- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Random Insertional Mutagenesis of a Serotype 2 Dengue Virus Clone
Published: Vol 8, Iss 16, Aug 20, 2018 DOI: 10.21769/BioProtoc.2975 Views: 6519
Reviewed by: Modesto Redrejo-RodriguezSesha Lakshmi Arathi PaluriAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

General Maintenance and Reactivation of iSLK Cell Lines
Ariana C. Calderón-Zavala [...] Ekaterina E. Heldwein
Jun 5, 2025 1922 Views

Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
Jade Jansen [...] Neeltje A. Kootstra
Jul 20, 2025 2473 Views

Assembly and Mutagenesis of Human Coronavirus OC43 Genomes in Yeast via Transformation-Associated Recombination
Brett A. Duguay and Craig McCormick
Aug 20, 2025 3057 Views
Abstract
Protein tagging is a powerful method of investigating protein function. However, modifying positive-strand RNA virus proteins in the context of viral infection can be particularly difficult as their compact genomes and multifunctional proteins mean even small changes can inactivate or attenuate the virus. Although targeted approaches to functionally tag viral proteins have been successful, these approaches are time consuming and inefficient. A strategy that has been successfully applied to several RNA viruses is whole-genome transposon insertional mutagenesis. A library of viral genomes, each containing a single randomly placed small insertion, is selected by passaging in cell culture and the insertion sites can be identified using Next Generation Sequencing (NGS). Here we describe a protocol for transposon mutagenesis of the 16681 strain of dengue virus, serotype 2. Mutant dengue virus libraries containing short randomly placed insertions are passaged through mammalian cells and insertions are mapped by NGS of the viable progeny. The protocol is divided into four stages: transposon mutagenesis of a dengue cDNA clone, viral genome transfection into permissive cells, isolation of viral progeny genomes, and sequencing library preparation.
Keywords: TransposonBackground
A key aspect of understanding viral pathogenesis is elucidating the viral proteins’ functions during infection. However, viral proteins, particularly those encoded by compact viral genomes, are often multifunctional and therefore more challenging to study, in part because they are often difficult to tag (epitope tags, fluorescent proteins, etc.) in the context of an infectious virus genome without compromising viral infection. One workaround is to express individually tagged proteins in cells, which may result in an incomplete picture of the viral protein’s function as the other viral proteins are not present. It also does not directly determine if the functional tag interferes with the tagged protein’s function(s) in viral infection. Another approach is the empiric tagging of viral proteins in the context of an infectious virus genome, which ensures viral viability but is an inefficient process that is difficult to scale.
Transposon mutagenesis can help dissect the functions of proteins under various experimental conditions and has been used at a whole genome scale to elucidate the role of various proteins during microbial infections. This approach has been successfully applied to a number of positive-strand RNA viruses (Arumugaswami et al., 2008; Beitzel et al., 2010; Teterina et al., 2011; Thorne et al., 2012; Remenyi et al., 2014; Eyre et al., 2017; Fulton et al., 2017). By coupling the transposon mutagenesis approach to Next Generation Sequence, a map of sites in the viral genome that tolerate insertions can be determined with unprecedented resolution. Once these sites are identified, functional tags can be introduced into these sites through site-directed mutagenesis. This protocol describes whole-genome transposon insertion mapping applied to the 16681 strain of serotype 2 dengue virus.
Materials and Reagents
- Pipette tips (Fisher Scientific, catalog numbers: 02-707-80 ; 02-707-167 )
- 6-well plate (Corning, catalog number: 3506 )
- 0.22 μm sterile filter (Corning, catalog number: 431219 )
- Cell scraper (Corning, catalog number: 3010 )
- 10-beta chemically competent E. coli (New England Biolabs, catalog number: C3019H ) or equivalent–genotype used Δ(ara-leu) 7697 araD139 fhuA ΔlacX74 galK16 galE15 e14- ϕ80dlacZΔM15 recA1 relA1 endA1 nupG rpsL (StrR) rph spoT1 Δ(mrr-hsdRMS-mcrBC)
- Primers
- Fragment A (SacI/NarI) forward with T7 sequence
GAAATTAATACGACTCACTATAAGTTGTTAGTCTACGTG - Fragment A (SacI/NarI) reverse
GTCATAGTGGCGCCTACCATAACCATCACTCTTCCC - Fragment B (NarI/EcoRV) forward
GGTTATGGTAGGCGCCACTATGACGGATGAC - Fragment B (NarI/EcoRV) reverse
CTGCTTCCTGATATCTCTGCCTGGTCTTCCC - Fragment C (EcoRV/XbaI) forward
GACCAGGCAGAGATATCAGGAAGCAGTCCAATCC - Fragment C (EcoRV/XbaI) reverse
AGAACCTGTTGATTCAACAGCAC - Ion Torrent P1 adaptor top oligo
CCACTACGCCTCCGCTTTCCTCTCTATGGGCAGTCGGTGAT - Ion Torrent P1 adaptor bottom oligo
<phos>ATCACCGACTGCCCATAGAGAGGAAAGCGGAGGCGTAGTGGTT - Modified Ion Torrent A adaptor top oligo
<phos>GGCCGCCTGAGTCGGAGACACGCAGGGATGAGATGGTT - Modified Ion Torrent A adaptor bottom oligo
CGGACTCAGCCTCTGTGCGTCCCTACTCTACC
- Fragment A (SacI/NarI) forward with T7 sequence
- SacI (New England Biolabs, catalog number: R3156 )
- NarI (New England Biolabs, catalog number: R0191 )
- EcoRV (New England Biolabs, catalog number: R3195 )
- XbaI (New England Biolabs, catalog number: R0145 )
- NotI (New England Biolabs, catalog number: R0189 )
- Mutation Generation System kit (Thermo Fisher Scientific, catalog number: F-701 )
- Carbenicillin (Fisher Scientific, catalog number: BP26481 )
- Kanamycin (Thermo Fisher Scientific, catalog number: 11815024 )
- Promega Wizard Maxiprep kit (Promega, catalog number: A7270 )
- Promega Gel Purification kit (Promega, catalog number: A9281 )
- m7g(5')ppp(5')A RNA Cap Structure analog (New England Biolabs, catalog number: S1405S )
- Agarose (Thermo Fisher Scientific, catalog number: 16500100 )
- Q5 polymerase (New England Biolabs, catalog number: M0493 )
- T7 MEGAscript in vitro RNA translation kit (Thermo Fisher Scientific, catalog number: AM1334 )
- ZYMO Quick-RNA Viral kit (ZYMO RESEARCH, catalog number: R1034 )
- Applied Biosciences High Capacity cDNA kit (Thermo Fisher Scientific, catalog number: 4368814 )
- Ion Library Taqman Quantitation kit (Thermo Fisher Scientific, catalog number: 4468802 )
- 10x TBE (Fisher Scientific, catalog number: BP1333 )
- T4 DNA ligase (New England Biolabs, catalog number: M0202 )
- T4 PNK (New England Biolabs, catalog number: M0201 )
- dNTPs (Thermo Fisher Scientific, catalog number: R1121 )
- AMPure XP Beads (Beckman Coulter, catalog number: A63881 )
- LB medium (Fisher Scientific, catalog number: BP1425-500 )
- PEG 8000 (Thermo Fisher Scientific, catalog number: BP233-100 )
- Vero cells (ATCC, catalog number: CCL-81 )
- TransMessenger transfection reagent (QIAGEN, catalog number: 301525 )
- Full-length dengue virus clone 16681 serotype 2 cDNA (in plasmid pD2/IC-30P-NBX, Huang et al., 2010).
Note: This cDNA was a generous gift of Dr. Huang from the Centers for Disease Control.
Equipment
Note: No specific equipment is necessary and any model of the following should suffice.
- Balance
- Pipettes
- Heat block
- Incubator
- Thermal cycler
- Water bath
- Microcentrifuge
- Magnet for Ampure XP bead purification
Procedure
- Transposon Mutagenesis
This section describes the process of generating a library consisting of a randomly inserted transposon in a cDNA clone of the viral genome on a plasmid. By excising the bulk of the 1.2 kb transposon using unique NotI sites, the randomly inserted transposon is reduced to 15 nucleotides containing a NotI site that introduces a 5-amino acid insertion without introducing a frameshift or nonsense mutation. This insertion is TGCGGCCGCAN1N2N3N4N5 where N1N2N3N4N5 denotes a duplication of the five nucleotides directly upstream of the insertion.
For our studies, the dengue cDNA clone was fragmented to eliminate genetic instability and toxicity in E. coli observed with full-length flavivirus clones. However, viral genome fragmentation is not required if the full-length viral genome is genetically stable and nontoxic in E. coli. Note that the starting plasmid must not contain any NotI restriction sites; if any exist they should be destroyed by site-directed mutagenesis.- Divide the full-length dengue virus clone 16681 serotype 2 cDNA (in plasmid pD2/IC-30P-NBX, Huang et al., 2010) into three fragments flanked by SacI and NarI (Fragment A), NarI and EcoRV (Fragment B), EcoRV and XbaI (Fragment C) (Figure 1, step 1) and subclone back into the original vector with a modified multiple cloning site containing SacI, NarI, EcoRV, and XbaI sites (Figure 1, step 2).
- Mutagenize the plasmids using MuA Transposase supplied in the Mutation Generation Kit to introduce an engineered transposon randomly into each plasmid. Under these conditions, only one transposon should be introduced into each DNA plasmid.
- Calculate amount of target DNA per reaction by multiplying the plasmid size (in kb) by 40 ng.
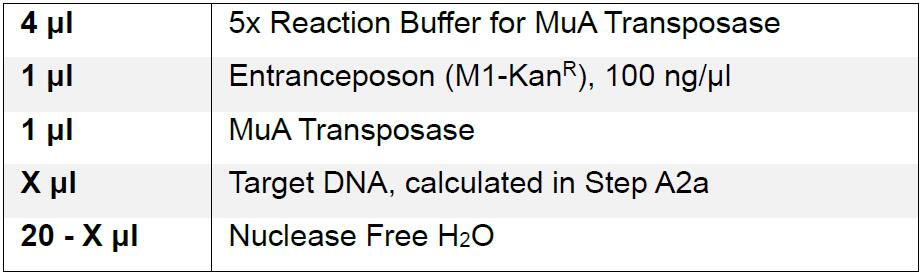
- Incubate reactions at 30 °C for 60 min, and heat inactivate at 75 °C for 10 min.
- Calculate amount of target DNA per reaction by multiplying the plasmid size (in kb) by 40 ng.
- Dilute the transposase reaction tenfold in sterile nuclease-free water to a total of 200 μl and transform into high-efficiency recA- competent cells following the manufacturer’s protocol using a maximum of 5 μl per transformation. We used chemically competent cells, but electrocompetent cells may be used, as long as the transformation efficiency is at least 108 cfu/μg. If electroporation is used, the reactions should be diluted by ten-fold to avoid arcing. Twenty reactions should be sufficient to generate an estimated nucleotide coverage of at least 10 (see Step A5).
- Select transformed E. coli on LB plates containing carbenicillin (to select for the plasmid) and kanamycin (to select for the transposon).
- Count the number of independent colonies to determine the library coverage. At this stage, the library coverage can be calculated by dividing the number of observed colonies by the total number of nucleotides in the plasmid (viral cDNA fragment plus backbone). We recommend a nucleotide coverage of at least 10-fold. Scrape the colonies from the plates into resuspension buffer from a Promega Wizard Maxiprep kit and prepare plasmid DNA. One Promega Wizard Maxiprep column should be used per 10,000 colonies.
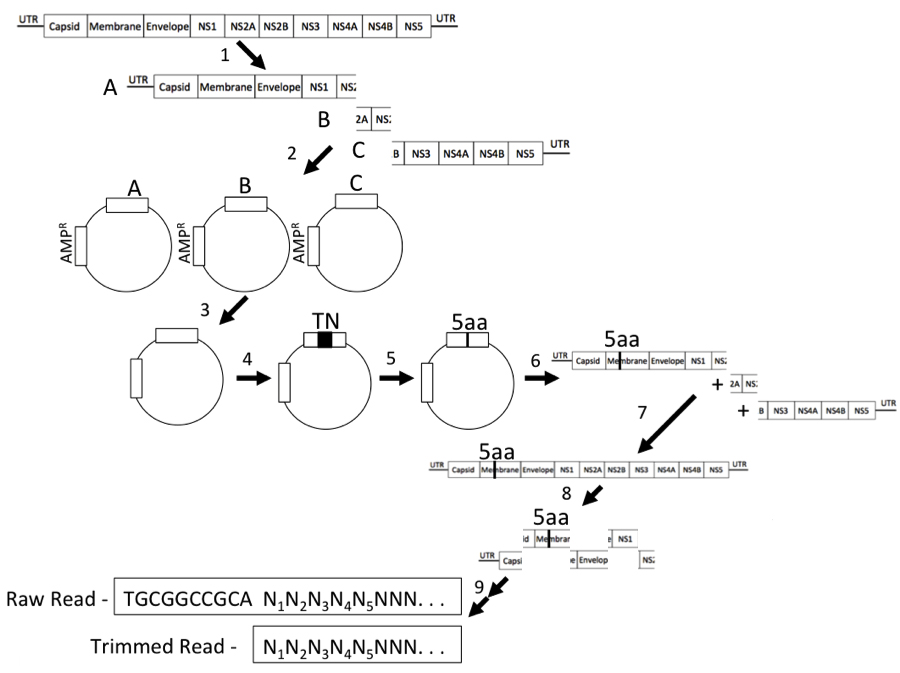
Figure 1. Protocol Schematic. 1. Fragment the full-length dengue virus genome into three parts using unique restriction enzyme cut sites. 2. Clone the fragments into a clean vector backbone. 3. Mutagenize each of these constructs independently using a mutation generation kit to generate randomly inserted transposon containing antibiotic resistance, and select mutated constructs for antibiotic resistance contained on the transposon. 4. Excise the dengue virus fragment from the vector backbone and ligate back into a clean vector to ensure that the transposon was located only in the dengue virus fragment and not the vector backbone. 5. Next, remove the transposon using a unique enzyme restriction site introduced by the transposon and self-ligate constructs to leave a small 15 nucleotide genetic scar (5aa). 6. Amplify the genetic scar containing fragment by PCR in parallel with corresponding wildtype fragments. 7. Stitch together using PCR the genetic scar containing fragment and wildtype fragments to obtain a full-length dengue virus genome. 8. Transcribe the genome in vitro and transfect RNA into permissive cells. Recover viable progeny virus from the transfection, isolate RNA, and synthesize cDNA. Amplify the fragment containing the genetic scar. 9. Next Generation Sequencing libraries were constructed from the PCR amplified genetic scar containing fragments, and sequenced. Reads and trimmed reads used for alignment are displayed. - The transformants will contain a transposon insertion in either the viral cDNA fragment or in the vector backbone. Steps C6-C8 will isolate only the transposon insertions within the viral cDNA fragments (Figure 1, step 4). Digest 5 μg of each plasmid library with Sac1 and Nar1 (Fragment 1), NarI and EcoRV (Fragment 2), or EcoRV and XbaI (Fragment 3) and separate on a 1% TBE-agarose gel. Each digest should contain four different bands consisting of the vector backbone, the vector with transposon insertion, the viral cDNA fragment, and the viral cDNA fragment with the transposon insertion. Excise the bands corresponding to the viral cDNA fragments with the transposon insertion and purify.
- Ligate the gel purified bands back into the original clean vector backbone digested with the appropriate compatible restriction enzymes using T4 DNA Ligase following the manufacturer’s protocol.
- Transform the ligation reaction into high-efficiency competent cells following the manufacturer’s protocol and select on LB plates with carbenicillin and kanamycin. The library coverage can be again calculated in a similar method to Step A5. To maintain library coverage above 10-fold coverage, ensure that at least as many colonies are recovered in this step as were recovered in Step A5. Purify plasmid DNA as in Step A5.
- Digest 5 μg of isolated plasmids with NotI and run on a 1% agarose gel. This digestion removes the bulk of the 1.2 kb transposon, leaving behind the 15-nucleotide insertion (Figure 1, step 5). Excise the larger band and discard the 1.2 kb transposon fragment.
- Recircularize the gel-purified band using T4 DNA ligase following the manufacturer’s protocol.
- Transform the ligation reaction into high-efficiency competent cells following the manufacturer’s protocol and select on LB-carbenicillin plates.
- Count E. coli colonies and purify plasmid DNA as in Step A5. To ensure high coverage ensure that at least as many colonies are counted and recovered as in Steps A5 and A8.
- Viral genome transfection into permissive cells
This section describes the process of generating full-length mutant dengue genomes in vitro from the fragments by splice overlap extension (SOE) PCR, in vitro transcribing full-length dengue genomes to RNA, and transfecting viral RNA into permissive cells. To maintain library complexity, reactions are performed in at least three replicates for each step and pooled. Several independent transfections of viral RNA into permissive cells should also be performed to minimize bottleneck effects.- PCR amplify the mutagenized dengue fragment libraries from the previous step (Figure 1, step 6).
- Amplify the mutagenized Fragment A, B, and C libraries with the forward and reverse primers listed in “Materials and Reagents.”
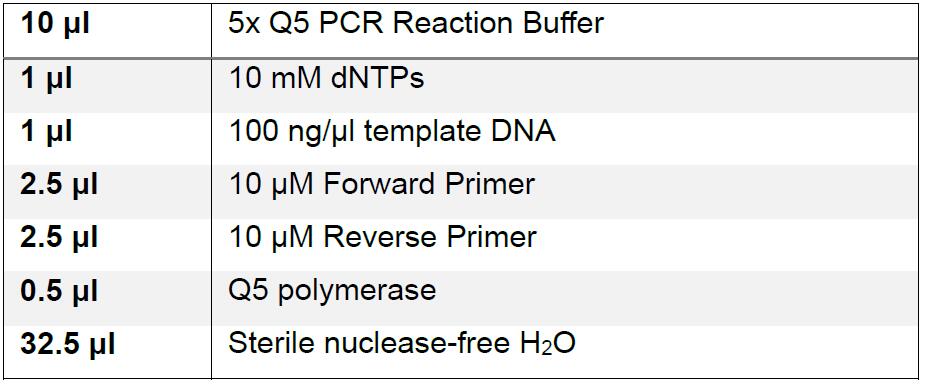
- In addition, amplify the corresponding wild-type viral fragments A, C, AB (Using Fragment A (SacI/NarI) forward with T7 sequence and Fragment B (NarI/EcoRV) reverse), and BC (Using Fragment B (NarI/EcoRV) forward and Fragment C (EcoRV/XbaI) reverse) fragments from an unmodified cDNA copy of dengue virus clone 16681 pD2/IC-30P-NBX.
Thermal cycler program: (1) 95 °C for 2 min; (2) 95 °C for 20 sec, 60 °C for 20 sec, and 72 °C for 1 min/kb product x 35 cycles; (3) hold at 4 °C.
- Amplify the mutagenized Fragment A, B, and C libraries with the forward and reverse primers listed in “Materials and Reagents.”
- Gel-purify PCR products on a 1% agarose gel.
- Construct full-length mutant dengue genomes using splicing by overlap extension (SOE) PCR (Figure 1, step 7) in the following PCR reactions. Splice mutagenized fragment A with wildtype fragment BC, mutagenized fragment B with wildtype fragments A and C, and mutagenized fragment C with wildtype fragment AB. The resulting full-length genomes will contain a random insertion within fragment A, B, or C, respectively. To ensure enough material for subsequent steps, perform three independent reactions.
- Thermal cycler program: (1) 95 °C for 2 min; (2) 95 °C for 20 sec, 72 °C for 1 min/kb product x 10 cycles.
- Add 2.5 μl of 10 μM fragment A forward primer and 2.5 μl of 10 μM fragment C reverse primer to each reaction.
- Thermal cycler program: (1) 95 °C for 2 min; (2) 95 °C for 20 sec, 60 °C for 20 sec, and 72 °C for 11 min x 35 cycles; (3) hold at 4 °C.
- Gel-purify the full-length dengue virus genomes on a 1% agarose gel.
- Use 200 ng of purified full-length genome for in vitro transcription using the T7 MEGAscript kit. Note that an m7G(5’) ppp(5’)A RNA cap analog is used in the in vitro transcription reaction as the dengue virus RNA has a 5’ cap, but is not needed for the synthesis of uncapped viral RNA genomes (e.g., hepatitis C virus).

- Incubate reactions at 37 °C for 6 h. To increase viral RNA yield, add 1 μl of 10 mM ATP, supplied in the T7 MEGAscript kit, to the reaction 1 h after beginning the incubation.
- Add 1 μl of the DNase solution supplied in the T7 MEGAscript kit, 30 min prior to completion of the incubation.
- Purify the in vitro transcribed viral RNA using the ZYMO Quick-RNA Viral kit following the manufacturer’s protocol. Elute in 50 μl of nuclease-free water prewarmed to 70 °C.
- Quantitate the viral RNA. We observed best results from freshly made RNA, although storing at -80 °C only negligibly decreases transfection efficiency.
- One day before viral RNA transfection, plate permissive cells in a 6-well plate such that they will be 80-90% confluent on the day of transfection. For dengue virus, plate 3.3 x 105 Vero cells per well of a 6-well plate.
- Transfect 2.5 μg of viral RNA per well using the QIAGEN TransMessenger kit with 4 μl enhancer and 8 μl transfection reagent.
Note: The ratio of RNA to enhancer and transfection reagent should be optimized if cells other than Vero cells will be used. - Wash off transfection complex 2 h after transfection.
Note: Duration of transfection will vary based on cell type and should be determined experimentally. - After three days, collect supernatant from wells, pool, and spin at 500 x g for 10 min to clarify. Use half of the clarified supernatant to infect naïve cells and store the other half at -80 °C as passage 1. Infect naïve cells by adding undiluted supernatant to Vero cells plated the day before infection at 1.7 x 105 cells per well of a 6-well plate, and replace with fresh medium the day after infection.
- After three days, collect supernatant from wells, pool, and spin at 500 x g for 10 min to clarify. Use half of the clarified supernatant to infect naïve cells and store the other half at -80 °C as passage 2. Infect naïve cells as described in Step B11. After three days, collect supernatant from wells, pool, and spin at 500 x g for 10 min to clarify. Store at -80 °C as passage 3.
- PCR amplify the mutagenized dengue fragment libraries from the previous step (Figure 1, step 6).
- Isolation of viral progeny genome and Next Generation Sequencing library preparation
- Concentrate virus from passage 1, 2, and 3 supernatants by PEG precipitation.
- Make a 40% (w/v) PEG8000 solution in PBS and filter through a 0.22 μm sterile filter.
- Add 40% PEG8000 to the supernatant to a final concentration of 8% PEG8000, and incubate overnight at 4 °C.
- Centrifuge at 7,500 x g for 4 h at 4 °C.
- Remove the supernatant and gently resuspend the pellet in 1 ml of sterile PBS by pipetting.
- Split the resuspended virus into three equal volumes and isolate viral RNA from each using the ZYMO Quick-RNA Viral kit following the manufacturer’s procedure. Elute in a volume of 20 μl per column and pool the purified viral RNAs.
- Reverse transcribe the purified viral RNA into cDNA using the Applied Biosciences High Capacity cDNA kit following the manufacturer’s procedure. Use all 60 μl of the eluted RNA from Step C2 to make cDNA, and pool after cDNA is made.
- PCR amplify the recovered cDNA for the genome fragment that contains the transposon insertion using primers designed to generate smaller ~250 bp amplicons for Next Generation Sequencing.
Thermal cycler program: (1) 95 °C for 2 min; (2) 95 °C for 20 sec, 60 °C for 20 sec, and 72 °C for 1 min/kb product x 35 cycles; (3) hold at 4 °C. - Clean up the amplicons using the PCR cleanup protocol for the Promega Gel Purification Kit.
- At this stage of the protocol, amplicons can be quantitated and submitted for library preparation and next-generation sequencing.
- Concentrate virus from passage 1, 2, and 3 supernatants by PEG precipitation.
- Next Generation Sequencing library preparation
This section of the protocol generates libraries compatible with Torrent PGM sequencing. Note that the library preparation protocol has been modified to select for NotI containing DNA fragments to enrich the sequencing reads for transposon insertion sites, which contain a NotI site. By blunt ligating one adaptor to each end of the DNA fragment, cutting with NotI, and ligating a NotI containing read adaptor, we created sequencing libraries where each read contained the insertion site. In principle, this strategy could be adapted to generate libraries compatible with Illumina sequencing, but this will have to be developed by the user.- Combine amplicons from step C5 in an equimolar fashion to generate a total of 1 μg of input DNA.
- Treat input DNA with T4 PNK to phosphorylate the ends for subsequent adapter ligation.
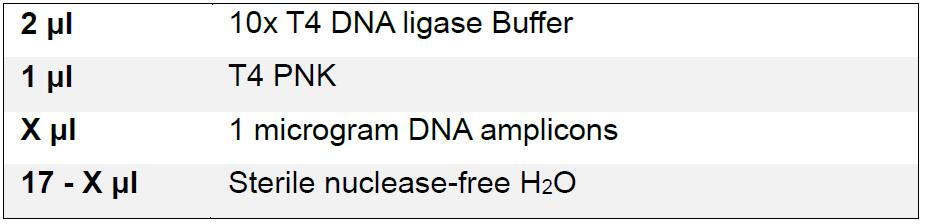
Incubate reaction at 37 °C for 30 min, and heat inactivate at 65 °C for 20 min. - Mix Ion Torrent P1 adaptor top and Ion Torrent P1 adaptor bottom together in sterile nuclease-free water to a final concentration of 44 μM for each oligo. Heat the tube containing the mixture in a water-filled beaker to 100 °C for 1 min, remove the beaker from heat, and allow it to cool to room temperature on a benchtop.
- Blunt ligate the phosphorylated amplicons to the Ion Torrent P1 adapter.
Incubate reaction for 60 min at 25 °C. - Clean up reactions using 72 μl of AMPure XP beads with a ratio of 1.8 bead volume to reaction volume, following the manufacturer’s protocol, and elute in 35 μl of sterile nuclease-free water.
- Digest reactions with NotI.
Incubate reactions at 37 °C for 60 min, and heat inactivate NotI at 65 °C for 20 min. - Clean up reactions using AMPure XP beads as in Step D4 and eluting in 34 μl of sterile nuclease-free water.
- Anneal Modified Ion Torrent A adaptor top and Modified Ion Torrent A adaptor bottom oligos as described in Step D3.
- Ligate reactions to the annealed Modified Ion Torrent A adaptor.
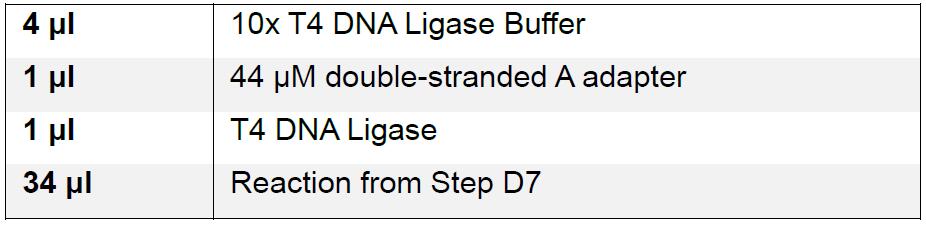
Incubate reaction for 90 min at 16 °C. - Clean up reactions using AMPure XP beads as in Step D4 and eluting in 35 μl of sterile nuclease-free water.
- Quantitate the libraries using the Ion Torrent Library quantitation kit following the manufacturer’s protocol.
- Submit the quantitated libraries for sequencing on Ion Torrent PGM.
Data analysis
Mapping sequencing reads to the viral genome, and enumerating these results can be challenging to individuals not proficient in bioinformatics. Collaboration with an experienced bioinformatician should be established to help produce accurate results. The following is a broad overview of the approach to determine sites in the genome that tolerate small insertions.
- Obtain the raw reads of the sequencing results (such as a FASTQ file). Next, split the reads into two groups; one containing TGCGGCCGCAN1N2N3N4N5 at the beginning of the read (Figure 1, step 9) and the other that did not contain that exact sequence.
- Trim the first ten nucleotides of each read to remove the transposon inserted sequence (Figure 1, step 9), and leave the Dengue genome sequence for alignment.
- Using the trimmed reads only, align reads to the dengue genome using the default settings of BOWTIE2. BOWTIE2 can be obtained from http://www.sourceforge.net.
- Obtain the alignment file, and extract the start position of the alignment for each read. This site is 5 bases upstream of the actual insertion due to the duplication of the insert caused by the transposase (see Procedure A), so these alignment sites should be reduced by 5.
- Enumerate the number of reads for each site in the viral genome.
- Normalize the number of reads by dividing the insertion sites counts by the total number of reads from the sequencing runs, so you can compare multiple sequencing runs.
Notes
Detailed recipes are supplied in the protocol above. Troubleshooting tips are available in each of the manufacturer’s protocols and should be read before performing experiments to highlight potential problems with the execution of the said experiment.
Acknowledgments
This protocol was adapted from several previous transposon insertional mutagenesis studies (Arumugaswami et al., 2008; Beitzel et al., 2010; Teterina et al., 2011; Thorne et al., 2012; Remenyi et al., 2014). We thank Dr. Claire Huang (CDC) for the pD2/IC-30-P-NBX infectious cDNA clone. This work was supported by National Institutes of Health grants R01DK097374 (A.W.T.), and the Michigan Institute for Clinical and Health Research (MICHR) grant UL1TR002240 (J.W.P.), and the University of Michigan Center for Gastrointestinal Research (UMCGR), grant 5P30DK034933.
Competing interests
The authors have no conflicts of interest to disclose.
References
- 1Arumugaswami, V., Remenyi, R., Kanagavel, V., Sue, E. Y., Ngoc Ho, T., Liu, C., Fontanes, V., Dasgupta, A. and Sun, R. (2008). High-resolution functional profiling of hepatitis C virus genome. PLoS Pathog 4(10): e1000182.
- Beitzel, B. F., Bakken, R. R., Smith, J. M. and Schmaljohn, C. S. (2010). High-resolution functional mapping of the venezuelan equine encephalitis virus genome by insertional mutagenesis and massively parallel sequencing. PLoS Pathog 6(10): e1001146.
- Eyre, N. S., Johnson, S. M., Eltahla, A. A., Aloi, M., Aloia, A. L., McDevitt, C. A., Bull, R. A. and Beard, M. R. (2017). Genome-wide mutagenesis of dengue virus reveals plasticity of the NS1 protein and enables generation of infectious tagged reporter viruses. J Virol 91(23).
- Fulton, B. O., Sachs, D., Schwarz, M. C., Palese, P. and Evans, M. J. (2017). Transposon mutagenesis of the zika virus genome highlights regions essential for RNA replication and restricted for immune evasion. J Virol 91(15).
- Huang, C. Y., Butrapet, S., Moss, K. J., Childers, T., Erb, S. M., Calvert, A. E., Silengo, S. J., Kinney, R. M., Blair, C. D. and Roehrig, J. T. (2010). The dengue virus type 2 envelope protein fusion peptide is essential for membrane fusion. Virology 396(2): 305-315.
- Remenyi, R., Qi, H., Su, S. Y., Chen, Z., Wu, N. C., Arumugaswami, V., Truong, S., Chu, V., Stokelman, T., Lo, H. H., Olson, C. A., Wu, T. T., Chen, S. H., Lin, C. Y. and Sun, R. (2014). A comprehensive functional map of the hepatitis C virus genome provides a resource for probing viral proteins. MBio 5(5): e01469-01414.
- Teterina, N. L., Lauber, C., Jensen, K. S., Levenson, E. A., Gorbalenya, A. E. and Ehrenfeld, E. (2011). Identification of tolerated insertion sites in poliovirus non-structural proteins. Virology 409(1): 1-11.
- Thorne, L., Bailey, D. and Goodfellow, I. (2012). High-resolution functional profiling of the norovirus genome. J Virol 86(21): 11441-11456.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Perry, J. W. and Tai, A. W. (2018). Random Insertional Mutagenesis of a Serotype 2 Dengue Virus Clone. Bio-protocol 8(16): e2975. DOI: 10.21769/BioProtoc.2975.
Category
Microbiology > Microbe-host interactions > Virus
Microbiology > Microbial genetics > Mutagenesis
Molecular Biology > DNA > Mutagenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









