. (2004). The synaptic vesicle cycle. Annu Rev Neurosci 27: 509-547.
- Thapliyal, S., Vasudevan, A., Dong, Y., Bai, J., Koushika, S. P. and Babu, K. (2018). The C-terminal of CASY-1/Calsyntenin regulates GABAergic synaptic transmission at the Caenorhabditis elegans neuromuscular junction. PLoS Genet 14(3): e1007263.
- White, J. G., Southgate, E., Thomson, J. N. and Brenner, S. (1976). The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 275(938): 327-348.
- White, J. G., Southgate, E., Thomson, J. N. and Brenner, S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314(1165): 1-340.
- Williams, S. N., Locke, C. J., Braden, A. L., Caldwell, K. A. and Caldwell, G. A. (2004). Epileptic-like convulsions associated with LIS-1 in the cytoskeletal control of neurotransmitter signaling in Caenorhabditis elegans. Hum Mol Genet 13(18): 2043-2059.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Thapliyal, S. and Babu, K. (2018). Pentylenetetrazole (PTZ)-induced Convulsion Assay to Determine GABAergic Defects in Caenorhabditis elegans. Bio-protocol 8(17): e2989. DOI: 10.21769/BioProtoc.2989.
Category
Neuroscience > Cellular mechanisms > Intracellular signalling
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link