- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measuring Secretion of Capsidiol in Leaf Tissues of Nicotiana benthamiana
Published: Vol 8, Iss 15, Aug 5, 2018 DOI: 10.21769/BioProtoc.2954 Views: 6132
Reviewed by: Arsalan DaudiMichael EnosKangquan Yin

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth
Zeinab Fotoohiyan and Ali Salehi Sardoei
Oct 5, 2025 1343 Views

Reproducible Emu-Based Workflow for High-Fidelity Soil and Plant Microbiome Profiling on HPC Clusters
Henrique M. Dias [...] Christopher Graham
Jan 20, 2026 406 Views
Abstract
Plant species produce a wide variety of antimicrobial metabolites to protect themselves against potential pathogens in natural environments. Phytoalexins are low molecular weight compounds produced by plants in response to attempted attacks of pathogens. Accumulation of phytoalexins in attacked plant tissues can inhibit the growth of penetrating pathogens. Thus phytoalexins play a major role in post-invasion defense against pathogens. Major phytoalexins produced by Solanaceous plants are sesquiterpenoids such as capsidiol produced by Nicotiana and Capsicum species, and rishitin produced by Solanum species, which are synthesized in the cytosol and secreted into the intercellular space of plant tissues. We previously reported that deficiency in capsidiol secretion causes enhanced susceptibility of Nicotiana benthamiana to potato late blight pathogen, Phytophthora infestans. Here, we describe a practical protocol to measure the secreted capsidiol in N. benthamiana.
Keywords: CapsidiolBackground
This protocol provides a quick and simple method to quantify the secretion of capsidiol as performed in Shibata et al. (2016). Because cyclohexane is commonly used as the solvent to wash off pollen coat layers but keeps pollen viable (Doughty et al., 1993), we developed this method to wash off secreted metabolites from the leaf surface to quantify extracellular capsidiol. The function of plasma-membrane localized transporters can be analyzed using this method, while also allowing it to be used in conjunction with other methods, such as biochemical transport analysis using plasma membrane vesicles (e.g., Sugiyama et al., 2007), in order to determine the substrate of the examined transporter.
Materials and Reagents
- Pipette tips (e.g., PIPETMAN Diamond Tips, Gilson)
- Needleless syringe (e.g., TerumoTM Tuberculin Syringes 1 ml, Terumo Medical, catalog number: SS-01T )
- 50 ml tubes (e.g., 50 ml Falcon centrifuge tube)
- 200 ml round-bottom flask
- 2 ml tubes (e.g., microcentrifuge tube, 2 ml with lid, BRAND, catalog number: 780550 )
- Leaves of 4-5 weeks old wild-type or gene-silenced Nicotiana benthamiana
Notes:- For Virus-induced gene silencing (VIGS) of N. benthamiana, see Zhang and Liu, (2014).
- Similar method can be applied for other plant species (e.g., see Khare et al., 2017).
- For Virus-induced gene silencing (VIGS) of N. benthamiana, see Zhang and Liu, (2014).
- INF1 elicitor produced by P. infestans or in E. coli
Notes:- For the method of INF1 preparation, see Takemoto et al. (2005) or Shibata et al. (2010).
- Other elicitors may be used as well.
- For the method of INF1 preparation, see Takemoto et al. (2005) or Shibata et al. (2010).
- Capsidiol
Note: Capsidiol is not commercially available. For the method of capsidiol purification, see Matsukawa et al. (2013). - 99.5% Cyclohexane (Wako Pure Chemical Industries, catalog number: 034-05006 )
- 99.5% Cyclohexane/Ethyl acetate (1:1, v/v) (Ethyl acetate, Wako Pure Chemical Industries, catalog number: 051-00351 )
- 99.5% acetonitrile (Wako Pure Chemical Industries, catalog number: 019-08631 )
- Liquid nitrogen
- Methanol (Wako Pure Chemical Industries, catalog number: 131-01826 )
Equipment
- Pipettes (e.g., PIPETMAN P, Gilson)
- Electronic scale (e.g., Mettler-Toledo International, model: AG245 Analytical balance)
- Freezer
- Rotary evaporator with water bath (e.g., Yamato Scientific, model: RE-301-BW )
- Centrifugal concentrator (e.g., TOMY DIGITAL BIOLOGY, model: CC-105 with Vacuum Pump)
- Microtube mixer (e.g., TAITEC, model: E-36 )
- Sonicator Bath (e.g., SND, model: US-101 )
- Mortar and pestle
- Microcentrifuge (e.g., KUBOTA, model: 3740 )
- High-performance liquid chromatography (HPLC) system (e.g., Prominence Modular HPLC system, Shimadzu, Japan)
- ODS column (e.g., Nomura Chemical, model: Develosil ODS-UG-3 )
Procedure
- Treatment of elicitor and preparation of extracellular metabolites of N. benthamiana leaves
- Treat leaves of Nicotiana benthamiana with 150 nM INF1 elicitor solution by injecting the solution into the intracellular spaces of the abaxial sides of the leaves using a needleless syringe (Figure 1A). Apply gentle pressure during infiltration, in order to avoid damaging the leaf tissue. Inject the solution at 5-10 locations to ensure sufficient coverage of the leaf. Incubate for 9 h at 23 °C under light.
- Cut off treated leaves from the plant (Figure 1B) and measure the weight of each leaf using an electronic scale (generally, approx. 1 g for leaf of middle-sized).
Note: Process Steps A2 to A4 one leaf at a time. - Put each leaf into a 50 ml tube (Figure 1C). Avoid rupture of the central vein.
- Washing step: Add approx. 50 ml cyclohexane into each tube. Shake gently for approx. 30 sec at RT (Figure 1D) and then pour the cyclohexane into a 200 ml round-bottom flask (or a fresh 50 ml tube for storage in the freezer) to collect cyclohexane-soluble metabolites on the leaf surface. Freeze and keep the leaf tissue in the same 50 ml tube to extract intracellular (intra-tissue) metabolites later (see steps in Procedure B).

Figure 1. Treatment of elicitor and preparation of extracellular metabolites of N. benthamiana leaves. A. Treatment of elicitor by injecting the solution into the intracellular spaces of the leaves using a needleless syringe. B. Cut off treated leaves from the plant. C. Put each leaf into a 50 ml tube. D. Add approx. 50 ml cyclohexane and shake gently for approx. 30 sec.
Note: Time for shaking can be longer (e.g., a couple of minutes) but it is important to fix the time for all samples. Test leakage of chlorophyll into cyclohexane to make sure that there was no major damage to leaf cells by cyclohexane. See Shibata et al., 2016 for details. - Evaporate the cyclohexane obtained in Step A4 using a rotary evaporator (at approx. 40 °C in a water bath).
- To collect the metabolites dried at the bottom of the round-bottom flask, wash the bottom twice with 1 ml of cyclohexane/ethyl acetate (1:1, v/v) by pipetting and collect the solution (total 2 ml) into a 2 ml tube.
Note: Let the flask cool down to room temperature before adding solvent. - Evaporate the solvent in the 2 ml tube using a centrifugal concentrator (This step usually takes about 1 h).
- Re-dissolve the precipitate in 100 µl acetonitrile, mix thoroughly and add 50 µl ddH2O.
- Shake the 2 ml tubes well using a microtube mixer for 5 min.
- Sonicate the samples by floating the tubes in a sonicator bath for 5 min.
- Centrifuge at 16,000 x g for 1 min at room temperature and collect the supernatant.
Note: Carefully collect 120 µl supernatant to avoid contamination with precipitates. - Analyze the metabolite by HPLC. See Procedure C.
- Treat leaves of Nicotiana benthamiana with 150 nM INF1 elicitor solution by injecting the solution into the intracellular spaces of the abaxial sides of the leaves using a needleless syringe (Figure 1A). Apply gentle pressure during infiltration, in order to avoid damaging the leaf tissue. Inject the solution at 5-10 locations to ensure sufficient coverage of the leaf. Incubate for 9 h at 23 °C under light.
- Preparation of intracellular (intra-tissue) metabolites of N. benthamiana leaves
- Grind leaves of N. benthamiana from Step A4 in liquid nitrogen with a mortar and pestle, and suspend in 50% methanol (1 ml/300 mg leaf tissue).
- Centrifuge at 16,000 x g for 1 min at room temperature and collect the supernatant.
- Add the same volume of cyclohexane/ethyl acetate (1:1, vol/vol) and vortex.
- Centrifuge at 3,000 x g for 5 min at room temperature, and collect the upper phase (ethyl acetate layer).
- Evaporate solvent using a centrifugal concentrator. (This step usually takes ~1 h).
- Re-dissolve precipitate in 100 µl acetonitrile and add 50 μl ddH2O.
- Shake 2 ml well using microtube mixer for 5 min.
- Sonicate samples by floating tubes in a sonicator bath for 5 min.
- Centrifuge at 16,000 x g for 1 min at room temperature and collect the supernatant.
Note: Carefully collect 120 µl supernatant to avoid contamination with precipitate. - Analyze the metabolite by HPLC. See Procedure C.
- Grind leaves of N. benthamiana from Step A4 in liquid nitrogen with a mortar and pestle, and suspend in 50% methanol (1 ml/300 mg leaf tissue).
- High-performance liquid chromatography (HPLC) analysis
Samples from Procedure A and B are subjected to HPLC analysis under the following conditions.
Note: Other conditions may be used though this will influence the elution times of the products.
Column: ODS column (e.g., Develosil ODS-UG-3, Nomura Chemical, Japan).
Solvent: 50% acetonitrile (0 to 3 min), linear increase from 50% to 80% acetonitrile (3 to 13 min), and from 80% to 100% acetonitrile (13 to 14 min).
Injection volume: 5 µl.
Flow rate: 1.0 ml/min.
Detection at 205 nm.
Capsidiol and capsidiol 3-acetate elute at 4.6 and 12.8 min, respectively (see Figure 2). Use peak areas for quantitative analysis.
Note: For the method of capsidiol 3-acetate purification, see Matsukawa et al. (2013).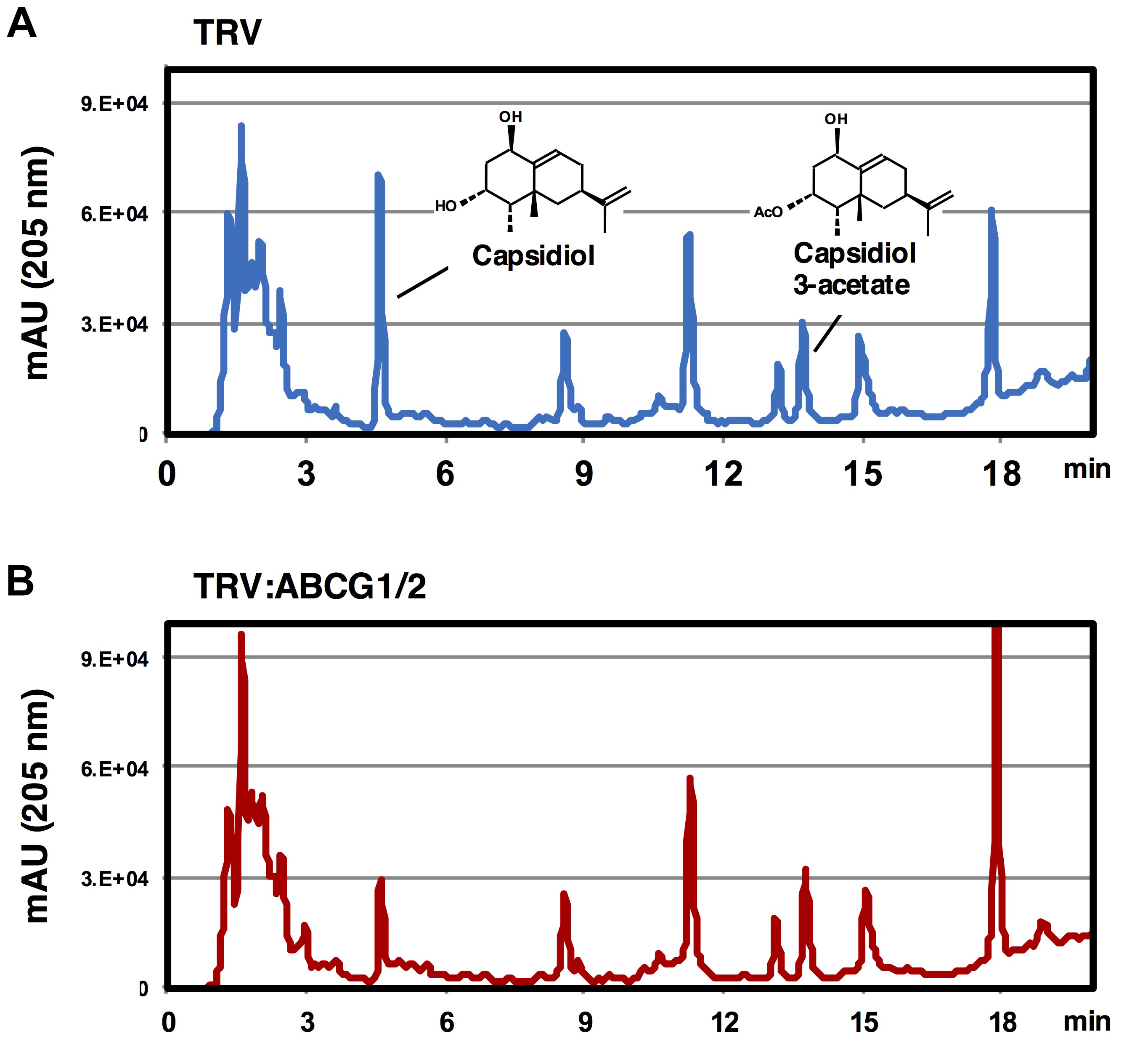
Figure 2. Elution profile of extracellular metabolites of TRV-infected or Nb-ABCG1/2-silenced N. benthamiana. Leaves of TRV-infected (A) or NbABCG1/2-silenced plants (B) were treated with 150 nM INF1. Elution profiles of washing fluids were analyzed by HPLC (detection at 205 nm).
Data analysis
- Test purified capsidiol first for HPLC analysis to determine the score of the peak area per amount of capsidiol, and elution time of capsidiol under your experimental condition.
- Calculate the ratio of secreted/intracellular capsidiol by comparing the amount of capsidiol detected in samples prepared by Procedure A and B from the same leaf.
- To compare the secretion of capsidiol between wild-type and transport deficient gene-silenced N. benthamiana, compare the elution profiles of extracellular metabolites between N. benthamiana lines. See example shown in Figure 1 for gene-silencing of probable capsidiol transporters NbABCG1/2. Peaks of the other metabolites can be used as internal standard to assure consistent extraction (washing) efficiency.
Notes
- The ratio of secreted/intracellular capsidiol of control N. benthamiana leaves treated with 150 nM INF1 for 9 h is around 1.5-2.5 under our experimental conditions. The ratio will be affected by the activity of the elicitor (lot-to-lot difference), the time point of sampling and the age of the leaf, etc. It is therefore important to fix your experimental condition.
- Avoid using elicitors which induce cell death of leaf tissue, since cell death can cause leakage of intracellular metabolites.
Acknowledgments
We thank Prof. Makoto Ojika (Nagoya University, Japan) for technical advice. The work was supported by a Grant-in-Aid for Scientific Research (B) (26292024 and 17H03771) from the Japan Society for the Promotion of Science. No potential conflicts of interest were disclosed.
References
- Doughty, J., Hedderson, F., McCubbin, A., and Dickinson, H. (1993). Interaction between a coating-borne peptide of the Brassica pollen grain and stigmatic S (self-incompatibility)-locus-specific glycoproteins. Proc Natl Acad Sci U S A 90(2): 467- 471.
- Khare, D., Choi, H., Huh, S. U., Bassin, B., Kim, J., Martinoia, E., Sohn, K. H., Paek, K. H. and Lee, Y. (2017). Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin. Proc Natl Acad Sci U S A 114(28): E5712-E5720.
- Matsukawa, M., Shibata, Y., Ohtsu, M., Mizutani, A., Mori, H., Wang, P., Ojika, M., Kawakita, K. and Takemoto, D. (2013). Nicotiana benthamiana calreticulin 3a is required for the ethylene-mediated production of phytoalexins and disease resistance against oomycete pathogen Phytophthora infestans. Mol Plant Microbe Interact 26(8): 880-892.
- Shibata, Y., Kawakita, K. and Takemoto, D. (2010). Age-related resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethylene- and salicylic acid-mediated signaling pathways. Mol Plant Microbe Interact 23(9): 1130-1142.
- Shibata, Y., Ojika, M., Sugiyama, A., Yazaki, K., Jones, D. A., Kawakita, K. and Takemoto, D. (2016). The full-size ABCG transporters Nb-ABCG1 and Nb-ABCG2 function in pre- and postinvasion defense against Phytophthora infestans in Nicotiana benthamiana. Plant Cell 28(5): 1163-1181.
- Sugiyama, A., Shitan, N. and Yazaki K. (2007). Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol 144(4): 2000-2008.
- Takemoto, D., Hardham, A. R. and Jones, D. A. (2005). Differences in cell death induction by Phytophthora elicitins are determined by signal components downstream of MAP kinase kinase in different species of Nicotiana and cultivars of Brassica rapa and Raphanus sativus. Plant Physiol 138(3): 1491-1504.
- Zhang, H. and Liu, Y. (2014). VIGS assays. Bio-protocol 4(5): e1057.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kuroyanagi, T., Camagna, M. and Takemoto, D. (2018). Measuring Secretion of Capsidiol in Leaf Tissues of Nicotiana benthamiana. Bio-protocol 8(15): e2954. DOI: 10.21769/BioProtoc.2954.
Category
Plant Science > Plant immunity > Host-microbe interactions
Cell Biology > Cell isolation and culture > Cell isolation
Molecular Biology > DNA > DNA cloning
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









