- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fusarium graminearum Inoculation on Wheat Head
Published: Vol 8, Iss 15, Aug 5, 2018 DOI: 10.21769/BioProtoc.2964 Views: 8968
Reviewed by: Feng LiRosario Gomez-GarciaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimized Protocol for DNA Extraction in Three Theobroma Species
Angie F. Riascos-España [...] Pedro A. Velasquez-Vasconez
May 5, 2025 2049 Views

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2634 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1568 Views
Abstract
Fusarium graminearum, the major causal agent of Fusarium head blight (FHB), causes serious wheat yield losses and a threat to human and animal health. The main efforts to combat the disease are the research of pathogenesis mechanisms and breeding for disease resistance plants. The efficiency of these actions could be evaluated by reliable inoculation assay, which is performed by accurate and repeatable inoculation methods. Hence, a standard procedure of effective wheat inoculation should improve the accuracy of pathogenicity evaluation. Here, we present a protocol for wheat spike inoculation with fungal conidial suspensions or fungus agar discs. These methods show highly reproducibility and accuracy on wheat infection experiment in laboratory conditions.
Keywords: Fusarium graminearumBackground
Fusarium graminearum is a destructing fungal pathogen which causes globally serious Fusarium head blight (FHB) disease on cereal crops. In recent years, the incidence of FHB increased with the global climate change and changes in farming practices. Additionally, F. graminearum produces several mycotoxins including trichothecene mycotoxin deoxynivalenol (DON), which threatens the health of humans and animals (Tanaka et al., 1988; Windels, 2000; Su et al., 2018). Up to date, the most effective tools to control FHB are derived from the studies on pathogenic mechanisms and breeding for disease resistant plant (Steiner et al., 2009; Son et al., 2013).
Because lack of the pathogen-specialized patterns that typically induce gene-for-gene-mediated resistance in the host (van Eeuwijk et al., 1995), the infection and spreading of F. graminearum are easily influenced by the environment. The study of pathogenesis mechanisms could be accelerated by analyzing of the F. graminearum pathogenicity. It is necessary to make a standard and an effective protocol of inoculation which is repeatable and accurate. One of the most important method to prevent FHB is breeding for resistant plants. Numerous studies have shown that inheritance of resistance of wheat to FHB is of a quantitative nature. Therefore, the major task of research FHB resistance is to map the quantitative trait loci (QTLs) region, and a large number of QTLs were described by genetic mapping in diverse wheat germplasm (Buerstmayr et al., 2009; Dhariwal et al., 2018). To verify the functions in QTLs region, an effective inoculation protocol is indispensable. However, the traditional wheat inoculation method for resistance identification is using the conidial suspension to widely sprinkle on the wheat surface instead of inoculation in the specific position of wheat in the field. This inoculation method is constrained by many factors, such as lower accuracy or stability, and sensitivity to environment (Fernando et al., 1997; Francl et al., 1999). The objectives of this protocol are to introduce a method of F. graminearum inoculation, which is standard, effective and repeatable on wheat in a growth chamber. It could help to improve the analysis accuracy and reduce the costs to some extent. In brief, the method can lay a foundation for the research of FHB pathogenesis and resistant breeding.
Materials and Reagents
- 20 μl pipette tips
- Centrifuge tube, 50 ml
- Petri dish, round (60 mm x 15 mm)
- Pots (25 cm in diameter)
- Grey cinnamon soil (obtained from wheat field of Yangling)
- 20 cm x 30 cm Plastic bag (Tuopu Daily Chemicals Company, Miaojie, catalog number: MBRL-A )
- Hole puncher (diameter: 2 mm)
- Miracloth (Merck, catalog number: 475855-1R )
- Round toothpick (yekee, catalog number: Y-9892 )
- Flowering stage Wheat (Triticum aestivum) cultivar of the Xiaoyan 22 (available upon request)
- F. graminearum wildtype strain PH-1 (NRRL 31084) (available upon request, Cuomo et al., 2007)
- Pathogenicity defective mutant Fgkin1 (Luo et al., 2014)
- Urea (GB 2004-2001) (Shaanxi Coal Chemical Corporation, Huashan)
- Carboxymethylcellulose sodium salt (Sigma-Aldrich, catalog number: C5678-500G )
- Ammonium nitrate (NH4NO3) (Spectrum Chemical Manufacturing, catalog number: YY975 )
- Mono potassium phosphate (KH2PO4) (Guangdong Guanghua Sci-Tech, catalog number: 1.01863.020 )
- Magnesium sulfate heptahydrate (MgSO4•7H2O) (Guangdong Guanghua Sci-Tech, catalog number: 1.03107.010 )
- Yeast Extract (Oxoid, catalog number: LP0021 )
- Potato dextrose agar medium (Beijing Aoboxing Bio-tech, Aobox®, catalog number: 02-023 )
- Carboxymethylcellulose liquid medium (CMC) (see Recipes; Cappellini and Peterson, 1965)
- PDA liquid medium (see Recipes; Booth, 1971)
Equipment
- Pipettes (Gilson, 20 μl)
- Hemocytometer (0.1 mm, 1/400 mm2) (QIUJING, model: XB-K-25 )
- Artificial climate growth chamber (Controlling temperature and humidity) (Percival Scientific, model: E-36HO )
- Centrifuge (Bench-top high speed centrifuge, Xiangyi, model: L530 )
- Shaker (Shaker-for flasks, Peiying, model: THC-C-1 )
- Microscope (Olympus, model: CX41RF )
- Camera (Nikon, model: D3000 )
Software
- SAS (Statistics Analysis System, ver. 9.2) or R (ver. R-3.5.0)
Procedure
- Wheat cultivation
- Place 6-8 wheat seeds in the pot (25 cm in diameter) with 2/3 Wheatfield soil and cover with soil (1.5-2 cm in height). Add 0.5 L H2O into each pot to make the soil moist.
- Grow the plants in a growth chamber with a photoperiod of 16 h light (20 °C)/8 h dark (18 °C), a relative humidity of 50-65%.
- After approximately 10-12 days at the 2-leaf stage, cultivate the plants at 20 h light (13 °C)/4 h dark (11 °C), with 40-60% relative humidity for 3 weeks. Water once per week.
- Cover the root with additional soil at 4-leaf stage (approx. 5-week-old plants), add 1.2 g urea into each pot and watering. Cultivate the plants at a photoperiod of 20 h light (16 °C)/4 h dark (13 °C), and a humidity of 40-60% for 3 weeks. Water once per week.
- At the booting stages (approx. 8-week-old plants), cultivate the plant at a photoperiod of 18 h light (18 °C)/6 h dark (16 °C), a humidity of 40-60% for 2 weeks, and apply 1-1.5 g urea to each pot. Water once a week.
- At the flowering stages, cultivate the plant at a photoperiod of 18 h light (20 °C)/6 h dark (18 °C), a humidity of 45-55% for 2 weeks. Use approximately 11-week-old plants for inoculating wheat head with fungal conidia.
- One day before inoculation, adjust the plant growth conditions to a photoperiod of 16 h light/8 h dark at 25 °C, and a humidity of 45-55%.
- Wheat head inoculation with F. graminearum
- Preparation of F. graminearum conidial suspension
- Prepare the PDA plate (6 cm), then inoculate tested fungus F. graminearum PH-1 with sterile hole puncher (~2 mm in diameter) onto the PDA plate in a sterile bench, and culture at 25 °C for 2-3 days in the incubator.
- Transfer the 1/4th square inch of fungal plug from PDA plate into a 250 ml conical flask with 50 ml CMC liquid medium in the sterile bench, and culture with shaking at 220 rpm at 25 °C for 3 days.
- Use one layer of sterile miracloth to filter mycelium, collect the filtrate, and wash the mycelium several times. Then transfer the filtrate into a 50 ml tube and centrifuge at 4,000 x g for 8 min at room temperature (if the volume of filtrate is over 50 ml, repeat this step).
- Discard the liquid supernatant carefully, and resuspend the conidia with sterilized water, count the number of conidia with a hemocytometer, and adjust the conidial concentration to 1.5-2.0 x 105/ml.
- Conidial inoculation
Ten microliter conidial suspension (1.5-2.0 x 105/ml) is inoculated to the floret in the middle of lemma and palea of fourth or fifth spikelet from the base of spike (Figure 1). The inoculation condition is at 25 °C, with a high relative humidity by covering the spikes with a plastic bag for conidia germination. Remove the plastic bags at 36 h post inoculation. At least ten wheat heads are inoculated for each strain. Cultivation lasts for 10-12 days before investigating the disease index.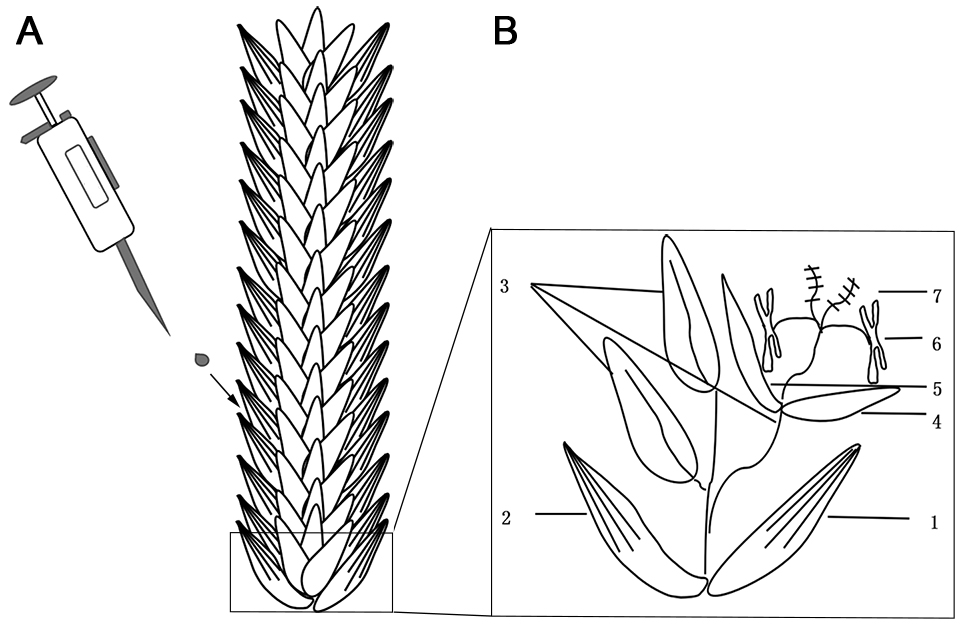
Figure 1. The diagram of conidial inoculation in wheat spike. A. One droplet (~10 μl) of conidial suspension was injected into one floret of a wheat spikelet. B. The enlarged figure is shown the structure of one wheat spikelet in wheat spike: 1. Outer glume; 2. Inner glume; 3. Floret; 4. Lemma; 5. Palea; 6. Stamen; 7. Pistil.
- Preparation of F. graminearum conidial suspension
- Wheat head inoculation by F. graminearum agar discs
This method is specially used for mutant strains which do not produce conidia although it can also be used as an alternative method for wheat head inoculation of normal strains.- Preparation of F. graminearum agar discs
Inoculate the agar block (~2 mm in diameter) of a 3-days pathogen culture onto a PDA plate (6 cm) in a sterile bench and cultivate at 25 °C for 2-3 days. Use a sterile hole puncher (~2 mm in diameter) to cut the plate agar to get the fungus agar block for inoculation. - Inoculation by fungus agar block
Inoculate the fungus agar block (~2 mm in diameter) with a toothpick to the floret in the middle of lemma and palea of fourth or fifth spikelet from the bottom of the wheat at blooming stage (Figure 2). The greenhouse temperature is maintained at 25 °C, and appropriate high relative humidity of the spikes is kept by covering with a plastic bag for conidia germination. Remove plastic bags at 36 h after inoculation. Cultivation lasts for 10-12 days before investigating the block inoculation result. At least ten wheat heads are inoculated for each strain.
Figure 2. The diagram of fungus agar disc inoculation in wheat spike. A mycelial agar disc (~2 mm in diameter) was inoculated into one floret of a wheat spikelet. 1. Mycelial agar disc; 2. Toothpick.
- Preparation of F. graminearum agar discs
- Inoculation result
About 10-12 days post-inoculation, the whole wheat head is generally diseased by inoculation of conidial suspension or agar block of F. graminearum PH-1, while only a part of the wheat head is diseased by inoculation of that of Fgkin1 mutant (Figure 3A). No obvious symptom is observed for the water control.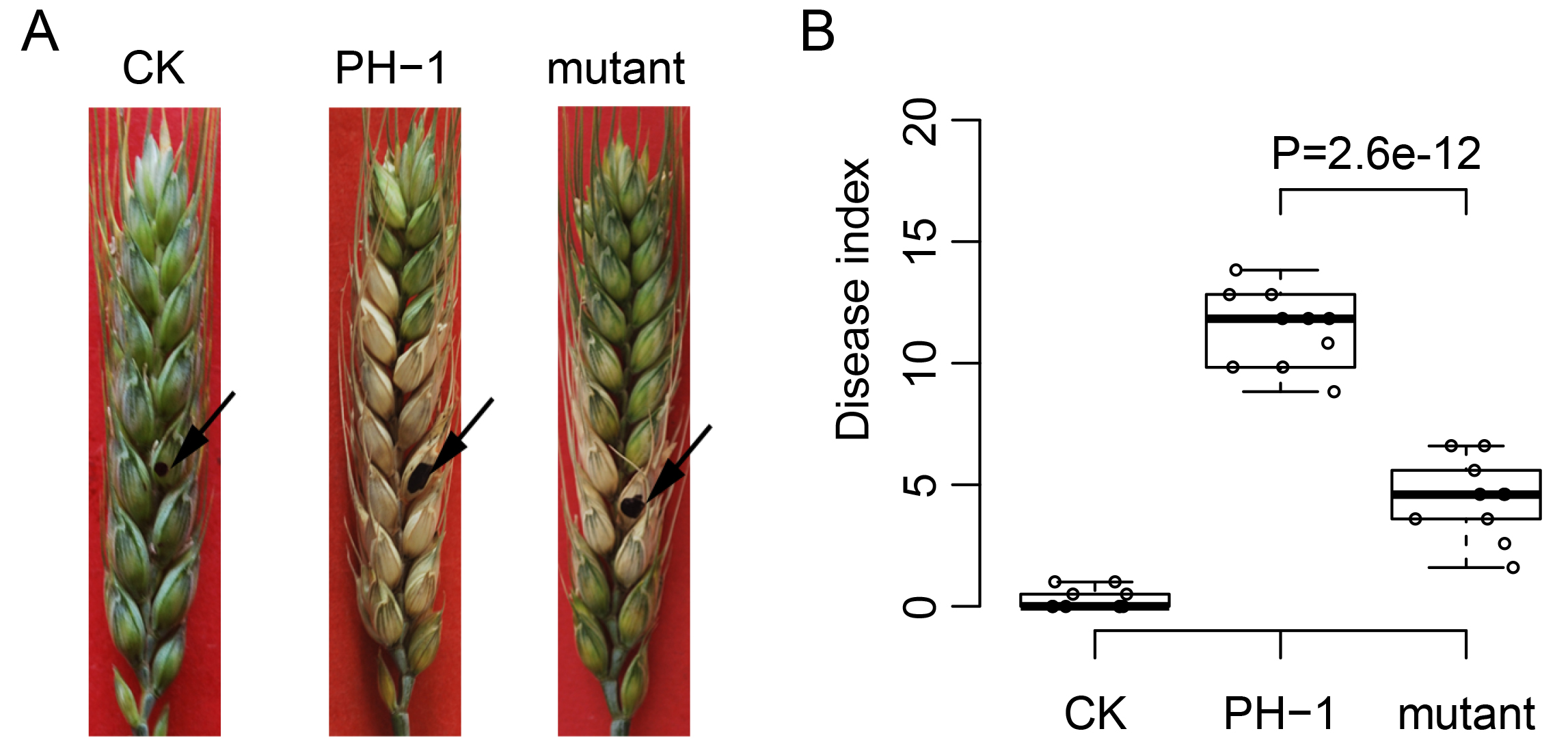
Figure 3. Disease symptom and statistical analysis of wheat head infection assay. A. Flowering wheat heads were drop inoculated with water (CK) or conidia of the wild type PH-1 and Fgkin1 mutant. Black arrows indicate the inoculated spikelet. Disease index was estimated as the number of diseased spikelets on each inoculated wheat heads. B. Box plots comparing the disease index of inoculation with PH-1 and Fgkin1 mutant. The data points are plotted as circles. P value from Student’s t-test is indicated.
Data analysis
Disease index is estimated as the number of diseased spikelets on each inoculated wheat heads. Typically, at least five wheat heads are used to calculate the disease index. Mean and standard deviation can be calculated. In the example of infection assay, the disease index reached 12.6 ± 1.5 for PH-1 while it is only 4.2 ± 1.6 for the Fgkin1 mutant. Bar Chart or Box plot can be used to compare the disease index of different inoculations. The statistical difference can be accessed by using Student’s t-test as developed in SAS or R (Figure 3B). P-value less than 0.05 is considered as statistically significant. The statistical results suggest that the pathogenicity of the Fgkin1 mutant be reduced significantly.
Notes
- According to the growth condition of wheat head, the volume of conidial suspension or fungus agar block could be increased or decreased, but the changes need to be kept consistent.
- Covering the wheat head with plastic bag should be taken carefully, because keep humidity is crucial steps for successful inoculation. Fasten the bag and do not hurt the wheat.
- After inoculation, the wheat should be watered once per 3-5 days.
Recipes
- CMC liquid medium (1 L)
15 g carboxymethylcellulose (CMC)
1.0 g NH4NO3
1.0 g K2HPO4
0.5 g MgSO4•7H2O
1.0 g Yeast Extract
Dissolve CMC in warm dH2O before adding the other ingredients
Autoclave at 121 °C for 20 min - PDA liquid medium (1 L)
37.0 g PDA powder
Dissolve PDA powder in boiling distilled water, then add up to 1 L with distilled water
Autoclave at 121 °C for 15 min
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 31671981). We like to thank Dr. Qinhu Wang for guiding data analysis. The authors declared that no competing interests exist.
References
- Booth, C. (1971). Chap. 1: Fungal culture media. In: Booth, C. (Ed.). Methods in Microbiology. Volume 4. Academic Press, London and New York, 1-48.
- Buerstmayr, H., Ban, T. and Anderson, J. A. (2009). QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed 128(1): 1-26.
- Cappellini, R. A. and Peterson, J. L. (1965). Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia 57(6): 962-966.
- Cuomo, C. A., Guldener, U., Xu, J. R., Trail, F., Turgeon, B. G., Di Pietro, A., Walton, J. D., Ma, L. J., Baker, S. E., Rep, M., Adam, G., Antoniw, J., Baldwin, T., Calvo, S., Chang, Y. L., Decaprio, D., Gale, L. R., Gnerre, S., Goswami, R. S., Hammond-Kosack, K., Harris, L. J., Hilburn, K., Kennell, J. C., Kroken, S., Magnuson, J. K., Mannhaupt, G., Mauceli, E., Mewes, H. W., Mitterbauer, R., Muehlbauer, G., Munsterkotter, M., Nelson, D., O'Donnell, K., Ouellet, T., Qi, W., Quesneville, H., Roncero, M. I., Seong, K. Y., Tetko, I. V., Urban, M., Waalwijk, C., Ward, T. J., Yao, J., Birren, B. W. and Kistler, H. C. (2007). The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317(5843): 1400-1402.
- Dhariwal, R., Fedak, G., Dion, Y., Pozniak, C., Laroche, A., Eudes, F. and Randhawa, H. S. (2018). High density single nucleotide polymorphism (SNP) mapping and quantitative trait loci (QTL) analysis in a biparental spring triticale population localized major and minor effect Fusarium head blight resistance and associated traits QTL. Genes (Basel) 9(1).
- Fernando, W. G., Paulitz, T. C., Seaman, W. L., Dutilleul, P. and Miller, J. D. (1997). Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology 87(4): 414-421.
- Francl, L., Shaner, G., Bergstrom, G., Gilbert, J., Pedersen, W., Dill-Macky, R., Sweets, L., Corwin, B., Jin, Y., Gallenberg, D. and Wirsma, J. (1999). Daily inoculum levels of Gibberella zeae on wheat spikes. Plant Dis 83(7): 662-666.
- Luo, Y., Zhang, H., Qi, L., Zhang, S., Zhou, X., Zhang, Y. and Xu, J. R. (2014). FgKin1 kinase localizes to the septal pore and plays a role in hyphal growth, ascospore germination, pathogenesis, and localization of Tub1 beta-tubulins in Fusarium graminearum. New Phytol 204(4): 943-954.
- Son, H., Kim, M. G., Min, K., Seo, Y. S., Lim, J. Y., Choi, G. J., Kim, J. C., Chae, S. K. and Lee, Y. W. (2013). AbaA regulates conidiogenesis in the ascomycete fungus Fusarium graminearum. PLoS One 8(9): e72915.
- Steiner, B., Kurz, H., Lemmens, M. and Buerstmayr, H. (2009). Differential gene expression of related wheat lines with contrasting levels of head blight resistance after Fusarium graminearum inoculation. Theor Appl Genet 118(4): 753-764.
- Su, P., Guo, X., Fan, Y., Wang, L., Yu, G., Ge, W., Zhao, L., Ma, X., Wu, J., Li, A., Wang, H. and Kong, L. (2018). Application of Brachypodium genotypes to the analysis of type II resistance to Fusarium head blight (FHB). Plant Sci 272: 255-266.
- Tanaka, T., Hasegawa, A., Yamamoto, S., Lee, U. S., Sugiura, Y. and Ueno, Y. (1988). Worldwide contamination of cereals by the Fusarium mycotoxins nivalenol, deoxynivalenol, and zearalenone. 1 Survey of 19 countries. Agric Food Chem 36(5): 979-983.
- van Eeuwijk, F. A., Mesterhazy, A., Kling, C. I., Ruckenbauer, P., Saur, L., Burstmayr, H., Lemmens, M., Keizer, L. C., Maurin, N. and Snijders, C. H. (1995). Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum and F. nivale using a multiplicative model for interaction. Theor Appl Genet 90(2): 221-228.
- Windels, C. E. (2000). Economic and social impacts of Fusarium head blight: changing farms and rural communities in the northern great plains. Phytopathology 90(1): 17-21.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Feng, C., Liu, H. and Tang, Z. (2018). Fusarium graminearum Inoculation on Wheat Head. Bio-protocol 8(15): e2964. DOI: 10.21769/BioProtoc.2964.
Category
Microbiology > Microbe-host interactions > Fungus
Plant Science > Plant immunity > Disease bioassay
Molecular Biology > DNA > DNA extraction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









